Published online Sep 15, 1997. doi: 10.3748/wjg.v3.i3.150
Revised: December 19, 1996
Accepted: January 22, 1997
Published online: September 15, 1997
AIM: To investigate the effect of cryopreservation at -50 °C on the human hepatoma SMMC-7721 cell line.
METHODS: With 15% DMSO as a cryoprotectant, the SMMC-7721 cells were cryopreserved at -50 °C, then thawed and recultured. The survival rate, mitotic index and LDH isoenzymes were compared between pre- and post-cryopreservation.
RESULTS: Thirteen hours after the thaw, the mitotic index of cryopreserved SMMC-7721 cells decreased by 1.09%. The mode scope of chromosome number (46-53) after cryopreservation tended to transfer to that of normal human cells, and the percentage of metaphases containing 46 chromosomes changed from 0% to 16%. LDH isoenzymes changed from H-like model (LDH3(29.3%) > LDH4 (26.8%) > LDH2 (25.3%) > LDH5 (14.9%) > LDH1 (3.6%) to M-like model (LDH4 (48.3%) > LDH5 (28.3%) > LDH3 (18.9%) > LDH2 (4.4%) > LDH1 (0%)). This suggests that the survival rate could reach over 95%.
CONCLUSION: Cryopreservation at -50 °C can be a convenient method for the cryopreservation of cell lines. However, cryopreservation at -50 °C is likely involved in the changes of the malignant phenotypes of the human hepatoma SMMC-7721 cell line, and may induce the differentiation of malignant cells.
- Citation: Jiang SM, Xu ZH, Zhang Y, Shi XM. Survival and malignant phenotype changes of human hepatoma SMMC-7721 cell line induced by cryopreservation at -50 °C. World J Gastroenterol 1997; 3(3): 150-152
- URL: https://www.wjgnet.com/1007-9327/full/v3/i3/150.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i3.150
Cryopreservation and cryotherapy playimportant roles in the research and therapy of cancer. It has been reported that -50 °C is in the great ice crystal forming temperature region (-40 °C to -60 °C), in which cells are greatly damaged, even if they incubated at this temperature for a very short period of time (10 min)[1]. This is particularly damaging to the cellular membranes and the chromatin. Therefore, how to pass this temperature region successfully is a key step in the cryopreservation of not only for maintaining cell suspensions, but also whole organs, such as heart, kidney, etc. Many studies have shown that some chemical reagents, such as sodium butyrate, dimethyl sulfate (DMSO), cAMP Vitamin A, and Vitamin D3 can induce the differentiation of malignant cells in vitro[2-4]. Nothing is known about the differentiation of cancer cells induced by the cryopreservation, especially at -50 °C. In this report, we provide some evidence that cryopreservation at -50 °C may be involved in the changes of malignant phenotypes of human hepatoma SMMC-7721 cell line.
The human hepatoma SMMC-7721 cell line was obtained from the Shanghai Cell Bank of Academia Sinica and maintained in our laboratory. The cells were cultured in RPMI 1640 medium (Gibco Inc) supplemented with 10%-20% fetal calf serum (FCS) and incubated at 37 °C, with 5% CO2/95% air. The cells attached and spread well on either untreated plastic plates or untreated glass flasks. In the cryopreservation study, the cells were firstly passaged in glass flasks. When the cells grew to exponent stages, the culture medium was removed, and the cells were released from the flask using 0.25% trypsin (in D-Hank′s solution, pH 7.2) for 1 min. The cells were then re suspended in RPMI 1640 medium. The cells were counted using a Bueker′s chamber. Cell viability was detected by mixing the cell suspension with 0.5% aqueous solution of trypan blue (1:1). The cell suspension was adjusted to the concentration of 1-5 × 106/mL with 85% RPMI-1640 medium and 15% ice cold DMSO before being placed at -50 °C in an ultracold freezer (Sanyo, Japan).
After being cryopreserved at -50 °C for 6 mo, the cells were taken out from the freezer and thawed in a 40 °C water bath, swirling until the ice was dissolved. The cell suspension was transferred into the centrifugation tube, centrifuged at 500 rpm for 3 min, the preservers were removed and washed with RPMI-1640 medium for 3 times by centrifugation. The cell viability was determined as described above. The cells were recultured in RPMI-1640 medium. The rate of attachment was determined after 12 h in culture by counting the unattached cells.
After being cryopreserved and treated as described above, the cells were adjusted to the concentration of 2 × 105/mL, plated in 24-well Costar tissue culture plastic dishes, and incubated at 37 °C, with 5% CO2/95% air for 3 h. After that the cells on cover slips in 4 parallel wells were taken out at an interval of 2 h, fixed in cooled methanol:acetic acid (3:1) and stained with Feulgen stain, and hematoxylin and eosin. The mitotic index of 1000 cells from each well were counted. The control group was treated in the same way as the experimental group but the cryopreservation step was omitted.
After being cultured for three days, the cryopreserved cells were given colchicine at a final concentration of 0.01 μg/mL and cultured for 6 h. The cells were then released from the flask using 0.25% trypsin and resuspended in RPMI-1640. After centrifuging the cell suspension to remove the trypsin, 0.075 mol/L KCl was added to the cells, and the cells were then incubated in a 37 °C water bath for 10 min. Following this, the cells were fixed with cooled methanol: acetic acid (4 °C) for three times. The chromosomes were spread onto slides and the routine method for Giemsa stain was used to examine the chromosome sample. The mode scope of chromosomal number of 100 metaphase figures was examined under a microscope.
After being cultured to the exponent stage, the cryopreserved cells were collected and homogenized. The supernatant of the cells was collected and LDH isoenzyme analysis was done using PAGE electrophoresis. After staining, the LDH bands were scanned with a gel scanning detector, and the percentage content of each band of the LDH was recorded.
After being cryopreserved in different DMSO concentrations with RPMI-1640 medium, or different DMSO concentrations with 10% FCS and RPMI 1640 medium at -50 °C for 6 mo, the cells were rapidly thawed in 40 °C water bath and washed with RPMI-1640 medium 3 times. The survival and the rate of attachment after 12 h in culture are listed in Table 1.
| 5 | 5 | 10 | 10 | 15 | 15 | 20 | 20 | 30 | 30 | 40 | 40 | DMSO (%) |
| 0 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | FCS (%) |
| 90 ± 2 | 90 ± 3 | 95 ± 2 | 98 ± 2 | 98 ± 1 | 99 ± 1 | 93 ± 4 | 95 ± 2 | 87 ± 4 | 98 ± 2 | 80 ± 4 | 83 ± 2 | SR (%) |
| 60 ± 3 | 65 ± 2 | 85 ± 4 | 90 ± 3 | 85 ± 3 | 90 ± 2 | 55 ± 2 | 60 ± 4 | 20 ± 4 | 25 ± 5 | 1 ± 1 | 2 ± 1 | AR (%) |
In all cells frozen with DMSO, the viabilities detected by trypan blue resistant staining were all greater than 80%, which suggested that DMSO could protect cells from damage during cryopreservation at -50 °C. However, the rates of attachment were significantly different between DMSO concentrations, with a sharp decrease in attachment when treated with 30% DMSO, and very few cells attaching on the flasks in the group treated with 40% DMSO. Rather, the cells treated with 40% DMSO tended to gather in large cell clumps, remaining suspended in the medium, with many of the cells in a bubble like form. The addition of FCS to the cryoprotectant DMSO increased both the survival and the attaching rates at all concentrations. Based on the results of our study, we recommended using 10% and 15% DMSO with 85% RPMI 1640 medium as the in the freezing medium when cryopreserving a the cell line. The results clearly indicated that cryopreservation at -50 °C could be used as a practical and convenient method for cryopreserving SMMC-7721 cells.
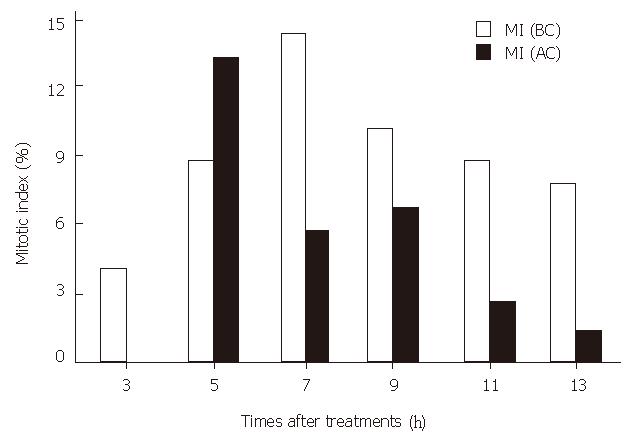
The comparison of mitotic index between SMMC-7721 cells that were not cryopreserved with those that were cultured for 13 h after 6 mo of cryopreservation is shown in Figure 1.
The MI of the cryopreserved cells was lower than that in controls at all time points examined, except for that in the 5th hour, when the MI was higher in the cryopreserved cells. The highest MI in the cryopreserved cells (13.31%) was 1.09% lower than that in the normal cultured cells. The results suggest that the cryopreservation at -50 °C could decrease the MI of SMMC-7721 cells.
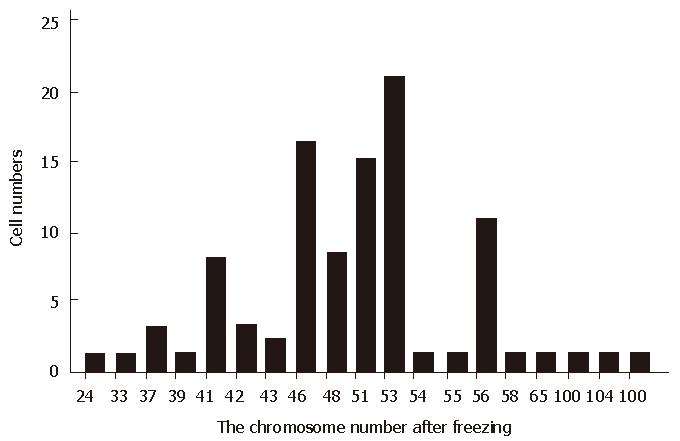
After being cryopreserved more than ten times, the mode scope of chromosomal numbers ranged from 24 to 109, the main scope being 46-53 (58%) (Figure 2), and the percentage of cells with 46 chromosomes was 16%, which was significantly different from the SMMC-7721 cells in the time of establishment[5].
The changes of LDH patterns after cryopreservation at -50 °C are shown in Table 2.
| LDH5 | LDH4 | LDH3 | LDH2 | LDH1 | |
| SC (AC) | 28.3 | 48.3 | 18.9 | 4.4 | 0 |
| SC (E) | 14.9 | 26.8 | 29.3 | 25.3 | 3.6 |
| BEL7404 cell | 1.7 | 22.95 | 37.76 | 27.21 | 10.38 |
After being cryopreserved at -50 °C, LDH2 and LDH3 contents decreased and the LDH4 and LDH5 contents increased as compared with the LDH patterns in the time of establishment of the SMMC-7721 cell line. These same differences were also evident in the cryopreserved cells when compared to the human hepatoma BEL7404 cell line. After cryopreservation, the content of LDH4 was the highest (48.3%), LDH5 the second (28.3%), with the sequence of the LDH activity in these cells being LDH4 > LDH5 > LDH3 > LDH2 > LDH1.
Many chemical reagents, such as sodium butyrate, DMSO, dBcAMP, vitamin A, and vitamin D3 can induce the differentiation of cultured malignant cells, which suggested that these agents might be useful in the treatment of cancer. To our knowledge, the differentiation induced by cryopreservation at -50 °C has not been reported. In this report, we have found that the survival rate of human hepatoma cells SMMC-7721 at -50 °C is 95%, as indicated by the trypan blue resistant staining. These cells also had an 85% attachment rate, spreading on the surface o f culture dishes when treated with 10%-15% DMSO as a cryoprotectant. DMSO could penetrate rapidly into the cells and decrease the great ice crystal formation, suggesting that DMSO concentration of 5%-40% could protect the cells from damage of great ice crystal at -50 °C, and the cell survival rates were all more than 80% with trypan blue resistant staining. However; high concentration of DMSO was toxic to cells, with a lower survival rate than lower concentrations of DMSO, but more importantly, the rates of attachment on the flasks after cryopreservation was much lower at 40% DMSO. These low rates of attachment might be induced by the high osmotic pressure in cells treated with high concentration of DMSO, which caused the cells to swell up and may have destroyed the cells in the process of revitrification. It is suggested that the cryopreservation at -50 °C with 10%-15% DMSO as a cryoprotectant is a practical and convenient method.
It is important to remember that -50 °C is in the great-ice-crystal forming temperature region in which cells can suffer great damage, and the cell membrane system might be changed greatly. This damage may induce many changes in physiology and biochemistry, maybe even in chromosomal and gene level. The success of cryopreservation at -50 °C and the high survival rate provided may not only be a convenient method for cryopreservation, but may also offer a new means for the study of gene expression and control in response to lower temperature.
We observed a decrease in the mitotic index of cryopreserved SMMC-7721 cells during the 13 hour culture at all time points examined except for that in the 5th hour, in which the MI was higher than that in control group. This time point discrepancy might be due to the synchronism of some cells after cryopreservation, as many cells might be going to the M phase together. These results suggested that after the culture period, the growth rate in the cryopreserved group might be lower than that in the control group.
After being cryopreserved at -50 °C more than 10 times, the chromosomal number, the main scope of chromosomal number and LDH isoenzymes of SMMC-7721 cells were compared between cells both before and after cryopreservation. The cell line in the establishment was a superdiploid cell line, its scope of chromosomal number was 44-107, the main scope 54-58,and the percentage of cells with 46 chromosomes was 0%. After being cryopreserved, chromosomal number changed to a range of 24-109, the main scope to 46-53, the percentage of cells with 46 chromosomes to 16%. These suggested that the cells with superdiploid chromosomes had a lower tolerance to -50 °C than those with a normal chromosome complement, and after being cryopreserved at -50 °C several times, both the main scope and the scope of chromosomal number returned to a normal level. The LDH isoenzymes of SMMC-7721 cells in the establishment tended towards H pattern (LDH3 (29.3%) > LDH4 (26.8%) > LDH2 (25.3%) > LDH5 (14.9) > LDH1 (3.6%) (H pattern > M pattern). After being cryopreserved at -50 °C for several times, the LDH isoenzyme contents were changed to LDH4 (48.3%) > LDH5 (28.3%) > LDH3 (18.9%) > LDH2 (4.4%) > LDH1 (0%), but the normal LDH isoenzymes of human hepatocyte were of M pattern, that was LDH5 > LDH4 > LDH3 > LDH2 > LDH1. The comparison showed that the LDH pattern of SMMC-7721 cells was also returned to the normal pattern of hepatocyte cells after being cryopreserved with the distinct decreasing of LDH2 content and increasing of LDH4 and LDH5. This LDH pattern, which is different from that of human hepatoma cells, has showed that genes controlling LDH M pattern are located in the chromosome 11, and the M pattern genes are located on chromosome 12. Both the LDH pattern and the change of main scope of chromosome suggested the changes of LDH isoenzyme were induced by the change of chromosomes.
From our results, we may conclude that the cryopreservation at -50 °C with 10%-15% DMSO as a cryoprotectant is not only a practical and convenient method for storage, but also might induce the differentiation of human hepatocarcinoma SMMC-7721 cells, thus opening a new way for researching into the gene expression and control in response to lower temperature.
Shi-Ming Jiang, Male, born on 1961-11-26. Master of science, graduated from Department of Biology, Shandong Normal University in 1985. Associate professor, having 28 papers and 1 book published.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Hu S
| 1. | Sakai A, Kobayashi S, Oiyama I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990;9:30-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 197] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Yang SM. [DMSO induced differentiation of human gastric adenocarcinoma cell line MGC 80-3]. Shiyan Shengwu Xuebao. 1994;27:281-287. [PubMed] |
| 3. | Rizzo AM, Gornati R, Rossi F, Bernardini G, Berra B. Retinoic acid induces changes in Xenopus embryo glycolipid pattern. Cell Biol Int. 1995;19:895-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Okazai T, Bell RM, Hannun YA. Sphngomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989;264:19076-19080. [PubMed] |
| 5. | Dong RC, Zhou RH, Lu FD. The establishment and biological study of human hepatoma SMMC-7721 cells. Academic Journal of Second Military Medical University. 1980;1:5-9. |