Published online Apr 15, 2000. doi: 10.3748/wjg.v6.i2.231
Revised: December 16, 1999
Accepted: December 29, 1999
Published online: April 15, 2000
AIM: To investigate the expression of integrins in rats liver during 3'-Me-DAB induced hepatocarcinogenesis and to find out the relationship between integrins and liver cancer metastasis.
METHODS: The expressions of integrins α1, α2, α3 and α5 and epidermal keratin (EK) were observed by immunohistochemical PAP method.
RESULTS: In the normal liver tissues, hepatocytes express integrins α1 and α5 and in the bile duct epithlium, EK. In liver cirrhosis, hepatocytes highly express integrins α1, α2, α3 and α5 and in hyperplastic bile duct epithelium, integrins α1, α5 and EK. Expression of integrins α1, α2, α3 and α5 were obviously decreased in the preneoplastic nodules and primary carcinoma but expressions of integrins α1 and α5 in metastasis in the lung and diaphragma were higher than those in primary carcinoma.
CONCLUSION: Integrins α1 and α5 may play a major role in chemically induced hepatocarcinogenesis and metastasis in rats.
- Citation: Yuan ST, Hu XQ, Lu JP, KeiKi H, Zhai WR, Zhang YE. Changes of integrin expression in rat hepatocarcinogenesis induced by 3’-Me-DAB. World J Gastroenterol 2000; 6(2): 231-233
- URL: https://www.wjgnet.com/1007-9327/full/v6/i2/231.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i2.231
The integrin superfamily of heterodimeric cell-surface receptors composed of distinct α and β subunits mediates the adhesion of cells to the extracellular matrix and in some instance, the intercellular adhesion. The integrins are thought to play important roles in differentiation and development, cell migration, and the complex process of tumor cell invasion and metastasis, as well as adhesion[1]. Alterations in integrin expressions upon malignant transformation and in naturally occurring human malignances are now well established. However, the mechanisms that give rise to altered integrin protein expression following malignant transformation are poorly understood. We investigate the expression of integrins α1, α2, α3 and α5 in rat liver during 3’-Me’-DAB induced hepatocarcinogenesis and expect to find out the relationship between integrins and liver cancer metastasis.
Male Wistar rats (n = 100, provided by Experimental Animal Center, Shanghai Medical University), weighing 100 g-150 g, were divided into four groups: group A (n = 44), fed with low choline maize powder and 0.03% 3’-Me-DAB for 12 wk; group B (n = 28), fed with low choline maize powder only for 12 wk and group C (n = 28), fed with standard diet only. After 12 wk, the rats of group A and B were fed with standard diet. Four to rats were killed in each group at week 3, 6, 9, 12 and 16 and 8-12 at week 20.
Small pieces of liver samples (including tumor and metastasis) were divided in two parts, one embedded in O.C.T (Miles, USA), rapidly frozen and stored at -70 °C, and the other was fixed with 10% neutral buffered formalin, embedded in paraffin and used for routine histological examination.
The antibodies against integrins α1, α2, α3 and α5 were purchased from Chemicon International INC and those against epidermal keratin (EK) from Nichi Lei Co. Tokyo, Japan. The rabbit PAP kits were prepared by our department[4]. Integrins α1, α2, α3 and α5 and EK in normal liver, liver cirrhosis and liver tumor were detected by PAP method. Briefly, 5 μm cryostat sections of the frozen tissues were fixed with cold acetone and 10% neutral buffered formalin (9∶1) for 10 min, and washed in 0.01 mol/L phosphate- buffered saline (PBS, pH7.4) for 5 times, each for 3 min. The sections were treated with methanol (containing 0.2 mL/L H2O2) at 37 °C for 30 min and washed in PBS, and then incubated in PBS with 100 mL/L normal goat serum at 37 °C for 30 min. The sections were incubated with rabbit anti-integrins α1 (1∶1200 dilution), α2 (1∶1200 dilution), α3 (1∶1200 dilution), α5 (1∶1500 dilution) and EK (1∶1000 dilution) respectively at 37 °C for 30 min and then at 4 °C overnight. The sections were washed in PBS and incubated with goat anti-rabbit IgG (1∶200 dilution) at 37 °C for 40 min. After washing in PBS, the sections were incubated with rabbit PAP complex (1∶200 dilution). The color was developed with 0.5 g/L 3,3’diaminobenzidine/0.5 mL/L H 2O2/0.05 mol/L PBS (pH7.6) for 10 min. Normal rabbit serum instead of the specific primary antibodies was used as negative control.
Only cells with membranous staining were considered; cells with intense dotlike cytoplasm immunoreactivity caused by endogenous peroxidase were ignored. The intensity of staining was divided into +++ (strong), ++ (moderate), + (weak), +/- (equivocal), - (negative). Variable patterns were indicated by combining the two extremes of staining intensity, e.g. +++/+/-.
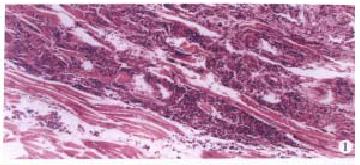
Liver cancer model of the rats in group A was successfully induced by fed 3’-Me-DAB with low choline maize powder. In the early experiment (week 1-9), the main morphologic changes were steatosis and necrosis of hepatocytes, proliferation of oval cells and cholangiofibrosis. In the middle of the experiment (week 10-16), massively proliferated oval cells, and extensive areas of cholangio fibrosis were visible. In some livers, few hepatocytes remained; they were either entrapped in cholangio fibrotic structures or located at the peripheries of these lesions. Then, liver cirrhosis and preneoplastic nodules were obvious. At the end of the experiment, most of the rats in group A had liver tumor, mainly mixed hepatocarcinoma (HCC) and cholangiocarcinoma (CCC). Tumor metastasized to the lung, spleen and diaphragm in some rats (Figure 1). There were no obvious morphologic changes in the livers of rats in group B and C.
The overall staining patterns are shown in Table 1.
| α1 | α2 | α3 | α5 | EK | |
| Normal liver tissue | |||||
| Hepatocytes | + | - | - | + | - |
| Sinusoidal endothelial cells | + | - | - | + | - |
| Bile duct epithelium | - | - | - | - | +++ |
| Liver cirrhosis | |||||
| Hepatocytes | +++ | ++ | ++ | +++ | - |
| Sinusoidal endothelial cells | ++ | + | + | ++ | - |
| Oval cell | +/- | +/- | +/- | +/- | +++/- |
| Hyperplastic bile duct epithelium | ++ | - | - | ++ | +++ |
| Preneoplastic nodules | + | +/- | +/- | + | - |
| Tumor | |||||
| Primary | -/+ | - | - | -/+ | +++/- |
| Metastasis | +++/- | - | - | +++/- | +++/- |
In the normal liver tissues, integrins α1 and α5 were weakly expressed in hepatocytes and sinusoidal endothelial cells but not in bile duct epithelium. The predominant staining was located on the sinusoidal side plasma membranes of hepatocytes. EK was highly expressed in the bile duct epithelium but not in hepatocytes.
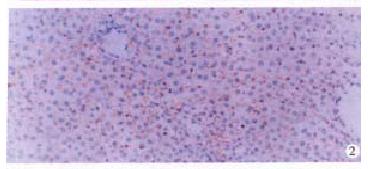
In liver cirrhosis, the expression of integrins α1, α2, α3 and α5 were increased in hepatocytes and sinusoidal endothelial cells but the distribution did not significantly change (Figure 2). However, hyperplastic bile duct epithelium only moderately expressed α1 and α5. Oval cells were equivocal on expression of integrins but most of them highly expressed EK.
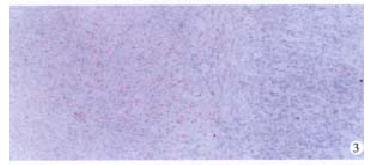
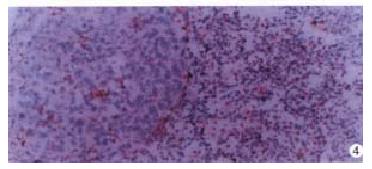
Expression of integrins α1, α2, α3 and α5 were obviously decreased in the preneoplastic nodules and primary carcinoma (Figure 3) but expression of integrins α1 and α5 in metastasis in the lung and diaphragm were higher than in primary carcinoma (Figure 4).
The β1 subgroup of the integrin superfamily of cell-surface receptors (also known as VLA integrins) contains six heterodimers (VLA-1 to VLA-6), in which different α-subunits share a common β1 subunit. They serve as receptor for extracellular matrix components as laminin (VLA-1, VLA-2, VLA-3, and VLA-6), collagen (VLA-1, VLA-2, and VLA-3), and fibronectin (VLA-3, VLA-4, and VLA-5) and are involved as counter-receptors for other cell-surface molecules (VLA-4 recognizes the vascular cell adhesio nmolecule-1 [VCAM-1]) in a cell-to-cell type of interaction[2].
The integrins are thought to play important roles in differentiation and development, cell migration, and the complex process of tumor cell invasion and metastasis, as well as adhesion. The integrin β1 and fibronectin were mainly distributed along sinusoids in normal liver, and the expression was weak in adult normal liver[5]. The distribution did not significantly change during the regeneration and proliferation, but the expression was increased. They acquire some characteristics of fetal and neonatal hepatocytes, including alternations in cell surface properties, such as the composition of glycoconjugates, and the level of receptors for hormones and growth factors and of pro teins involved in cell-cell contacts. In the early phase of liver carcinogenesis, proliferation of oval cells, cholangiofibrosis, proliferation of hepatocytes and the extracellular matrix (cirrhosis) were obvious. The high expression of integrins plays an important role in cirrhosis[6].
Alterations in integrins receptor expression upon malignant transformation and naturally occurring human malignances are now well established[7,8]. It was one of the properties of tumor cells that the expressions of integrins and fibronectin were decreased[9]. Integrins such as fibronection receptor transduce important growth inhibitory stimuli from the extracellular matrix. Integrins are involved not only in the adhesive, motile and invasive behavior of tumor cells but also in their growth regulation.
Expression of intergrins α1, α2, α3 and α5 was obviously decreased in the preneoplastic nodules and primary carcinoma but expression of integrins α1 and α5 in metastasis in the lung and diaphragma was higher than in primary carcinoma. Integrins α1 and α5 may play a major role in chemically induced hepatocarcinogenesis and metastasis in rats, and it was necessary for tumor cells to adher to the extracellular matrix in metastatic focus[10,11].
EK is a mark of epidermal cells. EK was highly expressed by the bile duct epithelium but not by hepatocytes and might be a helpful tool in the differential diagnosis between HCC and CCC. Some oval cells expressed EK but some not. It suggested that oval cells are the progeny of hepatic stem cells, which might differentiate into preneoplastic parenchyma cells and might give rise to HCC.
Edited by You DY
Proofread by Ma JY
Project supported by The Ninth Five-Year Key Program of the Ministry of Health (JB02 96-906-01-15)
| 1. | Albelda SM, Buck CA. Integrins and other cell adhesio nmolecules. FASEB J. 1990;4:2868-2880. [PubMed] |
| 2. | Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11-25. [PubMed] |
| 3. | Yuan ST, Hu XQ, Zhu QS, Lu JP, Lin ZH, Cha XL. Prelimnary studies of the possible mechanisms of the low choline diet on experimental hepatocarcinogenesis. Linchuang Yu Shiyan Binglixue Zazhi. 1996;12:57-59. |
| 4. | Wang XH, Zhang YE, Zhang JS, Xu ZD, Zhang XR. Purification of human type I and type III collagen and preparation of their antisera. Shanghai Yike Daxue Xuebao. 1994;21:405-408. |
| 5. | Voigt S, Gossrau R, Baum O, Löster K, Hofmann W, Reutter W. Distribution and quantification of alpha 1-integrin subunit in rat organs. Histochem J. 1995;27:123-132. [PubMed] |
| 6. | Reif S, Terranova VP, el-Bendary M, Lebenthal E, Petell JK. Modulation of extracellular matrix proteins in rat liver during development. Hepatology. 1990;12:519-525. [PubMed] |
| 7. | Volpes R, van den Oord JJ, Desmet VJ. Integrins as differential cell lineage markers of primary liver tumors. Am J Pathol. 1993;142:1483-1492. [PubMed] |
| 8. | Stamatoglou SC, Manson MM, Green JA, Mayol X, Hughes RC. Distribution of fibronectin and fibronectin-binding proteins, AGp110 and integrin alpha 5 beta 1, during chemically induced hepatocarcinogenesis in adult rats. J Cell Sci. 1991;100:599-604. [PubMed] |
| 9. | Schreiner C, Fisher M, Hussein S, Juliano RL. Increased tumorigenicity of fibronectin receptor deficient Chinese hamster ovary cell variants. Cancer Res. 1991;51:1738-1740. [PubMed] |
| 10. | Kohn EC, Liotta LA. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995;55:1856-1862. [PubMed] |
| 11. | Saiki I, Murata J, Iida J, Sakurai T, Nishi N, Matsuno K, Azuma I. Antimetastatic effects of synthetic polypeptides containing repeated structures of the cell adhesive Arg-Gly-Asp (RGD) and Tyr-Ile-Gly-Ser-Arg (YIGSR) sequences. Br J Cancer. 1989;60:722-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 1.8] [Reference Citation Analysis (0)] |