Published online Aug 14, 2023. doi: 10.3748/wjg.v29.i30.4685
Peer-review started: May 5, 2023
First decision: July 9, 2023
Revised: July 16, 2023
Accepted: July 27, 2023
Article in press: July 27, 2023
Published online: August 14, 2023
Processing time: 97 Days and 5.7 Hours
Upper gastrointestinal neoplasia mainly includes esophageal cancer and gastric cancer, both of which have high morbidity and mortality. Lymph node metastasis (LNM), as the most common metastasis mode of both diseases, is an important factor affecting tumor stage, treatment strategy and clinical prognosis. As a new fusion technology, endoscopic ultrasound (EUS) is becoming increasingly used in the diagnosis and treatment of digestive system diseases, but its use in detecting LNM in clinical practice remains limited.
To evaluate the diagnostic value of conventional EUS for LNM in upper gastro
Using the search mode of “MeSH + Entry Terms” and according to the prede
A total of 22 studies were included in our study, including 2986 patients. The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic score and diagnostic odds ratio of conventional EUS in the diagnosis of upper gastrointestinal neoplasia LNM were 0.62 [95% confidence interval (CI): 0.50-0.73], 0.80 (95%CI: 0.73-0.86), 3.15 (95%CI: 2.46-4.03), 0.47 (95%CI: 0.36-0.61), 1.90 (95%CI: 1.51-2.29) and 6.67 (95%CI: 4.52-9.84), respectively. The area under the summary receiver operating characteristic curve was 0.80 (95%CI: 0.76-0.83). Sensitivity analysis indicated that the results of the meta-analysis were stable. There was considerable heterogeneity among the included studies, and the threshold effect was an important source of heterogeneity. Univariable meta-regression and subgroup analysis showed that tumor type, sample size and EUS diagnostic criteria were significant sources of heterogeneity in specificity (P < 0.05). No significant publication bias was found.
Conventional EUS has certain clinical value and can assist in the detection of LNM in upper gastrointestinal neoplasia, but it cannot be used as a confirmatory or exclusionary test.
Core Tip: This meta-analysis examined the diagnostic value of conventional endoscopic ultrasound (EUS) for lymph node metastasis (LNM) in upper gastrointestinal neoplasia. The pooled analyses of 2986 patients from 22 studies performed herein show that conventional EUS has certain clinical value and can assist in the detection of LNM in upper gastrointestinal neoplasia, but it cannot be used as a confirmatory or exclusionary test. More high-quality studies are needed to further verify the diagnostic value of EUS and determine the best diagnostic criteria.
- Citation: Chen C, Song YL, Wu ZY, Chen J, Zhang Y, Chen L. Diagnostic value of conventional endoscopic ultrasound for lymph node metastasis in upper gastrointestinal neoplasia: A meta-analysis. World J Gastroenterol 2023; 29(30): 4685-4700
- URL: https://www.wjgnet.com/1007-9327/full/v29/i30/4685.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i30.4685
Upper gastrointestinal neoplasia mainly includes esophageal cancer and gastric cancer, and their morbidity and mortality have long been among the top ten of the global cancer list, bringing great pain and burden to countries all over the world, and they are major global public health problems[1-4]. The onset of esophageal cancer and gastric cancer is hidden, and the best time for treatment has often been passed by the time they are clinically diagnosed. Lymph node metastasis (LNM), as the most common metastasis mode of both diseases, is an important basis for tumor staging, which largely determines the treatment plan and clinical prognosis of patients[5-8]. For patients with early tumor stages and no LNM, we can attempt endoscopic minimally invasive treatment, but for patients with LNM or advanced tumor stages, it is often necessary to consider comprehensive treatment, including radiotherapy, chemotherapy or surgery[9-11]. One study showed that when esophageal cancer has 0, 1-2 or more than 2 malignant lymph nodes, the median patient survival time is 66 mo, 14.5 mo or 6.5 mo, respectively[12]. Therefore, it is very important to accurately predict LNM.
Endoscopic ultrasound (EUS) combines the advantages of endoscopic technology and ultrasound technology; that is, it can evaluate the mucous membrane of the digestive tract with the naked eye, and it can also be used to detect the hierarchical structure and surrounding tissues of the digestive tract wall with ultrasound wave. EUS has the advantages of close observation distance, high resolution, low price and few adverse events. Since the 1980s, EUS has been gradually used in the diagnosis and treatment of many digestive system diseases, including the staging of gastrointestinal tumors, the identification of submucosal tumors, and the study of pancreatic or biliary tract diseases[13,14]. Conventional EUS uses grayscale imaging technology for analysis, which can clearly display the status of lymph nodes near upper gastrointestinal neoplasia and identify the nature of lymph nodes according to the imaging features. When the endosonographic characteristics of lymph nodes are hypoechoic, round in shape, with a clear boundary and a size greater than 1 cm, the accuracy of conventional EUS in predicting malignant lymph nodes is more than 80%[15]. Some studies have shown that the ability of conventional EUS to detect LNM in upper gastrointestinal neoplasia is better than that of computed tomography and positron emission tomography, but some scholars believe that the diagnostic performance of conventional EUS is poor, and study results have varied widely[16-20]. The purpose of this meta-analysis was to explore the diagnostic value of conventional EUS for LNM in upper gastrointestinal neoplasia to guide clinical practice more effectively.
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis statement[21,22]. The study protocol was registered in the PROSPERO database with the number CRD42022372170.
We used the “MeSH + Entry Terms” search mode to conduct a comprehensive search of the PubMed, EMBASE and Cochrane Library databases before October 1, 2022. The specific search terms were as follows: (“esophageal neoplasms” OR “stomach neoplasms” OR “duodenal neoplasms”) AND (“lymphatic metastasis” OR “lymph nodes”) AND “endosonography” AND “diagnostic test search strategy”. We also manually searched the references of related studies.
We imported all the retrieved articles into EndNote software (Version X9.1; Clarivate Analytics; Philadelphia, United States). Two researchers independently conducted study selection according to the predetermined inclusion and exclusion criteria with the process of identification, screening, eligibility and inclusion. To ensure consistency, we conducted exercises and tests before the formal selection, and the data were verified for internal consistency with the Kappa test during the selection process. If there was any disagreement, the decision was made by the two researchers together through consultation.
The study inclusion criteria were as follows: (1) Patients older than 18 who had recently been diagnosed with upper gastrointestinal neoplasia such as esophageal cancer, gastric cancer, and duodenal cancer; (2) LNM detected by conventional EUS; and (3) Diagnostic testing.
The exclusion criteria were as follows: (1) Studies published before 2000; (2) Case reports, conference abstracts, reviews, comments, letters, meta-analyses and systematic reviews; (3) Animal or in vitro models used as the objects of the study; (4) Sample size less than ten cases; (5) Inclusion of only stage cN0 patients; (6) Patients with other malignant tumors; (7) Patients who received or may have received preoperative neoadjuvant therapy; (8) Use of assistive technologies such as fine needle aspiration (FNA); (9) LNM diagnosis not made with postoperative pathological examination as the gold standard or radical surgery not performed for all patients; (10) Per patient not used as the analysis unit; (11) Inability to extract 2 × 2 tables of true positives (TP), false positives (FP), false negatives (FN), and true negatives (TN); (12) Repeated publication of the same data; and (13) Full text of English literature not found.
Two researchers independently extracted the study data using the predetermined data extraction form, and when they faced disagreement, a third researcher was consulted. Extracted data included: (1) Study characteristics such as first author, publication year, study country, study design and participating center; (2) Diagnostic test characteristics such as EUS model, EUS scan type, EUS examination method, EUS scan frequency, EUS diagnostic criteria, type and number of image interpretation experts, blinding, interval between EUS and surgery, gold standard and analysis unit; (3) Patient/tumor characteristics such as tumor type, tumor location, tumor stage, tumor histological type, neoadjuvant therapy, location of metastatic lymph nodes, age, sex and sample size; and (4) Statistical indicators such as TP, FP, FN, and TN. If the data were not reported directly, the sensitivity, specificity, accuracy and other indicators were used for reverse calculation.
Two researchers independently assessed the quality of the included studies using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool[23]. Disagreements were resolved through consultation. The results of the quality assessment were presented using Review Manager software (Version 5.3.5; Nordic Cochrane Centre; Copenhagen, Denmark).
All data evaluation and picture generation were completed by Stata software (Version 14.0; StataCorp LP; Texas, United States) using the MIDAS module of the bivariable mixed effects model. This model not only considers factors such as heterogeneity between studies, threshold effect and study size but also enables the bivariate nature of the original data to remain unchanged throughout the analysis process, thereby generating reliable statistical indicators. The pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic score (DS) and diagnostic odds ratio (DOR) were calculated by drawing forest plots. The higher the values of DS and DOR were, the better the diagnostic effect of conventional EUS. The area under the curve (AUC) was obtained by drawing a summary receiver operating characteristic (SROC) curve, and the diagnostic performance was considered low, moderate, and high for AUCs of 0.5-0.7, 0.7-0.9 and 0.9-1.0, respectively. Fagan’s nomogram was used to reveal changes in the posttest probabilities. Likelihood ratio scatter diagram was used to evaluate the diagnostic performance of conventional EUS. Sensitivity analysis was used to assess the influence of individual studies on heterogeneity and observe the stability of the summary statistics. The threshold effect was determined according to whether the ROC plane showed a “shoulder-arm” point distribution. The Q statistical test was applied to assess the heterogeneity among the included studies, and heterogeneity was considered statistically significant when P < 0.05. The degree of heterogeneity was estimated based on the I2 statistic, where I2 < 25%, 25%-50%, 50%-75%, and ≥ 75% were considered low, moderate, substantial, and considerable heterogeneity, respectively. If the heterogeneity was high, meta-regression and subgroup analysis were used to explore the most significant source of heterogeneity. Publication bias was assessed with Deeks’ funnel plot, and P < 0.05 indicated statistical significance. The statistical methods of this study were reviewed by Professor Yao Zhang from the Department of Epidemiology, College of Preventive Medicine, Army Medical University of China.
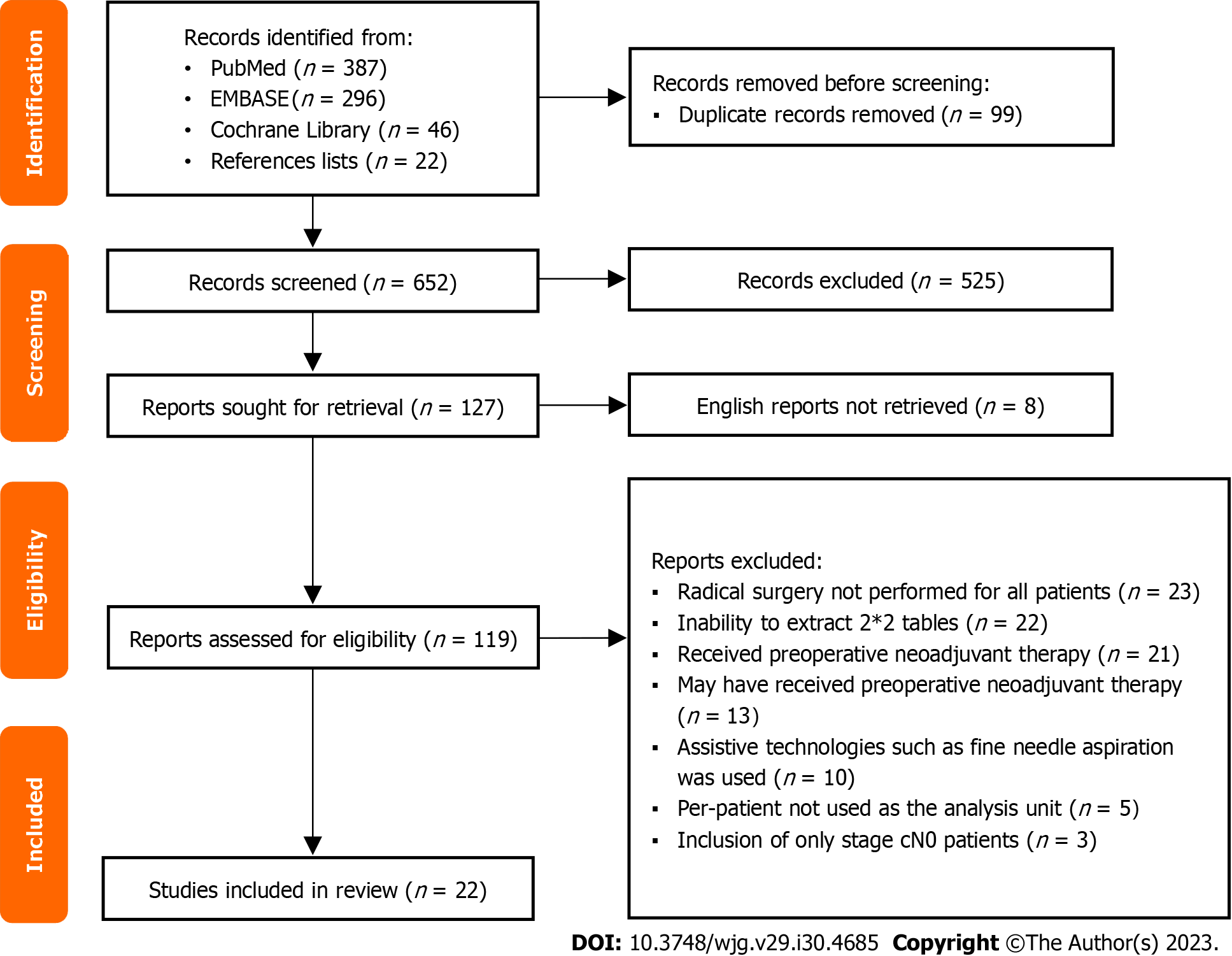
The study selection process is shown in Figure 1. A total of 729 articles were retrieved from three databases, and 22 articles were included in the manual search. The complete retrieval strategy of each database and manual search literature catalog can be found in Supplementary Table 1. Among them, 99 repeated articles were excluded after checking duplicates with EndNote software, 525 obviously irrelevant articles were excluded after reading the publication year, title and abstract, 8 articles were not published in English, 97 articles that did not meet the requirements were excluded after full-text reading, and 22 articles were included in the analysis according to the screening criteria[24-45]. In addition, the Kappa coefficient of the consistency test of the final selection results of the two researchers was 0.810 (P = 0.000).
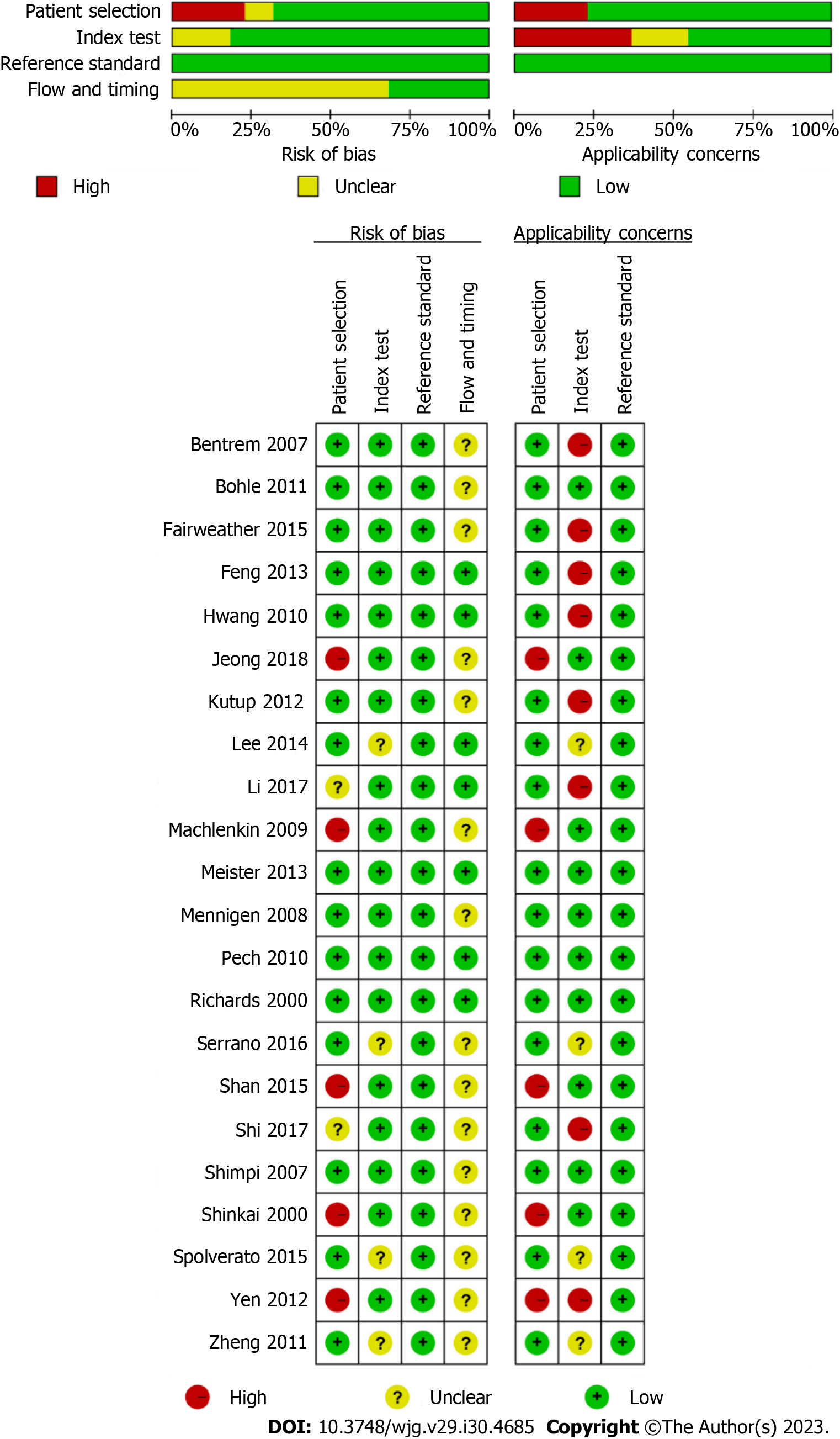
This meta-analysis included 22 studies with 2986 patients. The basic information of the studies is shown in Table 1, and the detailed information is shown in Supplementary Table 2. Among them, the vast majority of studies were retrospective studies (21/22, 95.5%) and single center studies (20/22, 90.9%); ten studies were conducted in eastern countries, and twelve studies were conducted in western countries; the objects of twelve studies and ten studies were esophageal cancer and gastric cancer, respectively; none of the patients received neoadjuvant therapy before EUS and surgery, and the gold standard for the diagnosis of LNM in all studies was postoperative pathology. The results of the quality assessment based on the QUADAS-2 tool are shown in Figure 2, and detailed quality assessment information is shown in Supple
| Ref. | Country | Study design | Center | EUS scan type | EUS scan frequency (MHz) | EUS diagnostic criteria1 | Gold standard | Tumor type | Age2 (yr) | Sample size (cases) | TP | FP | FN | TN |
| Jeong et al[24], 2018 | Korea | Retrospective | 1 | Radial | 12/20 | Criteria 1 | Postoperative pathology | Esophageal cancer | 64 | 435 | 57 | 31 | 80 | 267 |
| Shi et al[25], 2017 | China | Retrospective | 1 | Radial | - | Criteria 2 | Postoperative pathology | Esophageal cancer | 59 | 86 | 28 | 5 | 8 | 45 |
| Shan et al[26], 2015 | China | Prospective | 1 | Radial | 7.5 | Criteria 1 | Postoperative pathology | Esophageal cancer | ≥ 44 | 94 | 11 | 5 | 23 | 55 |
| Lee et al[27], 2014 | Korea | Retrospective | 1 | Radial | 7.5/12/20 | Criteria 2 | Postoperative pathology | Esophageal cancer | 69 | 12 | 2 | 0 | 3 | 7 |
| Meister et al[28], 2013 | Germany | Retrospective | 5 | Radial | 20 | Criteria 1 | Postoperative pathology | Esophageal cancer | ≥ 34 | 93 | 39 | 12 | 12 | 30 |
| Yen et al[29], 2012 | China | Retrospective | 1 | Radial | 12/20 | Criteria 2 | Postoperative pathology | Esophageal cancer | ≥ 43 | 27 | 5 | 12 | 0 | 10 |
| Pech et al[30], 2010 | Germany | Retrospective | 1 | Radial | 7.5-10 | Criteria 1 | Postoperative pathology | Esophageal cancer | 64 | 179 | 48 | 29 | 20 | 82 |
| Machlenkin et al[31], 2009 | Israel | Retrospective | 1 | Radial | 7.5-12 | Criteria 1 | Postoperative pathology | Esophageal cancer | ≥ 28 | 13 | 2 | 0 | 2 | 9 |
| Mennigen et al[32], 2008 | Germany | Retrospective | 1 | - | 7.5/15 | Criteria 1 | Postoperative pathology | Esophageal cancer | 65 | 97 | 49 | 15 | 10 | 23 |
| Shimpi et al[33], 2007 | United States | Retrospective | 1 | Radial | 20 | Criteria 1 | Postoperative pathology | Esophageal cancer | - | 37 | 9 | 1 | 3 | 24 |
| Shinkai et al[34], 2000 | Japan | Retrospective | 1 | Radial | 7.5/12/ | Criteria 1 | Postoperative pathology | Esophageal cancer | ≥ 42 | 102 | 41 | 20 | 13 | 28 |
| Richards et al[35], 2000 | United Kingdom | Retrospective | 1 | Radial | 7.5/12 | Criteria 1 | Postoperative pathology | Esophageal cancer | ≥ 35 | 69 | 19 | 9 | 23 | 18 |
| Li et al[36], 2017 | China | Retrospective | 1 | Radial | 5/7.5/ | Criteria 2 | Postoperative pathology | Gastric cancer | 57 | 81 | 48 | 4 | 3 | 26 |
| Serrano et al[37], 2016 | United States | Retrospective | 1 | Radial | 7.5/10 | Criteria 2 | Postoperative pathology | Gastric cancer | ≥ 42 | 46 | 8 | 6 | 8 | 24 |
| Spolverato et al[38], 2015 | United States | Retrospective | 7 | - | - | Criteria 2 | Postoperative pathology | Gastric cancer | - | 144 | 34 | 12 | 36 | 62 |
| Fairweather et al[39], 2015 | United States | Retrospective | 1 | Radial | 5-10 | Criteria 2 | Postoperative pathology | Gastric cancer | 67 | 49 | 2 | 3 | 25 | 19 |
| Feng et al[40], 2013 | China | Retrospective | 1 | - | 5/7.5/12/ | Criteria 2 | Postoperative pathology | Gastric cancer | 57 | 610 | 307 | 45 | 118 | 140 |
| Kutup et al[41], 2012 | Germany | Retrospective | 1 | Radial | 7.5/10/12 | Criteria 2 | Postoperative pathology | Gastric cancer | 61 | 123 | 64 | 18 | 17 | 24 |
| Zheng et al[42], 2011 | China | Retrospective | 1 | Radial | 7.5/12 | Criteria 2 | Postoperative pathology | Gastric cancer | 58 | 162 | 48 | 20 | 49 | 45 |
| Bohle et al[43], 2011 | Germany | Retrospective | 1 | Radial | 5-20 | Criteria 1 | Postoperative pathology | Gastric cancer | 63 | 62 | 30 | 5 | 9 | 18 |
| Hwang et al[44], 2010 | Korea | Retrospective | 1 | Radial | 5/7.5/ | Criteria 2 | Postoperative pathology | Gastric cancer | ≥ 49 | 247 | 16 | 6 | 67 | 158 |
| Bentrem et al[45], 2007 | United States | Retrospective | 1 | - | 7.5-12 | Criteria 2 | Postoperative pathology | Gastric cancer | - | 218 | 81 | 39 | 27 | 71 |
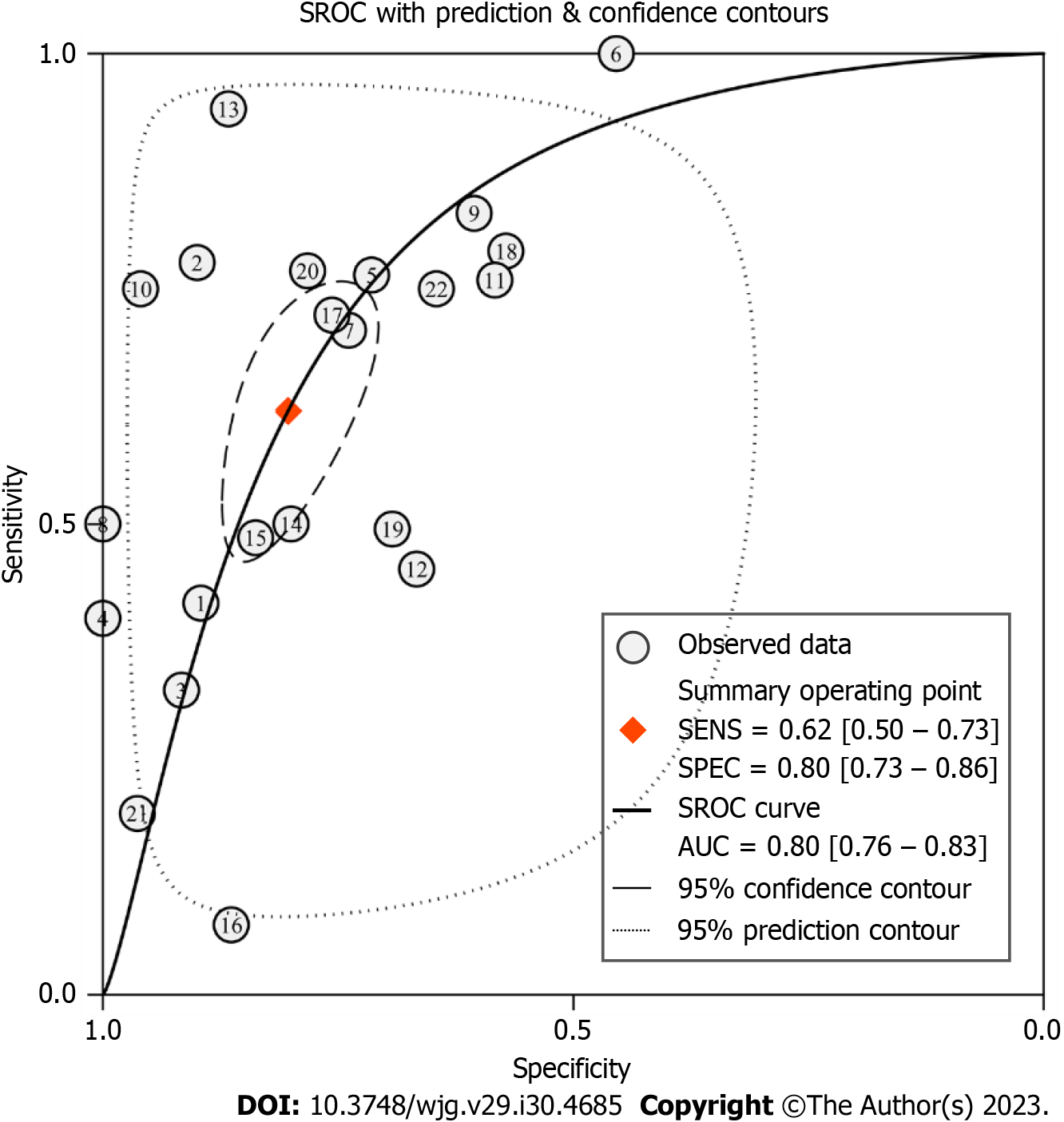
Primary outcomes: The pooled sensitivity and specificity of conventional EUS in the diagnosis of upper gastrointestinal neoplasia LNM were 0.62 [95% confidence interval (CI): 0.50-0.73, I2 = 91.50%] and 0.80 (95%CI: 0.73-0.86, I2 = 86.10%), respectively, as shown in Figure 3A. According to the SROC curve, the AUC was 0.80 (95%CI: 0.76-0.83), as shown in Figure 4.
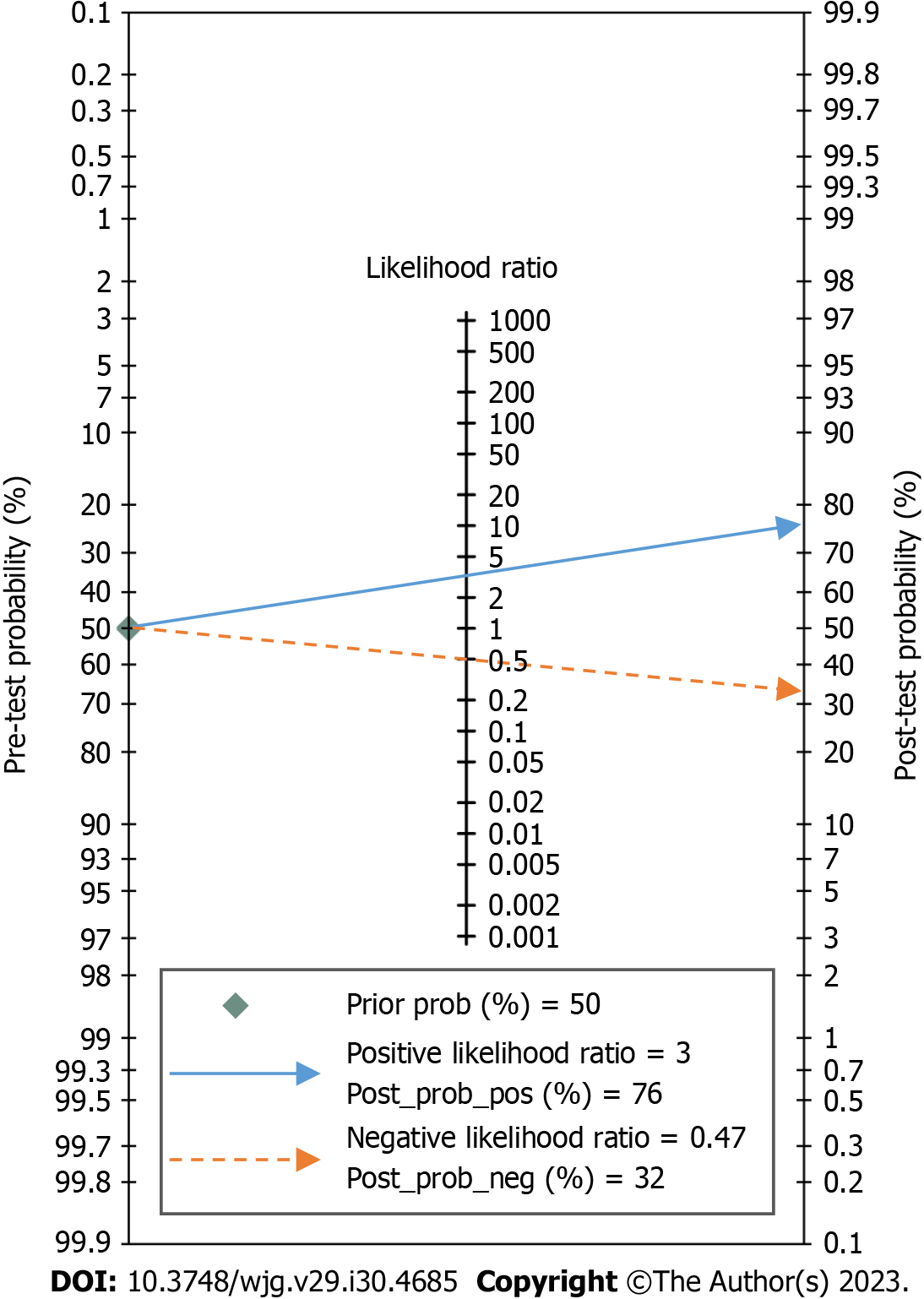
Secondary outcomes: The pooled PLR, NLR, DS, and DOR of conventional EUS in the diagnosis of upper gastrointestinal neoplasia LNM were 3.15 (95%CI: 2.46-4.03, I2 = 61.17%), 0.47 (95%CI: 0.36-0.61, I2 = 92.21%), 1.90 (95%CI: 1.51-2.29, I2 = 60.94%) and 6.67 (95%CI: 4.52-9.84, I2 = 99.99%), respectively, as shown in Figures 3B and C. The likelihood ratio scatter diagram showed that the summary PLR and NLR for the index test were in the fourth quadrant, suggesting that conventional EUS cannot be used as a confirmatory or exclusionary test, as shown in Figure 5. According to Fagan’s nomogram, when the EUS results were positive, the probability of diagnosing LNM increased from 50% to 76%; when the EUS results were negative, the probability of diagnosing LNM decreased from 50% to 32%, as shown in Figure 6.
Sensitivity analysis: We conducted sensitivity analysis by eliminating studies one by one, and the results showed that the pooled sensitivity change rate was ≤ 4.84% (I2 change rate ≤ 2.75%), and the pooled specificity change rate was ≤ 2.50% (I2 change rate ≤ 5.04%), indicating that the results of the meta-analysis were stable. Detailed data from the sensitivity analysis are shown in Supplementary Table 4.
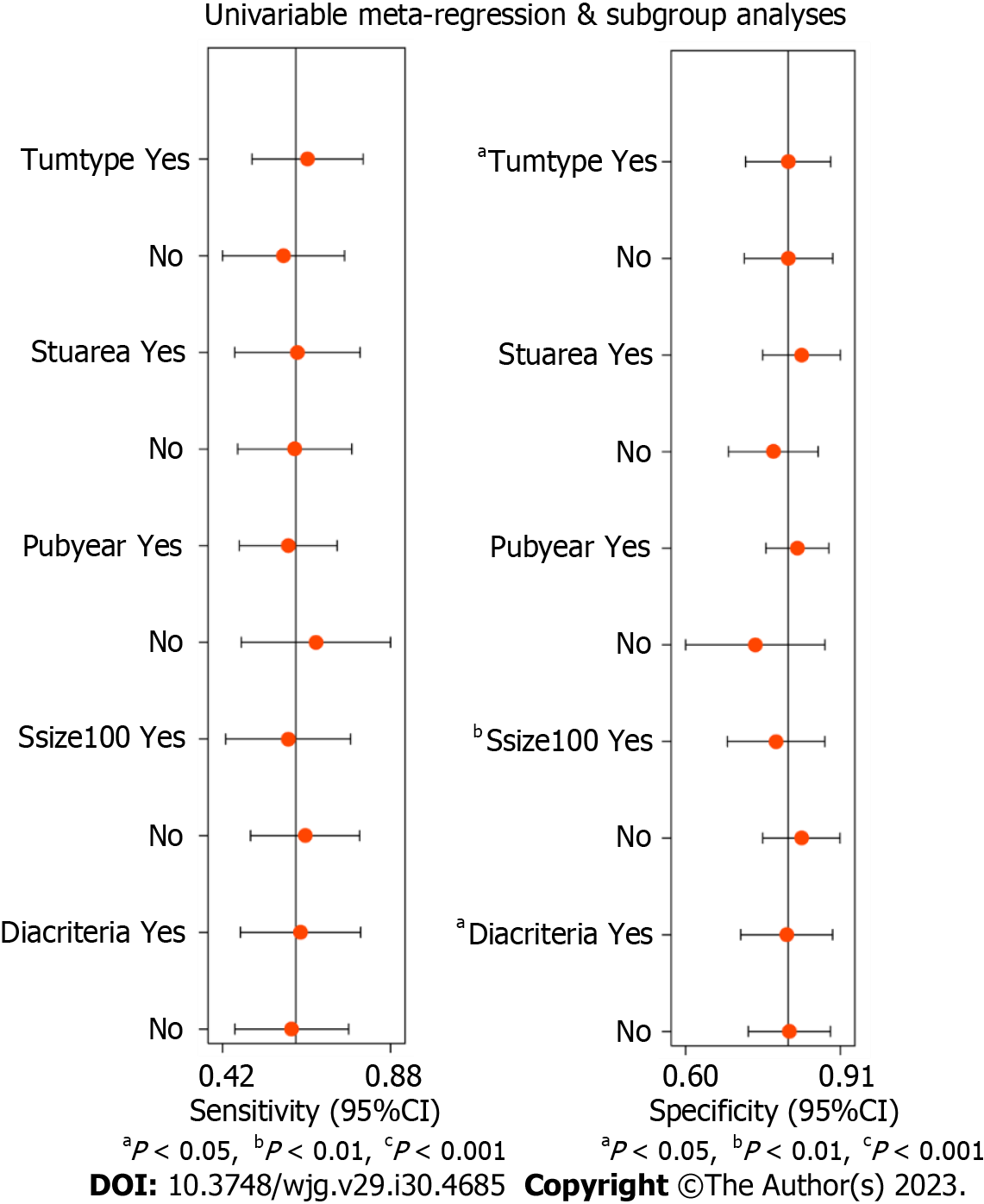
Heterogeneity: Based on the Q statistical test and I2 statistic, considerable heterogeneity was observed in the analysis for diagnostic sensitivity and specificity of conventional EUS. The ROC plane showed that the sensitivity was positively correlated with (1 - specificity), resulting in a “shoulder-arm” point distribution and indicating the existence of a threshold effect, as shown in Figure 7. According to the calculations from Stata software, the proportion of heterogeneity likely due to the threshold effect was 0.54. Because of the obvious heterogeneity among studies, we included five covariates: Tumor type (esophageal cancer or gastric cancer), study area (eastern country or western country), publication year (2010-2018 or 2000-2009), sample size (≥ 100 cases or < 100 cases) and EUS diagnostic criteria (criteria 1 or criteria 2). Univariable meta-regression and subgroup analysis were performed to identify the significant sources of heterogeneity. The results showed that tumor type, sample size and EUS diagnostic criteria were significant sources of heterogeneity in specificity (P < 0.05), as shown in Figure 8. The covariable assignment instructions are shown in Supplementary Table 5. The indicators for evaluating the diagnostic value of conventional EUS in each subgroup are shown in Table 2.
| Subgroup | Studies | Sensitivity (95%CI) | Specificity (95%CI) | PLR (95%CI) | NLR (95%CI) | DS (95%CI) | DOR (95%CI) | AUC (95%CI) | |
| All studies | 22 | 0.62 (0.50-0.73) | 0.80 (0.73-0.86) | 3.15 (2.46-4.03) | 0.47 (0.36-0.61) | 1.90 (1.51-2.29) | 6.67 (4.52-9.84) | 0.80 (0.76-0.83) | |
| Tumor type | Esophageal cancer | 12 | 0.64 (0.51-0.76) | 0.81 (0.70-0.88) | 3.33 (2.27-4.87) | 0.44 (0.33-0.59) | 2.02 (1.53-2.50) | 7.52 (4.64-12.18) | 0.79 (0.75-0.83) |
| Gastric cancer | 10 | 0.59 (0.38-0.76) | 0.80 (0.71-0.87) | 2.95 (2.09-4.17) | 0.52 (0.34-0.79) | 1.74 (1.09-2.40) | 5.71 (2.96-10.99) | 0.79 (0.75-0.82) | |
| Study area | Eastern country | 10 | 0.63 (0.42-0.80) | 0.84 (0.72-0.91) | 3.83 (2.46-5.95) | 0.44 (0.28-0.71) | 2.15 (1.48-2.83) | 8.62 (4.38-16.94) | 0.82 (0.79-0.85) |
| Western country | 12 | 0.61 (0.48-0.73) | 0.76 (0.69-0.82) | 2.57 (2.13-3.11) | 0.51 (0.38-0.67) | 1.62 (1.26-1.99) | 5.07 (3.53-7.30) | 0.77 (0.73-0.80) | |
| Publication year | 2010-2018 | 16 | 0.60 (0.44-0.74) | 0.82 (0.75-0.88) | 3.36 (2.58-4.38) | 0.48 (0.35-0.68) | 1.94 (1.47-2.41) | 6.93 (4.34-11.08) | 0.81 (0.77-0.84) |
| 2000-2009 | 6 | 0.70 (0.56-0.81) | 0.75 (0.56-0.88) | 2.79 (1.50-5.18) | 0.41 (0.27-0.62) | 1.93 (1.01-2.85) | 6.87 (2.74-17.22) | 0.78 (0.74-0.81) | |
| Sample size | ≥ 100 cases | 9 | 0.60 (0.46-0.73) | 0.78 (0.67-0.87) | 2.79 (2.14-3.65) | 0.51 (0.40-0.65) | 1.71 (1.45-1.97) | 5.52 (4.24-7.18) | 0.76 (0.72-0.80) |
| < 100 cases | 13 | 0.65 (0.61-0.90) | 0.83 (0.72-0.89) | 3.80 (2.45-5.89) | 0.42 (0.26-0.69) | 2.20 (1.42-2.99) | 9.04 (4.13-19.81) | 0.83 (0.80-0.86) | |
| EUS diagnostic criteria1 | Criteria 1 | 10 | 0.62 (0.50-0.73) | 0.79 (0.71-0.86) | 3.04 (2.38-3.88) | 0.47 (0.37-0.60) | 1.86 (1.58-2.14) | 6.43 (4.84-8.54) | 0.78 (0.74-0.82) |
| Criteria 2 | 12 | 0.61 (0.41-0.79) | 0.81 (0.70-0.88) | 3.17 (2.19-4.60) | 0.48 (0.30-0.75) | 1.89 (1.22-2.57) | 6.63 (3.37-13.05) | 0.80 (0.76-0.83) | |
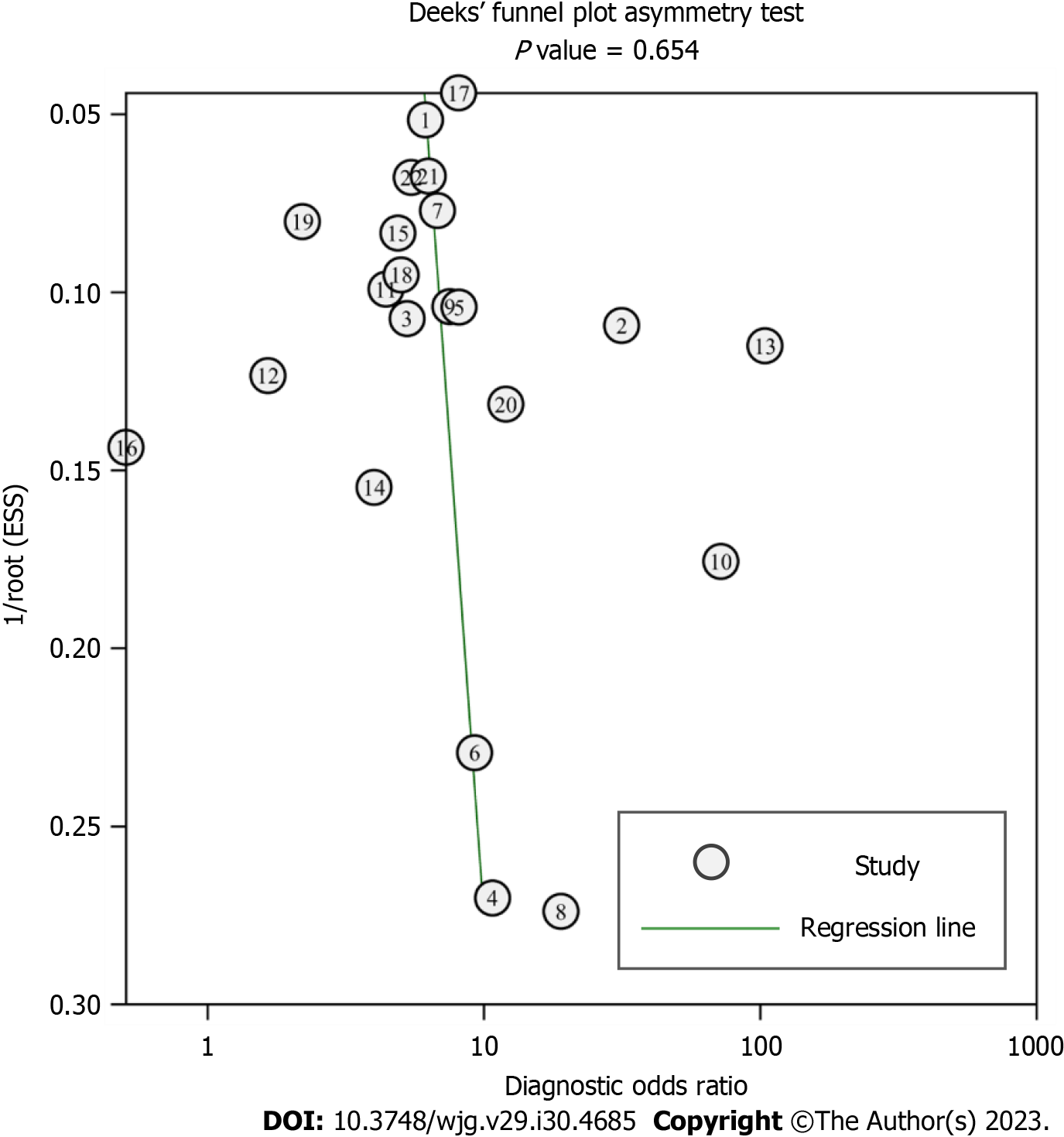
Deeks’ funnel plot showed that the distribution of all studies was relatively symmetrical; the asymmetry was not statistically significant (P = 0.654), indicating that there was no significant publication bias among the 22 studies, as shown in Figure 9.
In this meta-analysis, upper gastrointestinal neoplasia was considered as a whole, and the diagnostic value of conventional EUS for LNM was analysed. To ensure the reliability of the research results, we excluded studies that were old, had incomplete data, had small sample sizes and were published in languages other than English. The effects of incomplete surgical resection, neoadjuvant therapy, animal experiments, assistive technologies and other malignant tumors on the statistical results were excluded (which is also the reason for the small number of studies included in this meta-analysis). The results of conventional EUS were compared with postoperative pathology, and the data of 2986 patients in 22 studies were analyzed in detail.
The results of the quality assessment showed that many studies had a risk of bias, mainly because the proportion of retrospective studies was too high, and selective bias may have been present in the patient inclusion process. Four studies did not clearly describe the diagnostic criteria in the use of EUS, five studies unreasonably excluded some tumor patients (two studies limited the tumor location, two studies defined the location of metastatic lymph nodes, and one study only included esophageal cancer of ≤ pT2 stage), and fifteen studies did not specify the interval between EUS and surgery. However, we believe that since both esophageal cancer and gastric cancer are malignancies, examination and surgery should be arranged as soon as possible after clinical diagnosis. Although many studies did not specify the interval, it should not have had a significant impact on the research results. Regarding concerns regarding applicability, we think that the main reasons were the difference in diagnostic criteria of EUS and bias in patient selection. Therefore, caution should be taken in interpreting the results of the meta-analysis.
Due to the significant heterogeneity among the included studies, we used the bivariate mixed effect model to calculate statistics on various diagnostic evaluation indicators. The results showed that the pooled sensitivity and specificity of conventional EUS in diagnosing LNM in upper gastrointestinal neoplasia were 0.62 and 0.80, respectively, and the AUC of the SROC curve was 0.80, which indicated that the diagnostic value of conventional EUS was moderate. When EUS indicated positive or negative results, the posttest probability could be adjusted from the previous 50% to 76% and 32%, respectively. This result is meaningful for noninvasive examinations, indicating that conventional EUS has certain clinical value. However, it is undeniable that because the PLR < 10 and NLR > 0.1 in conventional EUS diagnosis and the DS and DOR were relatively small, this examination cannot be used to confirm or exclude LNM, which is consistent with the results of previous studies[46-49]. It is not difficult to understand that, as with other imaging examinations, it is difficult for conventional EUS to reach such a high diagnostic level without obtaining lymph node tissue.
We explored the sources of heterogeneity among the included studies. First, we believe that the threshold effect could lead to heterogeneity because the 22 studies adopted a variety of EUS diagnostic criteria, and the “shoulder-arm” point distribution in the ROC plane also confirmed our view; the threshold effect might contribute 54% of the heterogeneity. Then, in view of the differences among the various studies, we included five covariables that could be easily grouped according to the collected data for meta-regression and subgroup analysis. Considering the limited number of studies, it would have been difficult to guarantee the accuracy of the statistical results of the simultaneous inclusion of five covariates, so we included individual covariates one by one for analysis. Although we were unable to identify significant sources of heterogeneity in sensitivity, we found that the significant sources of heterogeneity in specificity included tumor type, sample size, and EUS diagnostic criteria. However, after excluding the influence of the above factors, the heterogeneity within each subgroup was still obvious. Therefore, we have reason to believe that the heterogeneity was caused by a combination of factors. Many unincluded factors may also have been sources of heterogeneity; examples and the reasons they were not analyzed in detail included the study design, participating center and EUS scan type (because of the proportion imbalance within the group), the qualifications of the endoscopists, the EUS model and scan frequency (because of the complexity of the data), and the tumor stage, tumor location and location of metastatic lymph nodes (because these could not be accurately distinguished). We also found that the study area was not a significant source of heterogeneity, indicating that the diagnostic performance of conventional EUS for LNM in patients with upper gastrointestinal neoplasia in eastern and western countries is comparable. Publication year was also not a significant source of heterogeneity, indicating that the diagnostic performance of conventional EUS has not changed significantly in the past 20 years and that there may be technical barriers in conventional EUS that limit opportunities to significantly improve the ability of conventional EUS to identify malignant lymph nodes by relying solely on the diagnostic criteria of size, shape, boundary and echo.
Although the performance of conventional EUS in diagnosing LNM in upper gastrointestinal neoplasia remains nonideal, the diagnostic ability can be greatly improved with the assistance of EUS-guided FNA (EUS-FNA), EUS elastography (EUS-E) and contrast-enhanced EUS (CE-EUS)[50-52]. EUS-FNA uses a slender biopsy needle to perform puncture biopsy for suspicious lesions under the guidance of EUS, which can provide histopathological information and is an accurate method to distinguish between benign and malignant lymph nodes. The sensitivity and accuracy of EUS-FNA in the diagnosis of regional LNM of upper gastrointestinal neoplasia are higher than those of conventional EUS. Chen et al[53] included 26 studies with 2753 patients for meta-analysis and found that the pooled sensitivity and specificity of EUS-FNA in differentiating benign and malignant lymph nodes were 87% and 100%, respectively, and the AUC was as high as 0.9912. EUS-E uses different colors to distinguish tissue hardness and displays different color images according to the elastic difference between lymph nodes and surrounding tissues, which can more clearly identify metastatic lymph nodes, improve the diagnostic performance of conventional EUS, and reduce unnecessary biopsies. Xu et al[54] included seven studies with 368 patients for meta-analysis and showed that the pooled sensitivity, specificity and AUC of EUS-E in the diagnosis of LNM were 88%, 85% and 0.9456, respectively. CE-EUS obtains enhanced images by using contrast agents, which can provide more information about the lesion tissue and can be used to identify metastatic lymph nodes. Lisotti et al[55] included four studies with 336 patients in their meta-analysis and indicated that the pooled sensitivity and specificity of CE-EUS in diagnosing LNM were 82.1% and 90.7%, respectively. However, our study only analyzed the diagnostic value of conventional EUS for LNM of upper gastrointestinal neoplasia, without considering the role of the above assistive technologies, which may underestimate the diagnostic value of EUS and affect the choice of clinicians. Therefore, we can carry out relevant studies in the next stage to evaluate the diagnostic value of various EUS assistive technologies in detail.
Our study only included patients who underwent radical surgery and did not receive preoperative neoadjuvant therapy, which inevitably led to case selection bias and excluded some patients with early tumors suitable for endoscopic treatment or patients with advanced tumors not suitable for surgical treatment. In addition, because preoperative neoadjuvant chemoradiotherapy can improve the treatment effect and prolong the survival time of some patients with upper gastrointestinal neoplasia, some patients with positive LNM may not have received the best treatment in this study. However, it is difficult to know the exact situation of LNM without obtaining complete pathological tissue, and preoperative neoadjuvant therapy will cause necrosis, fibrosis or inflammation of lymph nodes, which will affect the diagnostic effect of conventional EUS and the manifestations of postoperative histopathology. Therefore, to provide a reliable reference standard, we had to abandon the above cases in the study design stage.
Our study also has the following limitations. First, there were many retrospective studies with a long time span and use of different technologies and tools, which may have led to selection bias. Second, only studies published in English were included, which may have led to information bias. Third, there were differences in the study designs and implementation processes, which may have led to confounding bias. In addition, the significant heterogeneity may have affected the reliability and repeatability of the analysis results.
In conclusion, conventional EUS has certain clinical value and can assist in the detection of LNM in upper gastrointestinal neoplasia, but it cannot be used as a confirmatory or exclusionary test. There was great heterogeneity among the included studies, and more high-quality studies are needed to further verify the diagnostic value of EUS and determine its best diagnostic criteria. However, with the popularization of EUS technology, the use of assistive technologies such as EUS-FNA, EUS-E or CE-EUS, and the training of high-quality endoscopists, we believe that EUS will be increasingly valuable in the diagnosis of LNM in upper gastrointestinal neoplasia.
Upper gastrointestinal neoplasia, mainly including esophageal cancer and gastric cancer, is a common cancer with high mortality. Accurate prediction of lymph node metastasis (LNM) is of great significance for guiding clinical treatment and improving the prognosis of patients. In recent years, endoscopic ultrasound (EUS) has become increasingly used in the diagnosis and treatment of gastrointestinal diseases, but its application in the detection of LNM remains limited.
Although previous studies have reported the diagnostic value of conventional EUS for LNM in upper gastrointestinal neoplasia, the relevant research conclusions were controversial, and the research results have varied widely. Therefore, we intend to further carry out this research through meta-analysis.
This study aimed to systematically search the literature and examine the diagnostic value of conventional EUS for LNM in upper gastrointestinal neoplasia by summarizing and analyzing the data.
We conducted a comprehensive search and screening of the PubMed, EMBASE and Cochrane Library databases from January 1, 2000 to October 1, 2022. Then, relevant study data were extracted, and the quality of the included studies was assessed based on the Quality Assessment of Diagnostic Accuracy Studies tool. Afterward, a meta-analysis was performed using the statistical software Stata 14.0.
A total of 2986 patients in 22 studies were included. The results showed that the pooled sensitivity, specificity and area under the summary receiver operating characteristic curve of conventional EUS in the diagnosis of upper gastrointestinal neoplasia LNM were acceptable, which were 0.62 [95% confidence interval (CI): 0.50-0.73], 0.80 (95%CI: 0.73-0.86) and 0.80 (95%CI: 0.76-0.83), respectively. However, the pooled positive likelihood ratio and negative likelihood ratio were relatively poor, at 3.15 (95%CI: 2.46-4.03) and 0.47 (95%CI: 0.36-0.61), respectively. The pooled diagnostic score and diagnostic odds ratio were relatively small, at 1.90 (95%CI: 1.51-2.29) and 6.67 (95%CI: 4.52-9.84), respectively.
Conventional EUS has certain clinical value and can assist in the detection of LNM in upper gastrointestinal neoplasia, but it cannot be used as a confirmatory or exclusionary test. More high-quality studies are needed to further verify the diagnostic value of EUS and determine the best diagnostic criteria.
In the future, further clinical studies should be carried out to evaluate the diagnostic value of various EUS assistive technologies for LNM in upper gastrointestinal neoplasia and to evaluate the influence of neoadjuvant therapy on the diagnostic value of EUS for LNM in upper gastrointestinal neoplasia.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grgurevic I, Croatia; Massironi S, Italy; Redondo-Cerezo E, Spain S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64627] [Article Influence: 16156.8] [Reference Citation Analysis (176)] |
| 2. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2206] [Article Influence: 735.3] [Reference Citation Analysis (1)] |
| 3. | Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). 2021;41:1037-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 584] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 4. | Huang J, Koulaouzidis A, Marlicz W, Lok V, Chu C, Ngai CH, Zhang L, Chen P, Wang S, Yuan J, Lao XQ, Tse SLA, Xu W, Zheng ZJ, Xie SH, Wong MCS. Global Burden, Risk Factors, and Trends of Esophageal Cancer: An Analysis of Cancer Registries from 48 Countries. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 5. | Visser E, Markar SR, Ruurda JP, Hanna GB, van Hillegersberg R. Prognostic Value of Lymph Node Yield on Overall Survival in Esophageal Cancer Patients: A Systematic Review and Meta-analysis. Ann Surg. 2019;269:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 6. | Watanabe M, Toh Y, Ishihara R, Kono K, Matsubara H, Murakami K, Muro K, Numasaki H, Oyama T, Ozawa S, Saeki H, Tanaka K, Tsushima T, Ueno M, Uno T, Yoshio T, Usune S, Takahashi A, Miyata H; Registration Committee for Esophageal Cancer of the Japan Esophageal Society. Comprehensive registry of esophageal cancer in Japan, 2014. Esophagus. 2022;19:1-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 7. | Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41:747-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 463] [Article Influence: 115.8] [Reference Citation Analysis (1)] |
| 8. | Askari A, Munster AB, Jambulingam P, Riaz A. Critical number of lymph node involvement in esophageal and gastric cancer and its impact on long-term survival-A single-center 8-year study. J Surg Oncol. 2020;122:1364-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Li H, Fang W, Yu Z, Mao Y, Chen L, He J, Rong T, Chen C, Chen H, Chen K, Du M, Han Y, Hu J, Fu J, Hou X, Gong T, Li Y, Liu J, Liu S, Tan L, Tian H, Wang Q, Xiang J, Xu M, Ye X, You B, Zhang R, Zhao Y; Society of Esophageal Tumor, Chinese Anti-Cancer Association. Chinese expert consensus on mediastinal lymph node dissection in esophagectomy for esophageal cancer (2017 edition). J Thorac Dis. 2018;10:2481-2489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 960] [Article Influence: 320.0] [Reference Citation Analysis (0)] |
| 11. | Muro K, Lordick F, Tsushima T, Pentheroudakis G, Baba E, Lu Z, Cho BC, Nor IM, Ng M, Chen LT, Kato K, Li J, Ryu MH, Zamaniah WIW, Yong WP, Yeh KH, Nakajima TE, Shitara K, Kawakami H, Narita Y, Yoshino T, Van Cutsem E, Martinelli E, Smyth EC, Arnold D, Minami H, Tabernero J, Douillard JY. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic oesophageal cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 12. | Radlinski M, Shami VM. Role of endoscopic ultrasound in esophageal cancer. World J Gastrointest Endosc. 2022;14:205-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (3)] |
| 13. | Dhar J, Samanta J. Role of therapeutic endoscopic ultrasound in gastrointestinal malignancy- current evidence and future directions. Clin J Gastroenterol. 2022;15:11-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 14. | Serrani M, Calvanese C, Lisotti A, Caletti G, Abenavoli L, Fusaroli P. Basics in Endoscopic Ultrasound Part 1: Diagnostic Indications and Tissue Sampling. Rev Recent Clin Trials. 2018;13:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Krill T, Baliss M, Roark R, Sydor M, Samuel R, Zaibaq J, Guturu P, Parupudi S. Accuracy of endoscopic ultrasound in esophageal cancer staging. J Thorac Dis. 2019;11:S1602-S1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Zhou H, Li M. The Value of Gastric Cancer Staging by Endoscopic Ultrasonography Features in the Diagnosis of Gastroenterology. Comput Math Methods Med. 2022;2022:6192190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Schmidlin EJ, Gill RR. New frontiers in esophageal radiology. Ann Transl Med. 2021;9:904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Jayaprakasam VS, Yeh R, Ku GY, Petkovska I, Fuqua JL 3rd, Gollub M, Paroder V. Role of Imaging in Esophageal Cancer Management in 2020: Update for Radiologists. AJR Am J Roentgenol. 2020;215:1072-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Peng T, Lou Z, Wang X, Huang D, Zhang G, Gao H, Li S. Clinical Comparison of Endoscopic Ultrasonography and CT in Preoperative TN Staging of Esophagogastric Junction Cancer. Contrast Media Mol Imaging. 2022;2022:5810405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Yang H, Hu B. Recent advances in early esophageal cancer: diagnosis and treatment based on endoscopy. Postgrad Med. 2021;133:665-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40483] [Article Influence: 10120.8] [Reference Citation Analysis (2)] |
| 22. | Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4127] [Cited by in RCA: 4676] [Article Influence: 1169.0] [Reference Citation Analysis (0)] |
| 23. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 9579] [Article Influence: 684.2] [Reference Citation Analysis (0)] |
| 24. | Jeong DY, Kim MY, Lee KS, Choi JY, Kim SJ, Chung MJ, Min YW, Kim HK, Zo JI, Shim YM, Sun JM. Surgically resected T1- and T2-stage esophageal squamous cell carcinoma: T and N staging performance of EUS and PET/CT. Cancer Med. 2018;7:3561-3570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Shi H, Ma S, Zhao P, Jiang J, Cheng Y, Zhao J, Wang J, Qiao Z, Li S, Wu J. Endoscopic ultrasonography for preoperative staging of esophageal carcinoma. Scand J Gastroenterol. 2017;52:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Shan HB, Zhang R, Li Y, Gao XY, Lin SY, Luo GY, Li JJ, Xu GL. Application of Endobronchial Ultrasonography for the Preoperative Detecting Recurrent Laryngeal Nerve Lymph Node Metastasis of Esophageal Cancer. PLoS One. 2015;10:e0137400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Lee G, I H, Kim SJ, Jeong YJ, Kim IJ, Pak K, Park DY, Kim GH. Clinical implication of PET/MR imaging in preoperative esophageal cancer staging: comparison with PET/CT, endoscopic ultrasonography, and CT. J Nucl Med. 2014;55:1242-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Meister T, Heinzow HS, Osterkamp R, Wehrmann T, Kucharzik T, Domschke W, Domagk D, Seifert H. Miniprobe endoscopic ultrasound accurately stages esophageal cancer and guides therapeutic decisions in the era of neoadjuvant therapy: results of a multicenter cohort analysis. Surg Endosc. 2013;27:2813-2819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Yen TJ, Chung CS, Wu YW, Yen RF, Cheng MF, Lee JM, Hsu CH, Chang YL, Wang HP. Comparative study between endoscopic ultrasonography and positron emission tomography-computed tomography in staging patients with esophageal squamous cell carcinoma. Dis Esophagus. 2012;25:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Pech O, Günter E, Dusemund F, Origer J, Lorenz D, Ell C. Accuracy of endoscopic ultrasound in preoperative staging of esophageal cancer: results from a referral center for early esophageal cancer. Endoscopy. 2010;42:456-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Machlenkin S, Melzer E, Idelevich E, Ziv-Sokolovsky N, Klein Y, Kashtan H. Endoscopic ultrasound: doubtful accuracy for restaging esophageal cancer after preoperative chemotherapy. Isr Med Assoc J. 2009;11:166-169. [PubMed] |
| 32. | Mennigen R, Tuebergen D, Koehler G, Sauerland C, Senninger N, Bruewer M. Endoscopic ultrasound with conventional probe and miniprobe in preoperative staging of esophageal cancer. J Gastrointest Surg. 2008;12:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Shimpi RA, George J, Jowell P, Gress FG. Staging of esophageal cancer by EUS: staging accuracy revisited. Gastrointest Endosc. 2007;66:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Shinkai M, Niwa Y, Arisawa T, Ohmiya N, Goto H, Hayakawa T. Evaluation of prognosis of squamous cell carcinoma of the oesophagus by endoscopic ultrasonography. Gut. 2000;47:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Richards DG, Brown TH, Manson JM. Endoscopic ultrasound in the staging of tumours of the oesophagus and gastro-oesophageal junction. Ann R Coll Surg Engl. 2000;82:311-317. [PubMed] |
| 36. | Li JH, Shen WZ, Gu XQ, Hong WK, Wang ZQ. Prognostic value of EUS combined with MSCT in predicting the recurrence and metastasis of patients with gastric cancer. Jpn J Clin Oncol. 2017;47:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 37. | Serrano OK, Huang K, Ng N, Yang J, Friedmann P, Libutti SK, Kennedy TJ. Correlation between preoperative endoscopic ultrasound and surgical pathology staging of gastric adenocarcinoma: A single institution retrospective review. J Surg Oncol. 2016;113:42-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Spolverato G, Ejaz A, Kim Y, Squires MH, Poultsides GA, Fields RC, Schmidt C, Weber SM, Votanopoulos K, Maithel SK, Pawlik TM. Use of endoscopic ultrasound in the preoperative staging of gastric cancer: a multi-institutional study of the US gastric cancer collaborative. J Am Coll Surg. 2015;220:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Fairweather M, Jajoo K, Sainani N, Bertagnolli MM, Wang J. Accuracy of EUS and CT imaging in preoperative gastric cancer staging. J Surg Oncol. 2015;111:1016-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 40. | Feng XY, Wang W, Luo GY, Wu J, Zhou ZW, Li W, Sun XW, Li YF, Xu DZ, Guan YX, Chen S, Zhan YQ, Zhang XS, Xu GL, Zhang R, Chen YB. Comparison of endoscopic ultrasonography and multislice spiral computed tomography for the preoperative staging of gastric cancer - results of a single institution study of 610 Chinese patients. PLoS One. 2013;8:e78846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Kutup A, Vashist YK, Groth S, Vettorazzi E, Yekebas EF, Soehendra N, Izbicki JR. Endoscopic ultrasound staging in gastric cancer: Does it help management decisions in the era of neoadjuvant treatment? Endoscopy. 2012;44:572-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Zheng Z, Yu Y, Lu M, Sun W, Wang F, Li P, Zhang Y, Lin L, Huang P, Chen J, Zhang H, Xie Z, Dong Xda E. Double contrast-enhanced ultrasonography for the preoperative evaluation of gastric cancer: a comparison to endoscopic ultrasonography with respect to histopathology. Am J Surg. 2011;202:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Bohle W, Scheidig A, Zoller WG. Endosonographic tumor staging for treatment decision in resectable gastric cancer. J Gastrointestin Liver Dis. 2011;20:135-139. [PubMed] |
| 44. | Hwang SW, Lee DH, Lee SH, Park YS, Hwang JH, Kim JW, Jung SH, Kim NY, Kim YH, Lee KH, Kim HH, Park DJ, Lee HS, Jung HC, Song IS. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol. 2010;25:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 45. | Bentrem D, Gerdes H, Tang L, Brennan M, Coit D. Clinical correlation of endoscopic ultrasonography with pathologic stage and outcome in patients undergoing curative resection for gastric cancer. Ann Surg Oncol. 2007;14:1853-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Klamt AL, Neyeloff JL, Santos LM, Mazzini GDS, Campos VJ, Gurski RR. Echoendoscopy in Preoperative Evaluation of Esophageal Adenocarcinoma and Gastroesophageal Junction: Systematic Review and Meta-analysis. Ultrasound Med Biol. 2021;47:1657-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Chen J, Zhou C, He M, Zhen Z, Wang J, Hu X. A Meta-Analysis And Systematic Review Of Accuracy Of Endoscopic Ultrasound For N Staging Of Gastric Cancers. Cancer Manag Res. 2019;11:8755-8764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev. 2015;2015:CD009944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 49. | Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: a meta-analysis. Gastrointest Endosc. 2011;73:1122-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 50. | Tamanini G, Cominardi A, Brighi N, Fusaroli P, Lisotti A. Endoscopic ultrasound assessment and tissue acquisition of mediastinal and abdominal lymph nodes. World J Gastrointest Oncol. 2021;13:1475-1491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Dietrich CF, Jenssen C, Arcidiacono PG, Cui XW, Giovannini M, Hocke M, Iglesias-Garcia J, Saftoiu A, Sun S, Chiorean L. Endoscopic ultrasound: Elastographic lymph node evaluation. Endosc Ultrasound. 2015;4:176-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 52. | Ang TL, Kwek ABE, Wang LM. Diagnostic Endoscopic Ultrasound: Technique, Current Status and Future Directions. Gut Liver. 2018;12:483-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Chen L, Li Y, Gao X, Lin S, He L, Luo G, Li J, Huang C, Wang G, Yang Q, Shan H. High Diagnostic Accuracy and Safety of Endoscopic Ultrasound-Guided Fine-Needle Aspiration in Malignant Lymph Nodes: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2021;66:2763-2775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Xu W, Shi J, Zeng X, Li X, Xie WF, Guo J, Lin Y. EUS elastography for the differentiation of benign and malignant lymph nodes: a meta-analysis. Gastrointest Endosc. 2011;74:1001-9; quiz 1115.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 55. | Lisotti A, Ricci C, Serrani M, Calvanese C, Sferrazza S, Brighi N, Casadei R, Fusaroli P. Contrast-enhanced endoscopic ultrasound for the differential diagnosis between benign and malignant lymph nodes: a meta-analysis. Endosc Int Open. 2019;7:E504-E513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |