Published online Oct 15, 1998. doi: 10.3748/wjg.v4.i5.418
Revised: June 29, 1998
Accepted: August 14, 1998
Published online: October 15, 1998
AIM: To evaluate the killing effects of CDDP, 5-Fu and VCR on human hepaoma cell line (7721).
METHODS: The median-effect principle was used.
RESULTS: Killing effects of the individual drug were enhanced as the median concentration increased. Antagonism was produced when two drugs were used at a higher concentration (CI > 1), and synergism was achiened when CI < 1. Finally, the effect was influenced by both the ratios of drug concentration and the sequence of administration.
CONCLUSION: The drug administration order and drug concentrations are significant factors that need to be considered in clinical practice.
- Citation: Tang WX, Cheng PY, Luo YP, Wang RX. Interaction between cisplatin, 5-fluorouracil and vincristine on human hepatoma cell line (7721). World J Gastroenterol 1998; 4(5): 418-420
- URL: https://www.wjgnet.com/1007-9327/full/v4/i5/418.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i5.418
The combined chemotherapy for malignant carcinoma is desired to produce efficacious synergism between each drug, alleviate side effects of drugs and delay drug resistance. Clinically, the interaction (namely synergism, summation and antagonism) of different anticancer drugs in combination is usually evaluated by Chou-Talalay’s combination index ( i.e., median-effect principle)[1-9]. In this paper the combination effect between Cisplatin (Cis), 5-Fluorouracil (5-Flu) and Vincristine (VCR) on human hepatoma cell line 7721, was analyzed in vitro.
A human hepatoma cell line (7721 cells) was obtained from the Department of Bioengineering, Chongqing University. The cell line was cultured in RPMI 1640 (GIBCO, Grand Island, NY) with 10% (v/v) fetal calf serum, and 100 U/mL penicillin-streptomycin antibioties, and kept at 37 °C in a humindified atmosphere of 5% CO2.
Cis was purchased from Yunnan Gejiu Biochemical Pharmarcetics, 5-Fu from Tianjin Renming Pharmaceutical plant and VCR from Shanghai 11th Pharmacentical Factory. These drugs were freshly prepared, dissolved in 0.9% NaCl solution before use, at concentrations of 40, 20, 10, 5, 2.5 mg/L for Cis, 5-Fu 400, 200, 100, 50, 25 mg/L, and VCR 4, 2, 1, 0.5, 0.25 mg/L, respectively.
The cytotoxicity of the drugs on human hepatoma cell line 7721 was examined by MTT assay[10]. Cells (1-5 × 105 /mL) were suspended in 10% FCS RPMI1640 medium and 200 μL/well suspension was plated to 96 well microplate. Negative control and treatment groups were set up for each experiment.
When the drug was used alone, each drug at five different concentrations were added to 96 well microplate at 20 μL/well in three duplicates. When used in pairs, Cis and 5-Fu, Cis and VCR, 5-Fu and VCR at a ratio of 1 × 1 were added to microplate at a total of 20 μL/well (each drug 10 μL at two times concentration that of the drug used alone). Cells were incubated at 37 °C, 5% CO2 for 3 days.
Cis at five different concentrations 2.5, 5, 10, 20 and 40 mg/L were combined with 5-Fu 100, 200 mg/L or VCR 1, 2 mg/L, respectively; VCR 0.25, 0.5, 1, 2 and 4 interacted with 5-Fu at 100 and 200 mg/L. The process lasted 3 days at 37 °C in a humidified atmosphere of 5% CO2.
The time order for Cis and 5-Fu, Cis and VCR administration was as follows: two drugs were given simultancously and cells were cultured for 3 days; and one drug was given first, another was then administrated, and cells incubated for 2 days.
OD valucs of control and treatment groups were detected by Bio-Rad E2550 EIA Reader. Cytotoxicity = 1-( mean OD of treatment group/mean OD of control group).
According to the median equation fa/fu = (D/ Dm)m, the median effect curve, Y = bx + a ( Y = log fa/fu, x = log D) was plotted. D is the dose, Dm is the median effect dose required for 50% cytotoxicity, fa represents the fraction affected by the dose D, fu defined the fraction unaffected equals 1-fa, and m = b is the slope. The median effect dose is calculated by log Dm = -a/m, a = Y - bX. The interaction of two drugs is determined quantitatively by combination index (CI):
CI = (D)1/ (Dx)1 + (D)2/ (Dx) 2 + α (D) 1 (D) 2/ (Dx)1(Dx)2
D1 and D2 are the doses of drug 1 and 2, which are required to produce x% effect in combination. (Dx) 1 and (Dx)2 are the dose of drug 1 and drug 2 required to produce x% effect individually. If the drugs are mutually exclusive, α = 0 and non-exclusive, α = 1. CI = 1, summation; CI < 1, synergism; and CI > 1, antagonism[11].
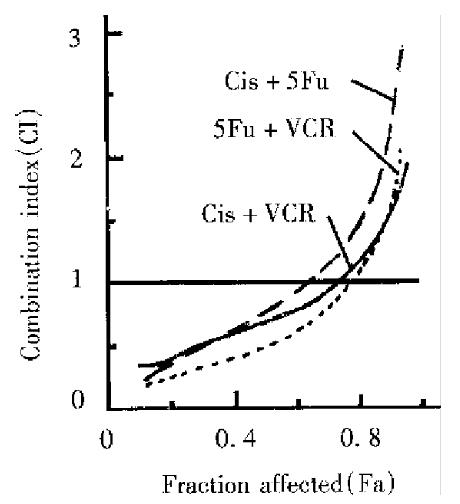
The cytotoxicity of three drugs progressively increased with increasing doses, either used alone or in pairs (Table 1). According to the median-effect equation, the median effect doses of these drugs used alone or in combination can be calculated. When used alone, Cis was 1.158 mg/L, 5-Fu 17.056 mg/L and VCR 5.22 mg/L, respectively. In contrast to the drug used alone, the median-effect doses were lower in combination. Cis plus VCR was 0.85 mg/L (containing Cis 0.82 mg/L and VCR 0.03 mg/L); Cis plus 5-Fu 7.43 mg/L (containing Cis 0.31 mg/L and 5-Fu 7.21 mg/L); and 5-Fu plus VCR was 8.45 mg/L (containing 5-Fu 8.44 mg/L and VCR 0.01 mg/L). The combined effect of two drugs at ratio 1:1 can be illustrated for synergism, summation or antagonism (Figure 1).
| Cis | 5-Fu | VCR | Cis + 5-Fu | VCR + 5-Fu | Cis + VCR |
| (2.5) 0.662 | (25) 0.600 | (0.25) 0.178 | (2.5 + 25) 0.709 | (2.5 + 25) 0.647 | (2.5 + 25) 0.527 |
| (5.0) 0.809 | (50) 0.784 | (0.50) 0.204 | (5 + 50) 0.859 | (5 + 50) 0.713 | (5 + 50) 0.781 |
| (10.0) 0.867 | (100) 0.834 | (1.00) 0.282 | (10 + 100) 0.877 | (10 + 100) 0.810 | (10 + 100) 0.844 |
| (20.0) 0.893 | (200) 0.853 | (2.00) 0.429 | (20 + 200) 0.902 | (20 + 200) 0.839 | (20 + 200) 0.873 |
| (40.0) 0.928 | (400) 0.871 | (4.00) 0.446 | (40 + 400) 0.951 | (40 + 400) 0.892 | (40 + 400) 0.898 |
Cis and 5-Fu The interaction of 5-Fu at a dose of 200 mg/L and Cis at five concentrations exhibited antagonism, the same results can be seen when 5-Fu at 100 mg/L was combined with Cis at ≥ 20 mg/L, but synergism was shown when Cis at 20 mg/L.
Cis and VCR Most cases in which Cis at various concentrations and VCR at 1 mg/L (except for Cis at 2.5 mg/L), were used, showed antagonism. When Cis at various concentrations (except for 2.5 mg/L) was combined with VCR at 2 mg/L, synergestic effect appeared.
VCR and 5-Fu The combination of VCR at > 0.5 mg/L and 5-Fu at 100 mg/L showed synergism. In contrast, when VCR at 0.5 mg/L or < 0.5 mg/L and 5-Fu 100 mg/L, were used in combination antagonism occurred. Moreover, when 5-Fu at up to 200mg/L was combined with Cis at various concentrations (except for Cis. the highest one) the interaction was also antagonism (Table 2).
| Drug 1 (mg/L) | Drug 2 (mg/L) | fa | CI | Drug 1 (mg/L) | Drug 2 (mg/L) | fa | CI | ||||
| Cis | 2.5 | 5-Fu | 100 | 0.821 | 0.75 | Cis | 2.5 | VCR | 2 | 0.554 | 1.15 |
| 5 | 100 | 0.856 | 0.85 | 5 | 2 | 0.858 | 0.80 | ||||
| 10 | 100 | 0.877 | 0.87 | 10 | 2 | 0.862 | 0.74 | ||||
| 20 | 100 | 0.883 | 1.09 | 20 | 2 | 0.873 | 0.71 | ||||
| 40 | 100 | 0.893 | 1.23 | 40 | 2 | 0.904 | 0.66 | ||||
| Cis | 2.5 | 5-Fu | 200 | 0.839 | 1.85 | VCR | 0.25 | 5-Fu | 100 | 0.657 | 2.39 |
| 5 | 200 | 0.867 | 2.93 | 0.5 | 100 | 0.761 | 1.15 | ||||
| 10 | 200 | 0.899 | 2.95 | 1 | 100 | 0.810 | 0.77 | ||||
| 20 | 200 | 0.902 | 3.00 | 2 | 100 | 0.826 | 0.64 | ||||
| 40 | 200 | 0.907 | 3.36 | 4 | 100 | 0.850 | 0.51 | ||||
| Cis | 2.5 | VCR | 1 | 0.498 | 0.32 | VCR | 0.25 | 5-Fu | 200 | 0.759 | 2.13 |
| 5 | 1 | 0.825 | 1.38 | 0.5 | 200 | 0.783 | 1.79 | ||||
| 10 | 1 | 0.844 | 2.05 | 1 | 200 | 0.825 | 1.28 | ||||
| 20 | 1 | 0.852 | 2.09 | 2 | 200 | 0.839 | 1.10 | ||||
| 40 | 1 | 0.894 | 3.40 | 4 | 200 | 0.867 | 0.92 | ||||
As can be seen in Table 2, the more prominent efficacy can be achieved when 5-Fu at lower concentration was used with other two drugs, while VCR could be used at higher concentrations.
| Drugs (mg/L) | Simultaneous exposure | Pre-Cis exposure | Post-Cis exposure | |
| Cis + 5-Fu | ||||
| 2.5 | 25 | 0.709 ± 0.015 | 0.683 ± 0.016 | 0.598 ± 0.010 |
| 5 | 50 | 0.859 ± 0.011 | 0.807 ± 0.027 | 0.773 ± 0.015 |
| 10 | 100 | 0.877 ± 0.013 | 0.824 ± 0.012 | 0.793 ± 0.026 |
| 20 | 200 | 0.902 ± 0.014 | 0.831 ± 0.017 | 0.805 ± 0.016 |
| 40 | 400 | 0.951 ± 0.012 | 0.853 ± 0.015 | 0.822 ± 0.010 |
| Cis + VCR | ||||
| 2.5 | 0.25 | 0.527 ± 0.025 | 0.470 ± 0.023 | 0.199 ± 0.023 |
| 5 | 0.5 | 0.781 ± 0.034 | 0.707 ± 0.022 | 0.407 ± 0.017 |
| 10 | 1 | 0.844 ± 0.015 | 0.789 ± 0.025 | 0.533 ± 0.013 |
| 20 | 2 | 0.873 ± 0.017 | 0.818 ± 0.029 | 0.651 ± 0.021 |
| 40 | 4 | 0.898 ± 0.025 | 0.893 ± 0.020 | 0.752 ± 0.025 |
Cis and 5-Fu When Cis and 5-Fu were used simultancously, the effect was more efficacious than that of Cis used for 24h followed by 5-Fu or 5-Fu followed by Cis (t test, P < 0.05). In contrast, there was no difference between Cis and 5-Fu no matter which was used first (P > 0.05).
Cis and VCR When Cis was given 24h after VCR, the fraction affected was less prominent than that of two drugs treated simultancously or Cis first (P < 0.05). There was no apparent difference between two drugs used simultaneously and Cis used first (P > 0.05).
Cis, a broad spectrus in anti-cancer drug, inhibits RNA and protein synthesis of tumor cells by cross-linking with DNA. 5-Fu, an antimetablic agent, halts DNA and RNA synthesis by inhibiting the activity of thymine nucleotide synthetase at various times in the cell cycle, especially in S phase. Thus, the common mechanism of the two drugs Cis and 5-Fu can stop RNA synthesis. VCR can inhibit cell microtubule integrity and therefore stops nucleospindle formation in cell division. In vitro, these three drugs exhibited pronounced cytotoxicity when used alone, in human hepatoma cell line 7721, but higher doses were needed. Compared with the drugs used alone, both the median effect concentration and the dose required were much lower when used in combination. The results indicate that lower dose in combination can achieve higher efficacy with less side-effects. When used in combination, the time order of administration affected drug cytotoxicity. If the cytotoxicity of the drug was pronounced when used alone, there was no difference when used in combination disregarding which was administered first. If one drug efficacy is more prominent than another, when used alone a higher combined effect can be achieved when the more efficacious drug was used first.
Anticancer drug sensitivity can analyze quantitatively the drug interaction, i.e. syncrgism, summation and antagonism, and hence guiding the clinical treatment.
We would like to thank Professor FAN Wei-Ke for preparing the manuscript.
Project supported by Chinese Ministry of Public Health.
| 1. | Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5258] [Cited by in RCA: 5701] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 2. | Chang TT, Gulati SC, Chou TC, Vega R, Gandola L, Ibrahim SM, Yopp J, Colvin M, Clarkson BD. Synergistic effect of 4-hydroperoxycyclophosphamide and etoposide on a human promyelocytic leukemia cell line (HL-60) demonstrated by computer analysis. Cancer Res. 1985;45:2434-2439. [PubMed] |
| 3. | Chang TT, Gulati S, Chou TC, Colvin M, Clarkson B. Comparative cytotoxicity of various drug combinations for human leukemic cells and normal hematopoietic precursors. Cancer Res. 1987;47:119-122. [PubMed] |
| 4. | Chang BK, Gutman R, Chou TC. Schedule-dependent interaction of alpha-difluoromethylornithine and cis-diamminedichloroplatinum(II) against human and hamster pancreatic cancer cell lines. Cancer Res. 1987;47:2247-2250. [PubMed] |
| 5. | Kohno N, Ohnuma T, Kaneko M, Holland JF. Interactions of doxorubicin and cis-platin in squamous carcinoma cells in culture. Br J Cancer. 1988;58:330-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Howell SB, Hom D, Sanga R, Vick JS, Abramson IS. Comparison of the synergistic potentiation of etoposide, doxorubicin, and vinblastine cytotoxicity by dipyridamole. Cancer Res. 1989;49:3178-3183. [PubMed] |
| 7. | Hofs HP, Wagener DJ, de Valk-Bakker V, van Rennes H, Ottenheijm HC, de Grip WJ. Concentration and sequence dependent synergism of ethyldeshydroxy-sparsomycin in combination with antitumor agents. Anticancer Drugs. 1994;5:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Smith KS, Folz BA, Adams EG, Bhuyan BK. Synergistic and additive combinations of several antitumor drugs and other agents with the potent alkylating agent adozelesin. Cancer Chemother Pharmacol. 1995;35:471-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Lee JT, Park S, Lee JH, Kim BK, Kim NK. Efficacy of in vitro treatment of chronic myelogenous leukemia cell line, K562 cells, using 4-hydroperoxycyclophosphamide, alpha-interferon and gamma-interferon. J Korean Med Sci. 1996;11:26-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 10. | Tang WX, Luo YP, Wang RX. Determination of anticancer drug sensitivity test by the MTT Colorimetric assay for human solid tumors. J Chongqing Med Univ. 1992;17:103-108. |
| 11. | Han R. The drug prophylaxis and chemotherapy for tumor. Beijing: United Publishing House of BMU and PUMC. 1991;315-320. |