Published online Jan 14, 2025. doi: 10.3748/wjg.v31.i2.97239
Revised: October 8, 2024
Accepted: November 26, 2024
Published online: January 14, 2025
Processing time: 205 Days and 17.7 Hours
The objective of the current study was to elucidate the clinical mechanism through which phospholipase D2 (PLD2) exerted a regulatory effect on neutrophil migra
To elucidate the clinical mechanism through which PLD2 exerted a regulatory effect on neutrophil migration, thereby alleviating the progression of acute pan
The study involved 90 patients diagnosed with acute pancreatitis, admitted to our hospital between March 2020 and November 2022. A retrospective analysis was conducted, categorizing patients based on Ranson score severity into mild (n = 25), moderate (n = 30), and severe (n = 35) groups. Relevant data was collected for each group. Western blot analysis assessed PLD2 protein expression in patient serum. Real-time reverse transcription polymerase chain reaction was used to evaluate the mRNA expression of chemokine receptors associated with neutrophil migration. Serum levels of inflammatory factors in patients were detected using enzyme-linked immunosorbent assay. Transwell migration tests were conducted to compare migration of neutrophils across groups and analyze the influence of PLD2 on neutrophil migration.
Overall data analysis did not find significant differences between patient groups
PLD2 exerted a crucial regulatory role in the pathological progression of acute pancreatitis. Its protein expression varied among patients based on the severity of the disease, and a negative correlation existed between PLD2 expression and disease severity. Additionally, PLD2 appeared to impede acute pancreatitis progression by limiting neutrophil migration.
Core Tip: This research substantiated the indispensable role of phospholipase D2 (PLD2) in the pathological progression of acute pancreatitis. Moreover, the varying protein expression levels of PLD2 among patients with distinct degrees of acute pancreatitis exhibited an inverse relationship with disease severity. Moreover, this study revealed PLD2’s potential to mitigate acute pancreatitis by restricting neutrophil movement.
- Citation: Niu JW, Zhang GC, Ning W, Liu HB, Yang H, Li CF. Clinical effects of phospholipase D2 in attenuating acute pancreatitis. World J Gastroenterol 2025; 31(2): 97239
- URL: https://www.wjgnet.com/1007-9327/full/v31/i2/97239.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i2.97239
Acute pancreatitis, a prevalent condition characterized by an acute pancreatic response with intricate and incompletely elucidated pathophysiology[1], poses a substantial global health burden. The United States grapples with severe cases of this condition, leading to significant clinical and economic repercussions[2,3]. Despite relentless research, the underlying causes of acute pancreatitis remains inadequately comprehended. Thus, delving into its pathogenesis and discovering effective treatment strategies is imperative from both theoretical and clinical perspectives. Phospholipase D2 (PLD2), a member of the phospholipase family, facilitates the breakdown of phosphatidylcholine into phosphatidylcholine and choline. It exerts influence over diverse cellular functions encompassing apoptosis, survival, migration, and adhesion[4-6]. PLD2 is associated with the development of various diseases, such as vascular, immune, and neurological disorders. Notably, PLD2 inhibition exhibits potential in ameliorating intestinal mucosal inflammation in inflammatory bowel disease[7]. Furthermore, PLD2 is implicated in cellular response to tissue damage, including acute inflammatory conditions. According to previous research, PLD2 induces the migration of macrophages from the vascular system in cases of acute pancreatitis[8]. While PLD2 appears to play a role in modulating inflammatory conditions like acute pancreatitis, the precise function of PDL2 remains uncertain.
Neutrophil migration plays a crucial role in the progression of acute pancreatitis[9,10], a sudden inflammation of the pancreas that involves a multitude of numerous cells and molecules, including neutrophil migration. Neutrophil migration constitutes a vital component of both inflammation and immune responses[11], a complex process regulated by various factors, including chemokines and their receptors. Chemokines, substances with the capability to attract and guide neutrophil movement, bind to receptors located on the exterior of neutrophils. In acute pancreatitis, the up-regulation of chemokine receptors can trigger neutrophil migration, driven by the release of inflammatory mediators[12]. This study is a retrospective analysis aimed at exploring the clinical mechanism of PLD2 in the development of acute pancreatitis, with a particular focus on its regulatory role in neutrophil migration.
A retrospective analysis was done on 90 hospitalized patients with acute pancreatitis at our hospital from March 2020 to November 2022. The age of the patients ranged from 25 to 64 years, with an average age of 46.24 ± 5.33 years. Among them, 53 were male and 37 were female. Based on the Ranson score, the severity of the patients’ conditions was categorized into three groups: Mild (n = 25), moderate (n = 30), and severe (n = 35) groups. The age range of the patients is 25 to 64 years, with an average age of 46.24 ± 5.33 years. There were 53 males and 37 females. According to the Ranson score, the severity of the patients was divided into three groups: Mild (n = 25), moderate (n = 30), and severe (n = 35). Notably, the difference was not statistically significant for this particular test (P > 0.05). This study has been approved by the ethics committee, and all participants have signed informed consent forms. The Ranson score is a scoring system used to assess the severity of acute pancreatitis, primarily based on the patient’s clinical presentation and laboratory test results (Table 1). This study was approved by the Ethics Committee of China-Japan Friendship Hospital and informed consent was waived because the research was retrospective and conducted on anonymized data.
| Parameter | Mild group (n = 25) | Moderate group (n = 30) | Severe group (n = 35) | Variance ratio | P value |
| Gender (male:female) | Eleven past two p.m. | Thirteen past five p.m. | Thirteen past ten p.m. | 3.109 | 0.413 |
| Age (years) | 45.17 ± 4.28 | 46.83 ± 4.66 | 45.92 ± 5.18 | 5.226 | 0.385 |
| BMI (kg/m2) | 22.46 ± 1.75 | 23.56 ± 1.49 | 22.85 ± 1.88 | 4.035 | 0.307 |
| Smoker | 8 (32.00) | 10 (33.33) | 12 (34.28) | 2.461 | 0.216 |
| Alcohol consumption | 6 (24.00) | 8 (26.67) | 11 (31.42) | 1.975 | 0.445 |
Patients at least 18 years old with characteristic abdominal pain and increased levels of amylase and/or lipase (> three times the normal upper limit), diagnosed with acute pancreatitis through clinical and imaging assessments.
Patients with pancreatitis caused by trauma, chronic pancreatitis, or pancreatic tumor; patients with congestive heart failure, chronic obstructive pulmonary disease, liver cirrhosis, renal insufficiency, autoimmune diseases, active acute infections, and malignant tumors were excluded due to potential confounding effects of systemic inflammatory markers.
The steps of collecting clinical data in this study include medical history collection, physical examination, and laboratory and auxiliary examinations. The prerequisite for obtaining effective data is proficient questioning and physical examination skills: (1) Medical history collection: The main method of collecting medical history is through consultation, which also includes reviewing various medical records of patients. The main body of medical history is symptoms, and the characteristics, occurrence, development, and evolution of symptoms play an important role in forming a diagnosis. But symptoms are not diseases, and physicians should combine medical knowledge and clinical experience in medical history collection to understand and explore the essence of the patient’s disease; (2) Physical examination: Based on the collection of medical history, a comprehensive, standardized, and correct physical examination is conducted on the patient. The positive signs and negative manifestations discovered can become important basis for diagnosing diseases. During the physical examination process, one should investigate and ask questions, think while examining, consider the relationship between symptoms, signs, and diagnosis, verify, supplement, and improve evidence; and (3) Laboratory and auxiliary examinations: Based on obtaining medical history and physical examination data, considering the available laboratory and auxiliary examinations, selecting necessary examinations reasonably will undoubtedly make clinical diagnosis more accurate and reliable.
Blood samples were obtained from each patient after fasting. The blood was collected in red cap tubes, left to clot for at least 20 minutes, and then centrifuged at a 3000 rpm for 10 minutes at room temperature. The resulting serum was stored in 1.8 mL freezing tubes for subsequent analysis.
Proteins were extracted from serum using RIPA lysis buffer and a protease inhibitor. The protein concentration was determined using the BCA protein method. Loading buffer measuring 5 L was added to each sample, followed by denaturation at 95 °C for 5 minutes and additional denaturation on ice for 5 minutes. Protein samples were loaded onto sodium-dodecyl sulfate gel electrophoresis gels for electrophoresis. Subsequently, proteins were transferred to a polyvinylidene fluoride membrane and blocked using 5% skim milk powder for 60 minutes. The primary antibody for PLD2 (diluted 1:2000) was applied to the membrane and incubated overnight at 4 °C. After a Tris-buffered saline with Tween wash, the membrane was incubated with alkaline phosphatase-coupled goat anti-rabbit secondary antibody (diluted 1:2000) for 1 hour. Following another Tris-buffered saline with Tween wash, the membrane was treated with 5 mL BCIP/NBT chromogenic agent, visualization was achieved using the Quality image analysis system.
Total RNA was extracted from serum samples using Trizol. The RNA underwent reverse transcription at 42 °C for 60 minutes, followed by an additional step at 70 °C for 5 minutes. The quantitative real-time polymerase chain reaction (qRT-PCR) reaction mixture consisted of 20 μL in total, comprising 10 μL SYBR Green PCR Master Mix (2 ×), 2 μL cDNA template, 0.5 μL forward primer, 0.5 μL reverse primer, and 7 μL DDH2O. The cDNA template was used for PCR and agarose gel electrophoresis to establish the PCR product’s standard curve. The qRT-PCR reaction program included an initial denaturation step at 95 °C for 5 minutes, followed by denaturation at 95 °C for 10 seconds and annealing at 60 °C for 30 seconds, repeated for 39 cycles. Amplification efficiency was approximately 90%-100%. Following qRT-PCR, the amplicon underwent electrophoresis on a 2% agarose gel, and analysis was performed using the 2-ΔΔCt method.
Plasma samples were obtained by centrifuging a 5 mL venous blood sample at 3000 rpm for 10 minutes and separating the plasma. Enzyme-linked immunosorbent assay (ELISA) kits (Thermo Fisher Scientific Shier Technology, United States) were utilized to measure the levels of C-reactive protein (CRP), tumor necrosis factor (TNF)-α, IL-6, and IL-1β in plasma, following the manufacturer’s instructions. Three samples were collected for each parameter.
After collecting peripheral blood samples from each patient group, the samples were centrifuged at 800 × g for 10 minutes to separate the serum. This serum was then divided into cryopreservation tubes and stored at -80 °C. Neutrophils were isolated using a commercial neutrophil isolation kit according to instructions. Isolated neutrophils were added into the culture medium, combined with 30% patient serum, and placed in a Transwell orifice plate. In the Transwell setup, neutrophils were placed in the upper chamber, and a culture medium-filled orifice plate was placed in the lower chamber. The Transwell orifice plate was placed in an incubator at a constant temperature, allowing neutrophils to migrate from the upper to the lower chamber in the culture medium over a 6-hour period. After incubation, the upper section of the Transwell was removed, and the cells in the lower section were immobilized and stained. The migrating cells in the lower chamber were observed and counted in several randomly chosen visual fields under a microscope. For the in vitro neutrophil migration test, a PLD2 inhibitor (Cmpd101) at a concentration of 5 μM was prepared by dissolving it in DMSO and adding it to the patient’s serum.
Statistical analysis was conducted using IBM’s SPSS software. The data were presented as the mean ± SD from a minimum of three trials. One-way ANOVA was initially conducted, followed by further analyses using Tukey’s and Student’s t-tests to explore the differences among the groups. A significance level of less than 0.05 was considered statistically significant.
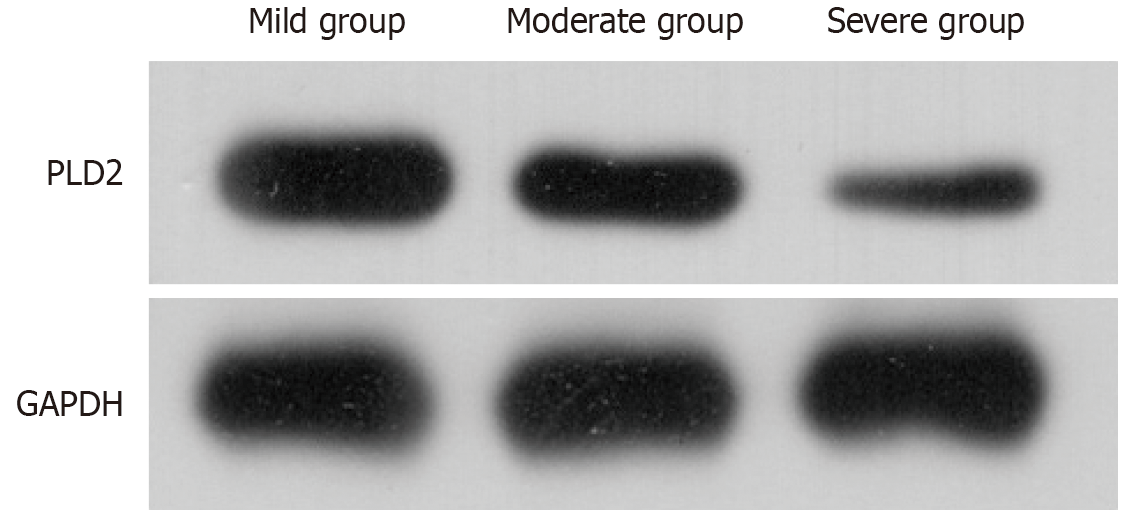
The analysis indicated that the severe group exhibited a decreased protein expression compared to both the moderate and mild groups (P < 0.05), while the moderate group also displayed a lower protein expression in comparison to the mild group (P < 0.05). Pearson correlation coefficient analysis revealed a negative correlation between the severity of acute pancreatitis and PLD2 expression (r = -0.75, P = 0.002) (Figure 1 and Table 2).
| Group | PLD2 |
| Mild group (n = 25) | 1.49 ± 0.10 |
| Moderate group (n = 30) | 1.03 ± 0.05 |
| Severe group (n = 35) | 0.54 ± 0.02 |
| Variance ratio | 13.226 |
| P value | 0.015 |
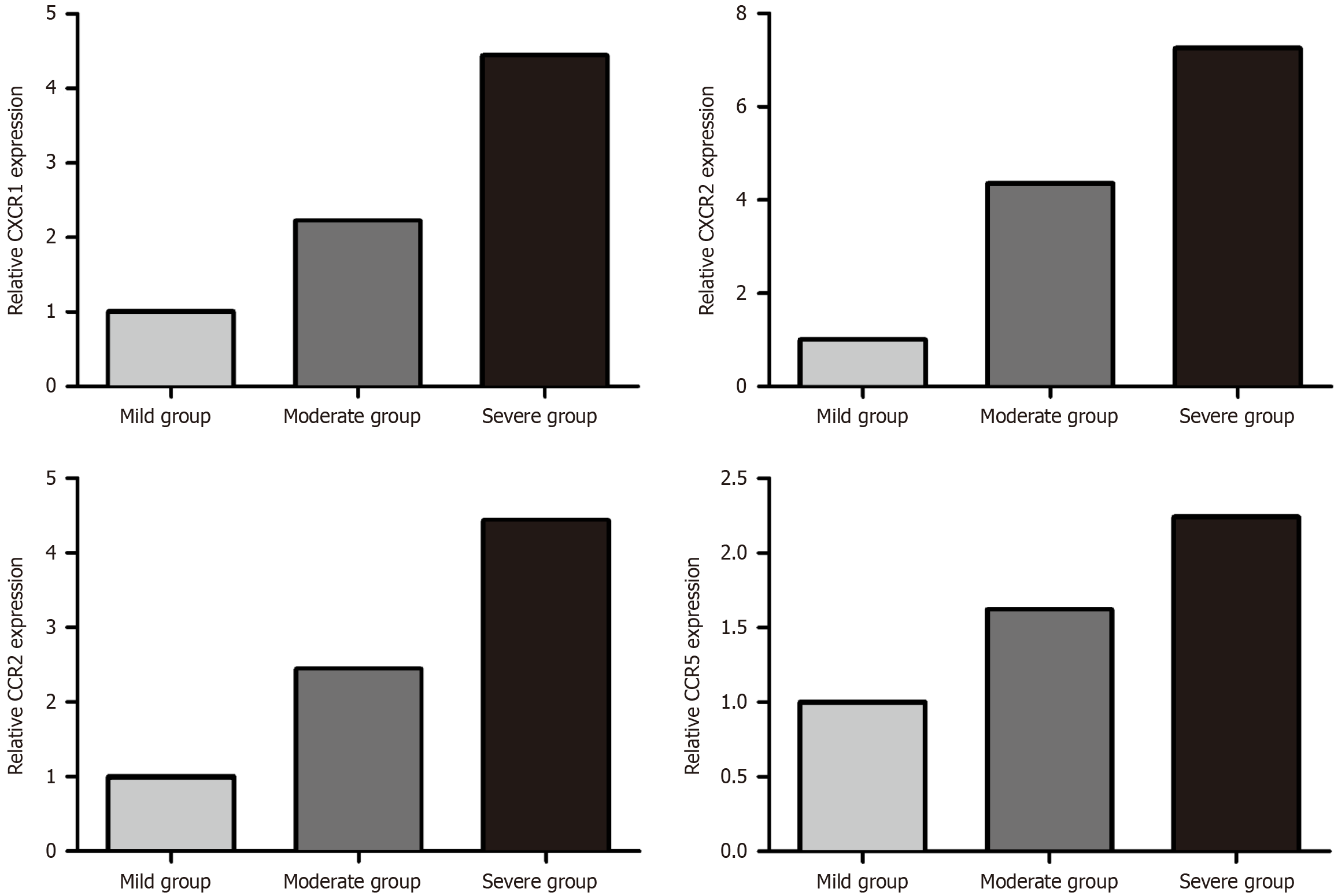
The mRNA expression of chemokine receptors linked to neutrophil migration was detected using RT-PCR. The results revealed significantly elevated mRNA expression levels of C-X-C chemokine receptor type 1 (CXCR1), CXCR2, C-C chemokine receptor type 2 (CCR2), and CCR5 in the severe group compared to both the moderate and mild groups (P < 0.05). Additionally, the moderate group displayed elevated mRNA expressions of CXCR1, CXCR2, CCR2, and CCR5 compared to the mild group (P < 0.05) (Figure 2 and Table 3).
| Group | CXCR1 | CXCR2 | CCR2 | CCR5 |
| Mild group (n = 25) | 1.02 ± 0.01 | 0.97 ± 0.01 | 1.06 ± 0.02 | 1.01 ± 0.01 |
| Moderate group (n = 30) | 2.28 ± 0.07 | 2.05 ± 0.03 | 1.98 ± 0.06 | 1.49 ± 0.05 |
| Severe group (n = 35) | 4.16 ± 0.16 | 4.94 ± 0.15 | 3.02 ± 0.13 | 1.98 ± 0.17 |
| Variance ratio | 9.336 | 12.208 | 13.115 | 10.624 |
| P value | 0.002 | 0.014 | 0.005 | 0.006 |
Serum inflammatory factors were detected using ELISA. The severe group exhibited elevated levels of CRP, TNF-α, IL-1β, and IL-6 compared to both the moderate and mild groups (P < 0.05). Additionally, the moderate group had higher levels of CRP, TNF-α, IL-1β, and IL-6 than the mild group (P < 0.05) (Table 4).
| Group | CRP (mg/L) | TNF-α (pg/mL) | IL-1β (pg/mL) | IL-6 (pg/mL) |
| Mild group (n = 25) | 75.68 ± 10.43 | 36.45 ± 8.24 | 14.39 ± 3.18 | 135.88 ± 15.05 |
| Moderate group (n = 30) | 136.29 ± 10.67 | 68.24 ± 10.67 | 36.27 ± 6.33 | 536.72 ± 25.46 |
| Severe group (n = 35) | 194.18 ± 17.34 | 105.67 ± 12.49 | 58.41 ± 8.62 | 768.55 ± 36.19 |
| Variance ratio | 15.389 | 11.663 | 9.419 | 12.056 |
| P value | 0.015 | 0.026 | 0.003 | 0.006 |
The investigation demonstrated that the severe group exhibited an increased count of migrating neutrophils compared to both the moderate and mild groups (P < 0.05). Additionally, the moderate group had a higher count of migrating neutrophils than the mild group (P < 0.05) (Table 5).
| Group | Number of neutrophil migration (n) |
| Mild group (n = 25) | 235.62 ± 23.15 |
| Moderate group (n = 30) | 568.39 ± 49.37 |
| Severe group (n = 35) | 856.27 ± 54.28 |
| Variance ratio | 13.226 |
| P value | 0.015 |
The Transwell migration test was applied to assess the impact of PLD2 on neutrophil migration. Results indicated that the neutrophil migration count was higher in the mild group + PLD2 inhibitor group when compared to the mild group (P < 0.05). Similarly, the moderate group + PLD2 inhibitor group showed higher neutrophil migration compared to the moderate group (P < 0.05). The severe group + PLD2 inhibitor group displayed increased neutrophil migration compared to the severe group (P < 0.05). The addition of the PLD2 inhibitor significantly promoted neutrophil migration in vitro (Table 6).
| Group | Number of neutrophil migration (n) |
| Mild group (n = 25) | 212.24 ± 24.66 |
| Mild group + PLD2 inhibitor group (n = 25) | 473.69 ± 35.17 |
| Moderate group (n = 30) | 536.19 ± 44.58 |
| Moderate group + PLD2 inhibitor group (n = 30) | 755.03 ± 50.19 |
| Severe group (n = 35) | 828.61 ± 58.21 |
| Severe group + PLD2 inhibitor group (n = 35) | 1126.75 ± 64.38 |
| Variance ratio | 16.204 |
| P value | 0.001 |
An array of triggers, including the activation of pancreatic digestive enzymes, gallstone-related conditions, unhealthy dietary habits, alcohol misuse, and other factors damage to the pancreatic tissue, give rise to a series of symptoms and complications[13,14]. The severe manifestations of pancreatitis can result in significant complications including pancreatic necrosis, abdominal infections, multiple-organ failure, and potentially fatal outcomes[15-17]. Amidst this backdrop, PLD2 emerges as a significant player. Belonging to the phospholipase group, PLD2 facilitates the cleavage of phospha
To assess the severity of the disease, we categorized the patients into mild, moderate, and severe groups based on their Ranson score. Significant differences in PLD2 protein expression were observed among patients with varying degrees of acute pancreatitis, particularly in the severe group. This observation indicated that PLD2 might exert a detrimental regulatory function in the inflammatory process. Alternatively, its expression could be suppressed due to inflammation. Furthermore, Pearson correlation coefficient analysis revealed a negative correlation between acute pancreatitis severity and PLD2 expression. The identification highlighted a possible control mechanism of PLD2 in the pathological progression of acute pancreatitis. The inverse relationship indicated that PLD2 might play a specific role in suppressing the inflammatory response, and its reduced expression might contribute to aggravated inflammation.
Substantial increases in mRNA expression of neutrophil chemokine receptors (such as CXCR1, CXCR2, CCR2, and CCR5) were observed in severe acute pancreatitis patients. This phenomenon might signify active immune cell infiltration regulation triggered by inflammation during the course of acute pancreatitis. Receptors CXCR1 and CXCR2 linked with chemokine IL-8 significantly influence inflammation and the immune responses[22]. Compared with similar studies, this study found that receptors CCR2 and CCR5 are tied to chemokines CCL2 and CCL5 playing a regulatory function in inflammation and immunity[23]. This increased receptor expression could represent the body’s response to inflammatory signals, resulting in neutrophil accumulation at inflammation sites and amplification of the inflammatory response[24]. Although increased receptor expression in severe patients could indicate a natural immune response, it might also lead to excessive neutrophil migration, aggravating inflammatory and tissue damage. This aligned with the pathophysiological mechanism of acute pancreatitis, characterized by cell infiltration and inflammation-induced tissue damage. Hence, increased chemokine receptor expression in neutrophils could significantly impact the pathological progression of acute pancreatitis. The escalation of inflammatory markers additionally reinforced the pathological features of acute pancreatitis. CRP, TNF-α, IL-1β, and IL-6 are common indicators of acute inflammation, and their rise is strongly associated with the inflammatory response. This surge in inflammatory markers may reflect the immune system’s reaction to inflammation, but it could also contribute to pancreatic tissue harm. Phosphatidylcholine specific PLD2 is a key signaling molecule involved in regulating various cellular processes, including cell survival, proliferation, and death. In pancreatitis, changes in the expression and activity of PLD2 may affect the fate of pancreatic cells[25]. The results indicated that PLD2 potentially regulated the pathological progression of pancreatitis. The decrease in PLD2 expression could potentially be associated with increased neutrophil chemokine receptor expression and elevated levels of inflammatory factors, consequently impacting neutrophil migration and the inflammatory process.
The pathways of antioxidant response transcription factors and key regulatory factors of inflammatory response play important roles in cellular response to oxidative stress and inflammation. During pancreatitis, PLD2 may affect the antioxidant capacity and inflammatory response of cells by regulating oxidative stress and inflammatory cyto-kine pathways, thereby affecting the activation of apoptosis pathways and intervening in the progression of inflammatory diseases[26]. The outcomes of the Transwell migration test strongly supported the significance of neutrophils in the progression of acute pancreatitis. Neutrophil migration plays a crucial role in the development and advancement of inflammation during acute pancreatitis. The observed disparities in the Transwell migration test results demonstrated the quantifiable changes in neutrophil migration across different severity groups. This indirectly reaffirmed the crucial involvement of neutrophil migration in the progression of acute pancreatitis. Simultaneously, the introduction of a PLD2 inhibitor led to an elevation in neutrophil movement across varying severity levels, potentially introducing a novel therapeutic intervention. The effect of PLD2 could be manifested through its capability to constrain neutrophil movement. Given that extensive neutrophil movement can exacerbate the inflammatory response during acute pancreatitis, the involvement of PLD2 might partially inhibit this process, exerting a beneficial influence on disease progression. This investigation uncovered the clinical mechanism of PLD2 in acute pancreatitis by conducting a retrospective analysis, specifically emphasizing on its role in restricting neutrophil movement. We uncovered the crucial governing function of PLD2 in the development of acute pancreatitis, as well as its impact on both neutrophil movement and the generation of inflammatory agents. Consequently, this deeper understanding of acute pancreatitis can offer valuable insights for the formulation of future therapeutic strategies involving PLD2.
In summation, this research substantiated the indispensable role of PLD2 in the pathological progression of acute pancreatitis. Moreover, the varying protein expression levels of PLD2 among patients with distinct degrees of acute pancreatitis exhibited an inverse relationship with disease severity. Moreover, this study revealed PLD2’s potential to mitigate acute pancreatitis by restricting neutrophil movement. PLA2 is an early biomarker for a series of complications in severe pancreatitis, and has important clinical significance for evaluating pancreatitis and various organ injuries. If the research results of PLD2 are applied to patient treatment in this study, it will help to develop more targeted nursing strategies for patients with pancreatitis. This study has some limitations, such as a small sample size and lack of regional representativeness. In subsequent research, multi center large sample studies will be organized to improve the credibility of the research results.
| 1. | Lu L, Feng Y, Liu YH, Tan HY, Dai GH, Liu SQ, Li B, Feng HG. The Systemic Immune-Inflammation Index May Be a Novel and Strong Marker for the Accurate Early Prediction of Acute Kidney Injury in Severe Acute Pancreatitis Patients. J Invest Surg. 2022;35:962-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Junare PR, Debnath P, Nair S, Chandnani S, Udgirkar S, Thange R, Jain S, Deshmukh R, Debnath P, Rathi P, Contractor Q, Deshpande A. Complete hemogram: simple and cost-effective in staging and predicting outcome in acute pancreatitis. Wien Klin Wochenschr. 2021;133:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Liu X, Luo W, Chen J, Hu C, Mutsinze RN, Wang X, Zhang Y, Huang L, Zuo W, Liang G, Wang Y. USP25 Deficiency Exacerbates Acute Pancreatitis via Up-Regulating TBK1-NF-κB Signaling in Macrophages. Cell Mol Gastroenterol Hepatol. 2022;14:1103-1122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 4. | Wu Y, Liao GY, Ke HJ, Liu P. Effects of Snake-Derived Phospholipase A2 Inhibitors on Acute Pancreatitis: In vitro and in vivo Characterization. Drug Des Devel Ther. 2020;14:4765-4774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Oliveira SRBD, Franco ÁX, Quaresma MP, de Carvalho CMM, da Cunha Jácome Marques F, da Silva Pantoja P, Mendonça VA, da Silva Osterne VJ, Correia JLA, Assreuy AMS, de Souza MHLP, do Nascimento KS, Cavada BS, Criddle DN, Soares PMG. Anti-inflammatory and anti-necrotic effects of lectins from Canavalia ensiformis and Canavalia brasiliensis in experimental acute pancreatitis. Glycoconj J. 2022;39:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Karabuga B, Gemcioglu E, Konca Karabuga E, Baser S, Ersoy O. Comparison of the predictive values of CRP, CRP/albumin, RDW, neutrophil/lymphocyte, and platelet/lymphocyte levels in determining the severity of acute pancreatitis in patients with acute pancreatitis according to the BISAP score. Bratisl Lek Listy. 2022;123:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Wang J, Zou Y, Chang D, Hong DQ, Zhang J. Protective effect of Dachengqi decoction on the pancreatic microcirculatory system in severe acute pancreatitis by down-regulating HMGB-TLR-4-IL-23-IL-17A mediated neutrophil activation by targeting SIRT1. Gland Surg. 2021;10:3030-3044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Han F, Chen H, Chen L, Yuan C, Shen Q, Lu G, Chen W, Gong W, Ding Y, Gu A, Tao L. Inhibition of Gasdermin D blocks the formation of NETs and protects acute pancreatitis in mice. Biochem Biophys Res Commun. 2023;654:26-33. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Gupta P, Das GC, Bansal A, Samanta J, Mandavdhare HS, Sharma V, Naseem S, Gupta V, Yadav TD, Dutta U, Varma N, Sandhu MS, Kochhar R. Value of neutrophil-lymphocyte ratio in evaluating response to percutaneous catheter drainage in patients with acute pancreatitis. World J Clin Cases. 2022;10:91-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (2)] |
| 10. | Liu GH, Chen J, Li LQ, Huan XS, Lei P. Development and validation of a nomogram for early assessment the severity of acute pancreatitis. Scand J Gastroenterol. 2022;57:990-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Susak YM, Dirda OO, Fedorchuk OG, Tkachenko OA, Skivka LM. Infectious Complications of Acute Pancreatitis Is Associated with Peripheral Blood Phagocyte Functional Exhaustion. Dig Dis Sci. 2021;66:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Ewers M, Epple D, Bugert P, Rosendahl J, Witt H. Genetic analysis of pancreatic phospholipase A2 (PLA2G1B) in patients with chronic pancreatitis. Pancreatology. 2022;22:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Gong M, Pan H, Yang X, Pan C, Ning Y, Li J. Prolonged Intermittent Renal Replacement Therapy Combined with Hemoperfusion Can Improve Early Recovery of Moderate and Severe Acute Pancreatitis, Especially in Patients with Acute Kidney Injury. Blood Purif. 2023;52:75-85. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Lu J, Wang Z, Maimaiti M, Hui W, Abudourexiti A, Gao F. Identification of diagnostic signatures in ulcerative colitis patients via bioinformatic analysis integrated with machine learning. Hum Cell. 2022;35:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Ren ZN, Yang J, Zhang MY, Huang YW, Song DX, Sun X, Pan LL, Sun J. A novel resveratrol analog upregulates sirtuin 1 and inhibits inflammatory cell infiltration in acute pancreatitis. Acta Pharmacol Sin. 2022;43:1264-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Rohith G, Sureshkumar S, Anandhi A, Kate V, Rajesh BS, Abdulbasith KM, Nanda N, Palanivel C, Vijayakumar C. Effect of Synbiotics in Reducing the Systemic Inflammatory Response and Septic Complications in Moderately Severe and Severe Acute Pancreatitis: A Prospective Parallel-Arm Double-Blind Randomized Trial. Dig Dis Sci. 2023;68:969-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Lin Y, Chen Y, Feng W, Zhang J, Hua R, Yin B, Yang X. STAT5 promotes chronic pancreatitis by enhancing GM-CSF-dependent neutrophil augmentation. J Leukoc Biol. 2021;110:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Nabeta R, Katselis GS, Chumala P, Dickinson R, Fernandez NJ, Meachem MD. Identification of potential plasma protein biomarkers for feline pancreatic carcinoma by liquid chromatography tandem mass spectrometry. Vet Comp Oncol. 2022;20:720-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Liu X, Guan G, Cui X, Liu Y, Liu Y, Luo F. Systemic Immune-Inflammation Index (SII) Can Be an Early Indicator for Predicting the Severity of Acute Pancreatitis: A Retrospective Study. Int J Gen Med. 2021;14:9483-9489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 20. | Li H, Qiao C, Zhao L, Jing Q, Xue D, Zhang Y. Epigallocatechin-3-gallate reduces neutrophil extracellular trap formation and tissue injury in severe acute pancreatitis. J Leukoc Biol. 2022;112:1427-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Tong J, Zhou J, Fang M, Wang G, Fu S, Sun B, Lv J. The anti-inflammatory mechanism of SAHA in acute pancreatitis through HDAC5/SLIT2/Akt/β-catenin axis. Hum Mol Genet. 2022;31:2023-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Tang J, Chen T, Ni W, Chen X. Dynamic nomogram for persistent organ failure in acute biliary pancreatitis: Development and validation in a retrospective study. Dig Liver Dis. 2022;54:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Kandikattu HK, Upparahalli Venkateshaiah S, Kumar S, Yadavalli CS, Mishra A. IL-18-mediated neutrophil recruitment promotes acute lung injury in inflammation-mediated chronic pancreatitis. Mol Immunol. 2023;155:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 24. | Hirota M, Murakami K, Koiwai A, Kawamura K, Yoshino Y, Takasu A, Kin R, Katayama T, Endo K, Kogure T, Meguro T, Tabata T, Murakami K, Satoh K. Neutrophil Infiltration and Acinar-ductal Metaplasia Are the Main Pathological Findings in Pembrolizumab-induced Pancreatitis. Intern Med. 2022;61:3675-3682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Kim HJ, Lee DK, Jin X, Che X, Ryu SH, Choi JY. Phospholipase D2 controls bone homeostasis by modulating M-CSF-dependent osteoclastic cell migration and microtubule stability. Exp Mol Med. 2022;54:1146-1155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Kanneganti TD. From pyroptosis, apoptosis and necroptosis to PANoptosis: A mechanistic compendium of programmed cell death pathways. Comput Struct Biotechnol J. 2021;19:4641-4657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 322] [Article Influence: 80.5] [Reference Citation Analysis (0)] |