TO THE EDITOR
The valuable study by Fan et al[1] on the role of transfer RNA (tRNA)-derived fragments (tRFs) 36 in acute pancreatitis (AP) piqued our interest. AP is a pancreatic disease characterized by a hyperinflammatory response[2]. The mortality rate associated with severe AP is high in patients with a hyper-inflammatory response[2]. Due to its complex pathogenesis, there is a lack of effective treatment strategies in clinical practice. Therefore, the condition imposes a significant health burden on humans[3]. Pyroptosis is a type of lytic programmed cell death associated with inflammation, which is typically triggered by inflammasomes[4]. The main features of pyroptosis are cell swelling, membrane perforation, and release of cellular contents[5]. Ferroptosis is a recently discovered form of cell death that is dependent on iron-mediated oxidative damage[6]. Pyroptosis induces ferroptosis, which is involved in AP pathogenesis[6,7]. Preventing pancreatic cell death and inhibiting inflammatory response are two effective therapies to cure AP[4].
Recently, the development of high-throughput sequencing technology has revealed a new class of non-coding RNAs, tRNA-derived small RNAs (tsRNAs), which are tRFs. Emerging evidence indicates that tsRNAs are involved in the regulation of disease development and cellular function. tsRNAs are potential therapeutic biomarkers in various cancers[8]. Liquid biopsy refers to the collection of nonsolid biological tissues, such as blood samples, for the detection of biomarkers and tumor analysis of the whole body[9]. Thus, tsRNA biomarkers are expected to become a new type of liquid biopsy markers[9]. Serum and exosomal tsRNAs may act as novel noninvasive biomarkers for multiple diseases[10]. For example, tsRNA sequencing has shown that tiRNA-VAL-CAC-2 levels are increased in the serum of patients with metastatic pancreatic cancer, and patients with high tiRNA-VAL-CAC-2 Levels have a poor prognosis[11]. Therefore, serum tiRNA-VAL-CAC-2 may serve as a promising prognostic biomarker and potential therapeutic target in pancreatic cancer[11]. Serum tRF-Pro-AGG-004 and tRF-Leu-CAG-002 levels are also powerful promising biomarkers for pancreatic cancer diagnosis[12]. Notably, tRNA-GlyGCC-5 can be used as a non-invasive biomarker of salivary exosomes in human esophageal squamous cell carcinoma[13]. However, there are few studies on tsRNAs that impact the regulation of AP pathogenesis. We elucidate, for the first time, the mechanism by which tsRNAs contribute to increased inflammation and result in the regulation of pyroptosis, ferroptosis, and the development of AP, as well as the characteristics of potential diagnostic markers.
tsRNA and classification
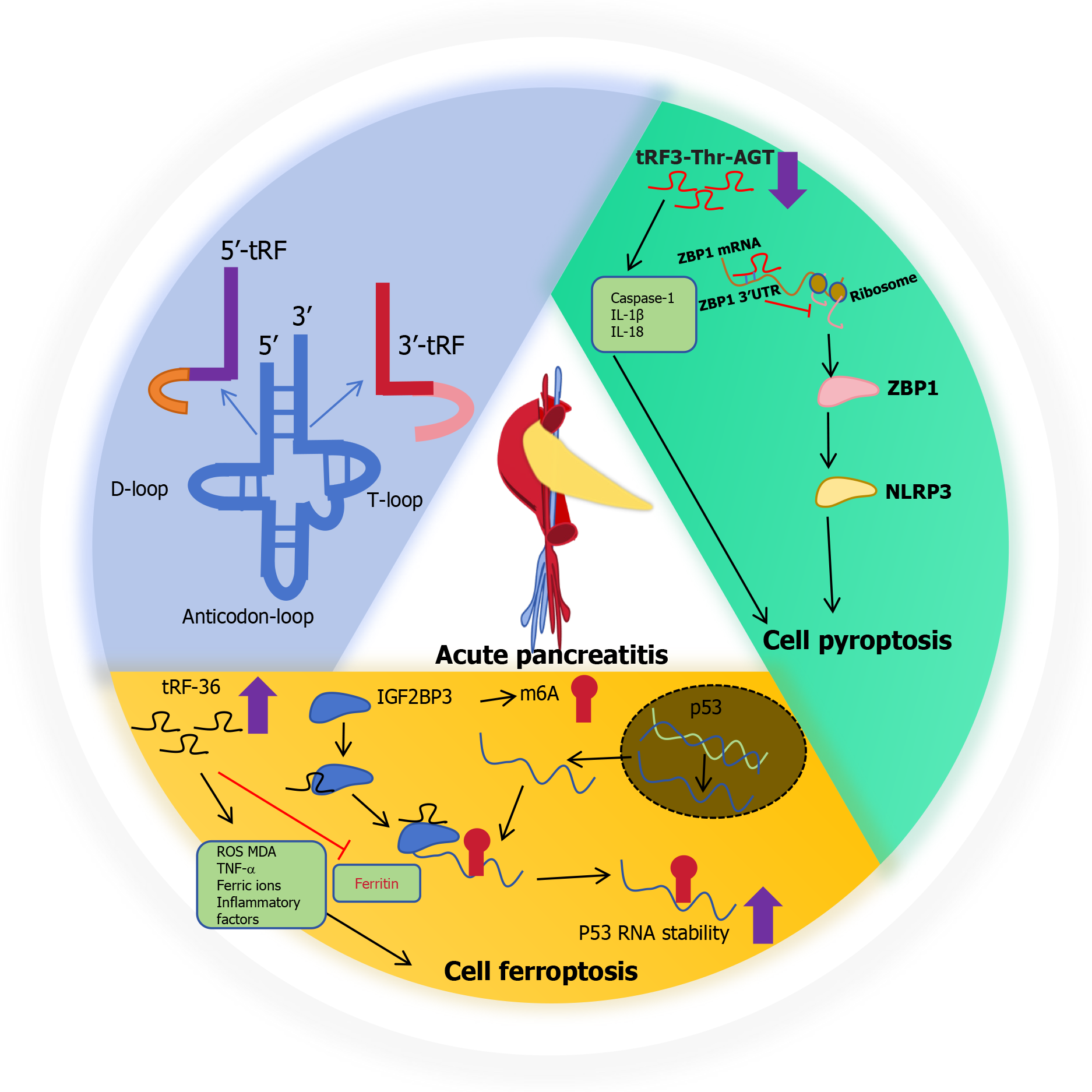
Stable tsRNAs generally exist in a variety of biological tissues, and their expression type, abundance, and modification, may be affected by sex, race, and even the current state of cells and disease[14]. tsRNAs undergo a wide range of processes and chemical modifications during the cell life cycle[14]. In oxygen, hunger, viral infection, arsenite, cells induced by heat shock or heavy metal toxic conditions, stress/angiogenin (ANG) as ribonuclease stress activation and secretion, which can be transferred to the cytoplasm from tRNA into tsRNA, results in tRNA cracking in the cytoplasm[13,14]. tRNA halves (tiRNAs) are 5' - and 3' -tRNA half-molecules of about 29-50 nt in length that are specifically cleaved by ANG at the anticodon loop of mature tRNA under a variety of stress conditions. The length of tRFs is approximately 16-28 nt, and they are derived from mature or precursor tRNA[13]. According to their corresponding positions on tRNA, tRFs can be further divided into: (1) tRF-5, which corresponds to the 5' end of mature tRNA, and cleavage occurs in the D-loop; (2) tRF-3, corresponding to the 3' end of mature tRNA, cleavage occurs in the T-loop; (3) tRF-1, derived from the 3' tail sequence of the precursor tRNA containing a poly-U sequence at the 3' end; and (4) i-tRF, mainly from the middle region of mature tRNA. It has been shown that tRFs and tiRNAs are directly involved in RNA silencing by a mechanism similar to that of miRNAs[15]. tsRNAs form complexes with argonaute (AGO) proteins[13]. tsRNA then can interact and inhibit the expression of target genes by binding to the 3'-untranslated region (3’-UTR) of target genes, and can also compete with mRNA to bind RBP proteins to regulate mRNA function[15]. For example, tRF-33-P4R8YP9 LON4VDP binds to AGO2 protein and negatively regulates the expression of STAT3 by targeting the STAT3 3’-UTR region, ultimately inhibiting the occurrence of gastric cancer[15]. They are involved in cell proliferation, migration, invasion and other processes in different diseases[13].
tsRNA as non-invasive biomakers in AP
With the development of sequencing technology, many diseases associated with tsRNAs have been identified, suggesting that aberrant tsRNAs play important roles in the development of AP. The functional characteristics of serum tsRNAs serve as diagnostic markers for AP and have an independent prognostic predictive value[1]. Liquid biopsy is a more convenient, efficient, and easy-to-obtain minimally invasive means to monitor the assessment of AP, and provides more comprehensive disease information to provide a diagnostic basis for disease treatment. Based on this research, serum samples from three patients with AP and three healthy controls were collected for RNA sequencing, and the specific tsRNAs with dysregulated expression were screened for analysis[1]. Samples were collected from 20 healthy controls and 20 patients with AP for verification[1]. The expression of tRF36 is upregulated in the serum of patients with AP, and its differential expression is the most significant[1]. These findings suggest that tRF36 plays an important role in the progression of AP[1]. Hence, tRF36 is a new discovery that is stable and abundant in vitro, in vivo, and in AP early settings, and has broad application prospects for the early diagnosis of AP.
Molecular mechanism of tsRNA regulating the progression of AP
Programmed cell death in pancreatic acinar cells is a major pathophysiological change in the early stage of AP, and the pattern of pancreatic acinar cell death plays a crucial role in determining its progression and prognosis[1]. Pyroptosis is inflammation-related cell death characterized by an acute inflammatory response that plays a key role in aggravating AP[16]. Ferroptosis is a novel type of cell death characterized by iron-dependent lipid peroxidation in cells[1]. Pyroptosis- and ferroptosis-induced inflammation are closely associated with the occurrence and development of AP.
tRF3-Thr-AGT and cell pyroptosis in AP
Pancreatic acinar cell trypsinogen activation (PAITA) is an important event in during the early stages of AP[17]. Trypsin in the acinar cells can lead to cell death and induce pancreatic inflammation. To establish the PAITA model, taurolithocholic acid 3-sulfate-treated AR42J cells and AP pancreatic tissues were induced in rats. By analysis of the RNA-sequencing, newly discovered tRF3-Thr-AGT is one of the most significant tsRNA, there is a powerful possibility to regulate the AP, and as a potential diagnostic marker and therapeutic target[17]. tRF3-Thr-AGT influences the progression of AP by regulating trypsinogen activation in pancreatic acinus[17]. Interestingly, overexpression of tRF3-Thr-AGT inhibits caspase 1, IL-1β and IL-18 expressions in AR42J cells and the supernatant. Therefore, tRF3-Thr-AGT is involved in regulating pyroptosis and inflammation in vitro[17]. tRF3-Thr-AGT binds to the 3'-UTR of Z-DNA binding protein 1 (ZBP1). Therefore, ZBP1 was identified as a downstream target of tRF3-Thr-AGT, which is closely associated with inflammation and cell pyroptosis[16]. Upregulated tRF3-Thr-AGT inhibits nod-like receptor protein 3 (NLRP3)-mediated pyroptosis in AR42J cells by degrading of ZBP1. Therefore, tRF3-Thr-AGT is mediated by the inhibition of ZBP1 expression to suppress NLRP3 expression in inflammatory cells. This is the role and potential underlying mechanism of a novel tRF3-Thr-AGT in regulating AP pathogenesis[15,16].
tRF-36 and cell ferroptosis in AP
Ferroptosis can trigger immune responses and the production of proinflammatory cytokines, thereby aggravating pancreatic acinar cell death and further aggravating AP[18]. Thus, tRF36 may become a novel diagnostic biomarker for clinical treatment[1]. After knockdown of tRF36, the levels of amylase and lipase in the cell supernatant and inflammatory factors TNF-α, IL-6 and IL-1β were significantly decreased, could significantly improve the cell viability. KEGG pathway enrichment was used to analyze the target genes of tRF36 involved in ferroptosis-related pathways, the P53 signaling pathway, and the mTOR signaling pathway. The expression of ferritin in the pancreatic tissue of AP and AP cell models was significantly decreased, while reactive oxygen species (ROS), ferric ion concentration, and malondialdehyde levels were significantly increased. These results highlighted the phenomenon of ferroptosis in AP. Otherwise, knockout of tRF36 resulted in ferroptosis-related ROS and malondialdehyde levels declining significantly, but increased ferritin expression. RNA pull-down assays and mass spectrometry were used to identify the proteins interacting with tRF-36, and tRF-36 was found to interact with the m6A methylation regulator IGF2BP3. IGF2BP3 is a unique m6A reading protein that promotes stable mRNA expression and prevents P53 mRNA degradation[1]. tRF36 regulates p53 gene expression by binding to IGF2BP3, which recruits p53 mRNA at the m6A modification site. tRF36 enhances p53 mRNA stability and promotes pancreatic follicular cell ferroptosis, thereby promoting AP progression[1].
Figure 1 shows a schematic of the potential mechanism of action of tsRNAs in the progression of AP. tsRNAs have the potential to serve as the gold standard for the prediction of a number of diseases[19]. Therefore, it is important to understand the potential mechanism of AP progression and the evolution of the disease, and to explore effective biomarkers for early diagnosis and progression[16]. The 2024 Nobel prize in physiology or medicine was awarded to scientists Victor Ambros and Gary Ruvkun for their discovery of miRNAs and their roles in post-transcriptional gene regulation. Both of miRNAs and tsRNAs can be detected by RNA sequencing, miRNA microarrays, and real-time fluorescent quantitative PC. miRNAs and tsRNAs exhibit high stability and great potential as diagnostic molecular markers. In living organisms, DNA is transcribed to produce mRNA, which is translated into protein. miRNAs and tsRNAs play important regulatory roles in transcriptional translation and can degrade mRNA or inhibit its translation, thereby controlling the expression of proteins. Similar to miRNAs, tsRNAs plays a key role in cell proliferation, cell death, individual growth, and homeostasis maintenance. Interestingly, tsRNA structure, size, and function are similar to those of miRNA, and it has been found that tsRNA derived from tRNALeu and tRNALys has the same sequence as those of miR-1280 and miR-1274a/b[20,21]. tsRNAs develop alongside microRNAs and play an important role in human diseases. We will focus on tsRNAs to explore more and better characteristics, including RNA modification, subtype classification, and the transition from animal models to human beneficial directions. Additionally, small RNA drugs may be a powerful therapeutic approach for targeted drugs[22]. Therefore, drugs targeting tsRNAs closely related to AP could be designed to further promote personalized precision treatment, especially because they can target the current state of tsRNA expression in the serum of patients. For example, tRF36 knockdown significantly increases cell viability and reduces cell death; therefore, it is possible to design suppressive drugs targeting tRF-36 to treat AP. To open new avenues for next-generation targeted small RNA therapy, precision medicine research should be conducted to reduce the toxic side effects of the long-term use of drugs and surgical trauma, resulting in double damage to physical and mental health. However, small RNA drug targets also face challenges because non-target cells may take up the resulting targeting effect, resulting in detachment from the target cell. In addition, tsRNAs with similar sequences in organisms may affect small-molecule drugs. In addition, naked, chemically unmodified RNA is unstable and lacks a delivery vector suitable for the target organ and cell type. Therefore, small-noncoding RNA drugs need to be further optimized to overcome these limitations and be truly efficient in the treatment of diseases.
Figure 1 Schematic of a potential mechanism of type of transfer RNA-derived small RNA in the progression of acute pancreatitis.
tRF: Transfer RNA-derived fragment; ROS: Reactive oxygen species; MDA: Malondialdehyde; ZBP1: Z-DNA binding protein 1.