Published online Dec 15, 1998. doi: 10.3748/wjg.v4.i6.503
Revised: October 20, 1998
Accepted: November 21, 1998
Published online: December 15, 1998
AIM: To determine the correlation between expression of androgen receptor (AR) gene and hepatocarcinogenesis.
METHODS: Male SD rats were used as experimental animals and the animal model of experimental hepatocarcinoma was established by means of 3’-me-DAB administration. Androgen receptor mRNA was detected by a non-radioactive in situ hybridization assay in neoplastic and non-neoplastic liver tissues.
RESULTS: The expression of androgen receptor mRNA was observed only in neoplasticcells and some atypical hyperplastic cells. In the liver tissue of control animal and the remaining normal liver cells adjacent to the carcinoma tissue, no positive signal was seen.
CONCLUSION: Androgen has an important correlation with hepatocarcinogenesis and the expression of androgen receptor gene might be a mark event during hepatocarcinogenesis.
- Citation: Zhao GQ, Xue L, Xu HY, Tang XM, Hu RD, Dong J. In situ hybridization assay of androgen receptor gene in hepatocarcinogenesis. World J Gastroenterol 1998; 4(6): 503-505
- URL: https://www.wjgnet.com/1007-9327/full/v4/i6/503.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i6.503
It is well known that sex hormone plays an important role in regulation of cell growth, organ development and carcinogenesis. But the most majority of studies were focused on the tumors of sex hormone-dependent organs (e.g., mammary carcinoma and prostate carcinoma). By now not much attention has been paid to sex hormone effects on hepatocarcinogenesis. However, some data showed that there is a difference in morbidity and mortality of patients with hepatocarcinoma between male and female[1,2]. Hepatic adenomas have been also demonstrated to have a clear relationship with oral contraceptive use, and it was presumed that there may be hormone receptors within the adenoma cells that mediate tumor growth in response to hormonal stimulation[3]. In addition, some reported that testosterone stimulated tumor growth and it may enhance the progression of chemically-induced hyperplastic nodules to frank malignancy[4]. All these suggested that hepatocarcinoma might also be a hormone-dependent tumor. In this study, male SD rate were used as experimental animals and the animal model of experimental hepatocarcinoma was established by means of 3’-me-DAB administration. Androgen receptor mRNA was detected by a non-radioactive in situ hybridization assay in neoplastic and non-neoplastic liver tissues in an attempt to detect the inner link between the expression of androgen receptor gene and hepatocarci nogenesis, and explore its exact mechanism.
Male SD rats were divided into two groups: ① control group: the animals were fed with standard food; ② experimental group: the animal were fed with the food containing 0.6% 3’-me-DAB, after 14 weeks the food were replaced with standard one. The experimental rats were sacrificed at 4, 8, 14, 17 and 24 weeks respectively. The liver tissue was fixed in 10% formaldehyde and embedded in paraffin.
A fragment of androgen receptor cDNA was cloned in a transcription vector Bluescript between EcoRI and Hind III. By means of transcription in vitro, a RNA probe complementary to the rat AR mRNA was labeled with digoxigenin. The probe was stored at -20 °C.
The sections, that represent different stages of hepatocarcinogenesis, were selected for in situ hybridization assay. After deparaffin and rehydrate, the sections were fixed in 4% paraformaldhyde again. The hybridization was carried out at 50 °C and placed overnight. The hybrids were then revealed by an alkaline phosphatase-conjugated anti-digoxigenin antibody and detected with the detection system of Boehringer Mannheim.
SD rats were divided into 10 groups as shown in Table 1. The specimen from various groups were first used for pathological examination. Results in different stages of experiment has demonstrated a gradual progression of hepatocarcinogenesis. At the 4th and 8th week, hyperplastic foci and nodules appeared, and the carcinoma nodules were seen at the 24th week of experiment.
| 4 weeks | 8 weeks | 14 weeks | 17 weeks | 24 weeks | |
| Experimental group | 5 | 5 | 5 | 5 | 5 |
| Control group | 3 | 3 | 3 | 3 | 3 |
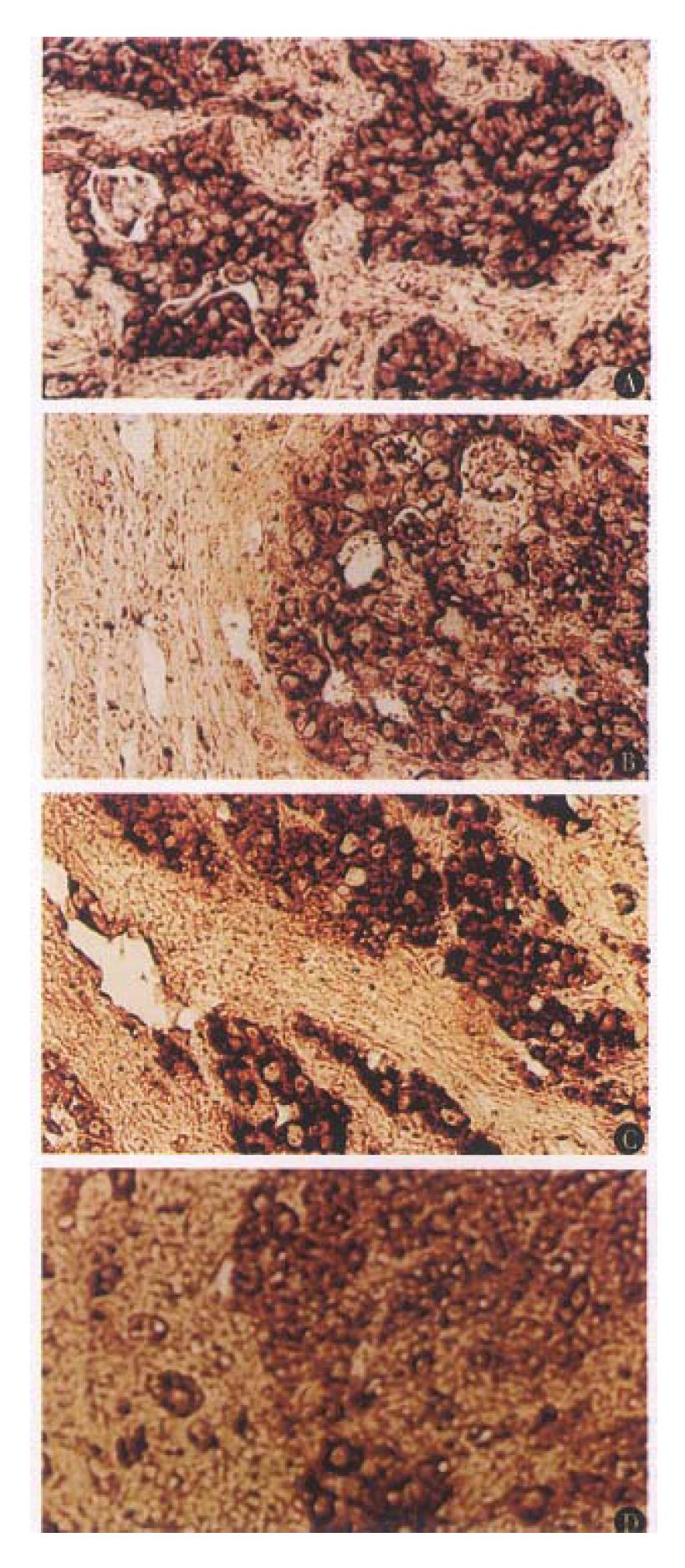
The selected sections from different groups, which reflect the pathological characteristics of hepatocarcinogenesis in different stages, were used for in situ hybridization assay. The rsults showed that no positive signal of AR expression was seen in the early stage of hepatocarcinogenesis (the 4th and 8th week). The signal of AR expression could be detected until the 14th week of experiment (Table 2), and all the hybridization signal was observed in the carcinoma tissues. In the liver tissues of control animal, and the remaining normal liver cells adjacent to the carcinoma tissue, no positive signal was detected (Figure 1A, B). In the experimental group of the 14th and 17th week, some atypical hyperplastic cells also displayed positive signal in varying degrees (Figure 1C, D). In all of control group, no positive signal was seen.
| 4 weeks | 8 weeks | 14 weeks | 17 weeks | 24 weeks | |
| Experimental group | - | - | + | + | + |
| Control group | - | - | - | - | - |
It is not clear yet whether androgen plays a role in regulation of hepatocarcinogenesis. But many data showed that there might be a correlation between androgen and hepatocarcinogenesis. The regional data in Cancer incidence in five continents demonstrated that the morbidity in men is generally higher than in women, no matter where the incidence is high, moderate or low[1]. Besides some men¡äs unhealthy hobby (e.g., excessive drinking) and work surroundings, the potential effects of sex hormone is also a factor which can not be ignored. Another data revealed that the danger of contracting hepatocellular adenoma increased among the women who use oral contraceptives[5]. Some also reported that the patients, who use the steroid-hormone stimulating metabolism of androgen over a long period of time for aplastic anaemia therapy, are susccptible to liver cancer. It is also observed that in some patients the survival was prolonged and the tumors were reduced in size after stopping the hormone-therapy[6]. In view of these data, it has been presumed that there might be some relationship between androgen and hepatocarcinogenesis. However, this conjecture was only based on the clinical observation and the statistical data, it lacks strong experimental proof yet. Our experimental results showed that the expression of androgen receptor mRNA was observed in the atypical hyperplastic cells and in the cells of hepatocellular carcinoma (the 14th, 17th and 24th week), yet in the normal liver tissue and the tissue from the early stage of hepatocarcinogenesis (the 4th and 8th week) no positive signal was seen. This result prompted us further that androgen probably issomewhat related to hepatocarcinogenesis. The strong expression of androgen receptor mRNA in hepatocarcinoma cells and no expression or weak expression (the level of expression might be lower than the threshold for detection) in the normal liver tissue and the remaining normal liver cells adjacent to the carcinoma tissue showed that androgen had an important bearing on hepatocarcinogenesis. It should be noted that androgen receptors express also in varying degrees in the atypical hyperplastic cells. Because the atypical hyperplsia is considered as a precancerous stage, the expression of androgen receptor gene in this stage give us much for thought. Is the synchronism of the appearance of precancerous cells and the expression of androgen receptor a mere coincidence or a necessity? This question is well worth further studying. According to these results, we infer further that androgen has an important correlation with hepatocarcinogenesis and the expression of androgen receptor gene might be a mark event during hepatocarcinogenesis.
Project supported by the National Natural Science Foundation of China, No. 39570348
| 1. | Okuda K, Ishak KG. Noeplasms of the liver. Shanghai Science and Technology Publishing House 1991: 3-9. . |
| 2. | Sun HX. Hepatology. Nanjing: Jiangsu Science and Technology Publishing House 1990; 676-678. |
| 3. | Masood S, West AB, Barwick KW. Expression of steroid hormone receptors in benign hepatic tumors. An immunocytochemical study. Arch Pathol Lab Med. 1992;116:1355-1359. [PubMed] |
| 4. | d'Arville CN, Johnson PJ. Growth factors, endocrine aspects and hormonal treatment in hepatocellular carcinoma--an overview. J Steroid Biochem Mol Biol. 1990;37:1007-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Jick H, Herman R. Oral-contraceptive-induced benign liver tumors--the magnitude of the problem. JAMA. 1978;240:828-829. [PubMed] [DOI] [Full Text] |
| 6. | Guy JT, Smith RE. Androgens and hepatocellular carcinoma. Prevention and detection of cancer, part II: Detection, vol.2: Cancer detection in specific sites. New York: Marcel Dekker 1980; 217-285. |