Published online Mar 28, 2023. doi: 10.3748/wjg.v29.i12.1899
Peer-review started: November 30, 2022
First decision: December 20, 2022
Revised: December 29, 2022
Accepted: March 9, 2023
Article in press: March 9, 2023
Published online: March 28, 2023
Processing time: 116 Days and 3.4 Hours
Lugol chromoendoscopy (LCE) has served as a standard screening technique in high-risk patients with esophageal cancer. Nevertheless, LCE is not suitable for general population screening given its side effects. Linked color imaging (LCI) is a novel image-enhanced endoscopic technique that can distinguish subtle diff-erences in mucosal color.
To compare the diagnostic performance of LCI with LCE in detecting esophageal squamous cell cancer and precancerous lesions and to evaluate whether LCE can be replaced by LCI in detecting esophageal neoplastic lesions.
In this prospective study, we enrolled 543 patients who underwent white light imaging (WLI), LCI and LCE successively. We compared the sensitivity and specificity of LCI and LCE in the detection of esophageal neoplastic lesions. Clinicopathological features and color analysis of lesions were assessed.
In total, 43 patients (45 neoplastic lesions) were analyzed. Among them, 36 patients (38 neoplastic lesions) were diagnosed with LCI, and 39 patients (41 neoplastic lesions) were diagnosed with LCE. The sensitivity of LCI was similar to that of LCE (83.7% vs 90.7%, P = 0.520), whereas the specificity of LCI was greater than that of LCE (92.4% vs 87.0%, P = 0.007). The LCI procedure time in the esophageal examination was significantly shorter than that of LCE [42 (34, 50) s vs 160 (130, 189) s, P < 0.001]. The color difference between the lesion and surrounding mucosa in LCI was significantly greater than that observed with WLI. However, the color difference in LCI was similar in different pathological types of esophageal squamous cell cancer.
LCI offers greater specificity than LCE in the detection of esophageal squamous cell cancer and precancerous lesions, and LCI represents a promising screening strategy for general populations.
Core Tip: Lugol chromoendoscopy (LCE) has served as the standard screening technique in high-risk patients with esophageal cancer. Nevertheless, LCE can induce esophageal spasms and chest pain and is associated with the risk of aspiration and iodine allergy. Therefore, LCE is not suitable for general population screening. Linked color imaging (LCI) is a novel image-enhanced endoscopy that can distinguish subtle differences in mucosal color. In this study, we compared the use of LCI and LCE in the screening of esophageal neoplastic lesions. Based on our analysis, we found that LCI is more specific than LCE and represents a promising screening strategy.
- Citation: Wang ZX, Li LS, Su S, Li JP, Zhang B, Wang NJ, Liu SZ, Wang SS, Zhang S, Bi YW, Gao F, Shao Q, Xu N, Shao BZ, Yao Y, Liu F, Linghu EQ, Chai NL. Linked color imaging vs Lugol chromoendoscopy for esophageal squamous cell cancer and precancerous lesion screening: A noninferiority study. World J Gastroenterol 2023; 29(12): 1899-1910
- URL: https://www.wjgnet.com/1007-9327/full/v29/i12/1899.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i12.1899
Esophageal cancer is the seventh most common cancer and the sixth leading cause of cancer-related deaths worldwide[1]. Most patients with esophageal cancer have progressed to an advanced stage when diagnosed, with poor quality of life and an overall 5-year survival rate of less than 20%[2,3]. Esophageal squamous cell carcinoma (ESCC) is the most common form of esophageal cancer worldwide, representing greater than 85% of all esophageal cancer cases[4,5]. Curative resection using endoscopic mucosal resection or endoscopic submucosal dissection is possible for lesions with high-grade intraepithelial neoplasia and most T1 tumors, offering less trauma, faster recovery and fewer complications[6]. Therefore, early diagnosis is critical for treatment success and prognostic improvement. Early identification and timely intervention of esophageal cancer or precancerous lesions are of great significance to delay the progression of the disease, improve the prognosis and improve the quality of life.
However, under white light imaging (WLI) endoscopy, early neoplastic lesions are easily missed due to the presence of small lesion areas and subtle differences in the surrounding mucosa color[7]. Historically, Lugol chromoendoscopy (LCE) has served as the standard diagnostic technique given its higher detection rate[8-12]. However, its specificity is low due to light staining under conditions of inflammation, which has been demonstrated in previous studies[13-15]. Furthermore, LCE occasionally induces esophageal spasms and chest pain and is associated with the risk of aspiration and iodine allergy[16,17].
With the advent of image-enhanced endoscopy, many studies have confirmed its efficacy in diagnosing upper gastrointestinal neoplasms[13,18]. The LASEREO system (Fujifilm Corporation, Tokyo, Japan), a new linked color imaging (LCI) technology, was recently developed. LCI images are illuminated with white light and short wavelength narrow-band light in an appropriate proportion simultaneously to realize the simultaneous expansion and contraction of colors. This procedure facilitates enhancement of the red and white colors and makes it easier for the operators to identify the subtle differences in mucosal color[18-21]. In our clinical work, it was found that LCI significantly improved the ability to identify lesions. In a large-scale randomized comparative study, Ono et al[20] reported the superiority of LCI over WLI in detecting neoplastic lesions in the upper gastrointestinal tract. Previous studies reported the usefulness of LCI for esophageal neoplasm detection and assessments of invasive depth[20-24]. Although Yi et al[25] compared LCI and LCE in the diagnosis of multiple primary esophageal cancers, no prospective study has compared the diagnosability of LCI with LCE in screening esophageal neoplastic lesions to date. Therefore, we conducted a prospective noninferiority study to compare the efficacy of non-magnifying LCI and LCE in detecting and diagnosing ESCC and precancerous lesions. We evaluated whether LCE can be replaced by LCI in detecting esophageal neoplastic lesions for the general population.
This was a single-center, prospective, registered clinical study (No. ChiCTR2100045636) conducted at the First Medical Center of Chinese PLA General Hospital that compared the detection of ESCC and precancerous lesions using LCI vs LCE after a conventional white light examination. Endoscopic biopsy is considered the gold standard for ESCC detection. The study was approved by the medical ethics committee of the Chinese PLA General Hospital (Beijing, China), and informed consent forms were obtained from all patients.
The regional screening studies of esophageal cancer in China revealed that endoscopic screening starting at 40 years of age was cost-effective[26,27]. We recommended 40 years as the beginning age for screening in the Chinese consensus[28]. The following inclusion criteria were employed: (1) Age > 40-year-old; and (2) Provision of written consent to participate. The exclusion criteria were as follows: (1) Contraindication for upper gastrointestinal endoscopy or general anesthesia; (2) Iodine allergy or hyperthyroidism; and (3) Previous surgical resection or chemotherapy, radiotherapy or chemoradiotherapy for ESCC (as these procedures may influence the mucosal surface, which is important for detecting these lesions).
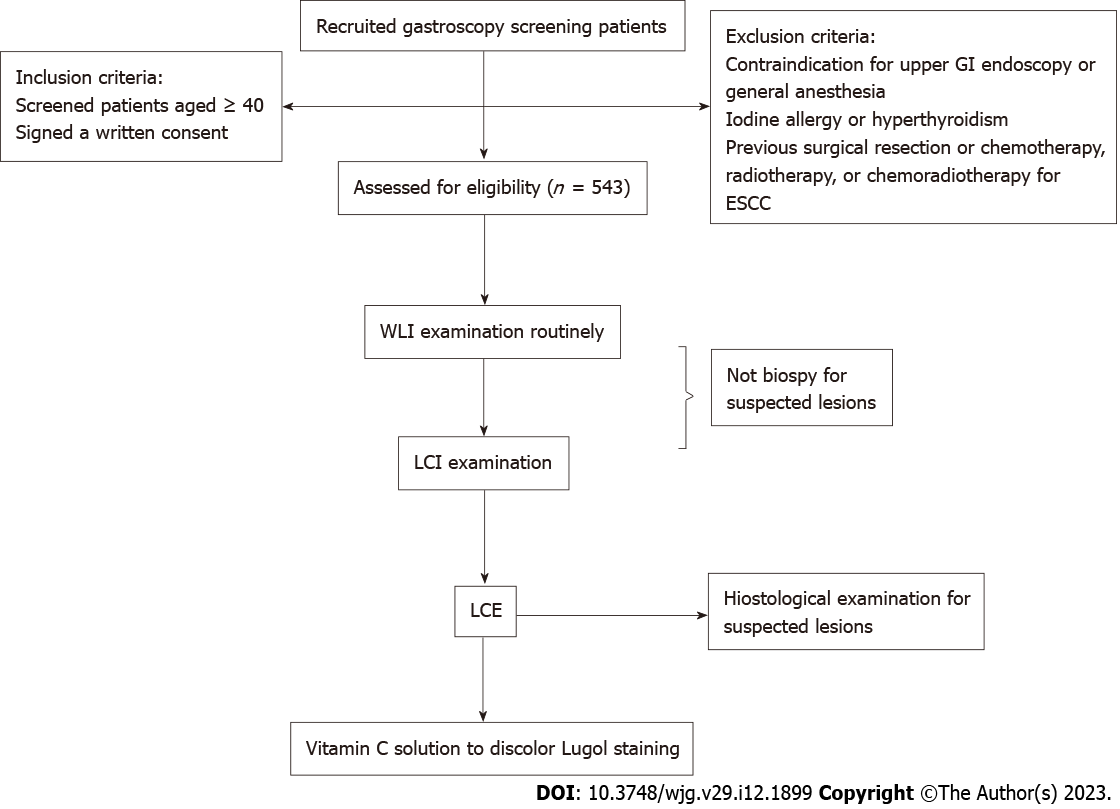
This study used an upper EG-590WR scope (Fujifilm) for gastroscopy. The specific examination procedure was performed as follows. First, WLI examination was performed, and all detected lesions were described. Second, LCI examination was performed, and all lesions detected were recorded in the report (another researcher) but not biopsied immediately to avoid bleeding that could influence the Lugol examination. Third, LCE was performed (2% Lugol dye was spread over the entire esophagus using a spray catheter except near the upper esophageal sphincter given the risk of aspiration), and we biopsied the suspected lesions detected by LCE. Finally, vitamin C solution was used to discolor the Lugol-stained mucosa[29], and lesions suspected only in LCI mode were biopsied based on the recorded location. All suspected lesions were biopsied, and the modality (only LCI, only LCE, or both LCI and LCE) that detected each lesion was indicated in the report. A flow chart of the study is described in Figure 1. All endoscopic operators were experienced and had completed at least 1000 endoscopies. The diagnosis modality (LCI or/and LCE), size, location and macroscopic type (Paris classification[30]) of each detected lesion were recorded and described after examination.
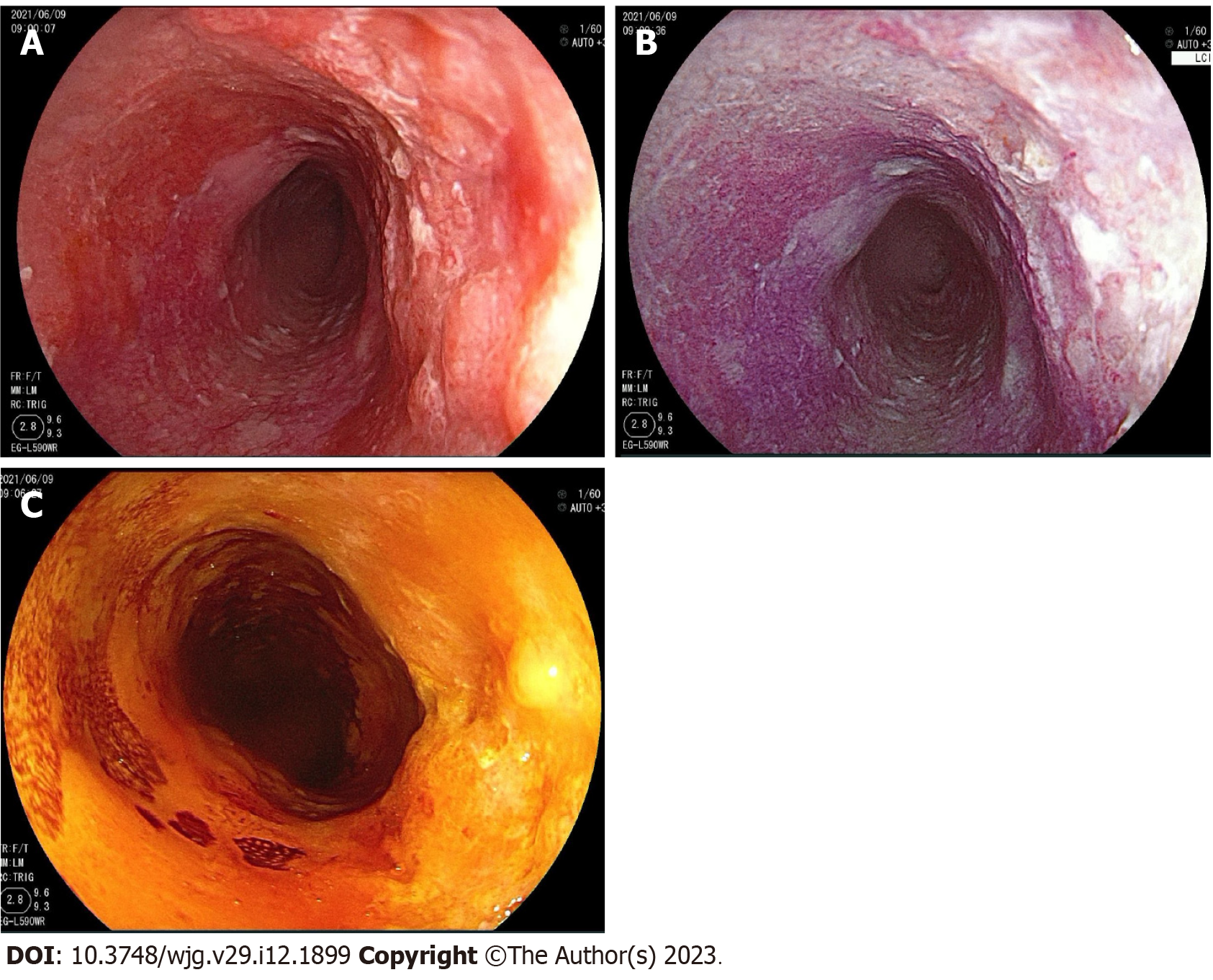
The diagnostic criteria for intraepithelial neoplasia and ESCC proposed by the Vienna Classification are as follows: Low-grade intraepithelial neoplasia (LGIN); high-grade intraepithelial neoplasia (HGIN), ESCC and negative for neoplasia, including chronic esophagitis[31]. The primary outcome was the sensitivity and specificity of the diagnostic strategies to detect squamous cell neoplastic lesions (LGIN, HGIN and/or ESCC) according to histology. Endoscopically suspicious ESCC lesions and precancerous lesions were defined as follows: (1) The presence of a reddish color change with a rough mucosal surface in WLI (Figure 2A); (2) The presence of a well-demarcated red or red-orange color region in LCI (Figure 2B)[21,22]; and (3) The presence of a well-demarcated unstained area ≥ 5 mm in diameter or a pink color area after iodine staining given that a Lugol-voiding lesion is more likely to be neoplastic with increasing size (Figure 2C)[32,33].
All suspected lesions were pathologically evaluated by biopsy to determine their neoplastic nature. If the lesion was confirmed to be neoplastic, it was a true positive for the detection modality. Conversely, for non-neoplastic lesions, an unconfirmed suspected lesion was defined as a false positive for the detection modality.
Major outcomes included sensitivity and specificity. The secondary outcome was procedure time. It was possible to determine the length of the imaging operation using a clock linked to the endoscopic equipment. The LCI procedure time was defined as the total examination time from the epiglottis to the dentate line in the image files. The LCE procedure time was defined as the examination time from spraying Lugol dye to complete discoloration.
In this study, we calculated the required sample size for a noninferiority test using the PASS 15.0.5 sample size software (NCSS, LLC, Kaysville, UT, United States). We regarded LCE as the reference endoscopy technique, and sensitivity was assumed to be 90.0%[13,14]. A sensitivity of 80.0% would be a clinically adequate diagnostic value for screening endoscopy for ESCC. The sample size required was 535 patients using the Z test with pooled variance for a statistical power of 90% with statistical significance defined as P < 0.05 (α = 0.05 and β = 0.10).
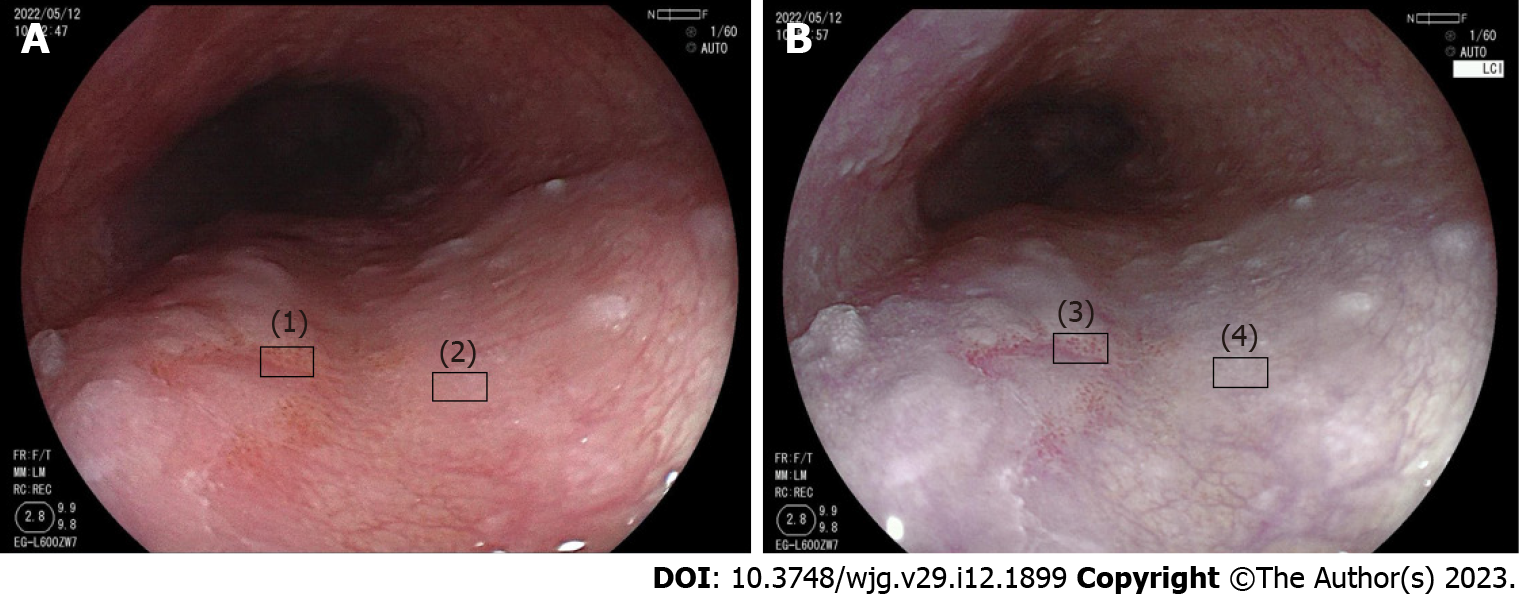
Color analysis was performed using Adobe Photoshop CC2017 (Adobe Systems Inc., San Jose, CA, United States). First, regions of interest of the lesion mucosa and surrounding mucosa were selected in WLI and LCI, separately (Figure 3). Second, the Commission International de l’Eclairage - L*a*b* color space was used to evaluate the mean color value in the regions of interest[34]. The three-dimensional color parameters L* (black to white; range: 0 to + 100), a* (green to red; range: 128 to + 127) and b* (blue to yellow; range: 128 to + 127) were used to define the color value. In Photoshop, L*, a* and b* represent color scores (Lab color unit). Third, the color difference between the lesion and the surrounding mucosa (ΔE) was represented by the distance connecting the two points:

To reduce the influence of color difference depending on imaging conditions, we chose similar distance and angle images in each mode to calculate color values. We calculated the ΔE of each image in LCI and WLI. In addition, we calculated the ΔE of different neoplastic pathologies in LCI and compared them.
All analyses were performed using SPSS 26.0 statistical software (IBM Corp., Armonk, NY, United States). The characteristics and diagnostic yields of patients were presented as percentages (%) or medians (interquartile range), and the color difference variables were expressed as the mean ± SD. Continuous variables were compared using the student’s t-test or the Mann-Whitney U test, whereas categorical variables were compared using Pearson’s χ2test or Fisher’s exact test. A two-tailed P value of < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by the staff of the Department of Epidemiology and Statistics from the First Medical Center of PLA General Hospital (Beijing, China).
From March 2021 to May 2022, a total of 543 patients were enrolled in our study (Figure 1). Among them, a total of 141 suspected lesions were identified in 115 patients. Of these lesions, 45 neoplastic lesions (17 ESCC, 3 HGIN and 25 LGIN) were histologically confirmed in 43 (7.8%) patients (Table 1). Among the 45 lesions, 38 lesions (17 ESCC, 3 HGIN and 18 LGIN) were endoscopically suspicious during LCI examination; further, 41 lesions (17 ESCC, 3 HGIN and 21 LGIN) were endoscopically suspicious during LCE examination. Regarding the diagnostic modality of the 45 lesions, 34 (75.6%) lesions were identified by LCI and LCE compared to 4 (8.9%) lesions that were detected only by LCI and 7 (15.6%) lesions that were detected only by LCE.
| Item | n | % (n/n total) |
| Patients with suspected lesions1 | 115 | 21.2 (115/543) |
| Inflammation | 72 | 13.2 |
| LGIN | 25 | 4.6 |
| HGIN | 3 | 0.5 |
| ESCC | 15 | 2.7 |
| Suspected lesions, including non-neoplastic | 141 | - |
| Neoplastic lesions2 | 45 | 31.9 (45/141) |
| LGIN | 25 | 17.7 (25/141) |
| HGIN | 3 | 2.1 (3/141) |
| ESCC | 17 | 12.1 (17/141) |
| Neoplastic lesions detected with LCI | ||
| LGIN | 18 | 72.0 (18/25) |
| HGIN | 3 | 100 (3/3) |
| ESCC | 17 | 100 (17/17) |
| Neoplastic lesions detected with LCE | ||
| LGIN | 21 | 84.0 (21/25) |
| HGIN | 3 | 100 (3/3) |
| ESCC | 17 | 100 (17/17) |
| Neoplastic lesions detected by different diagnosis modality | ||
| Only LCI | 4 | 8.9 (4/45) |
| Only LCE | 7 | 15.6 (7/45) |
| Both LCI and LCE | 34 | 75.6 (34/45) |
| Neoplastic lesions per patient, mean (SD) | 0.08 (0.29) |
In LCI mode, 74 suspected lesions underwent histological examination, and 38 lesions were confirmed histologically in 36 patients. In LCE mode, 104 suspected lesions were detected, and 41 lesions were histologically confirmed in 39 patients. In Table 2, the sensitivity, specificity, positive predictive value and negative predictive value for LCI for the diagnosis of ESCC/HGIN/LGIN were 83.7%, 92.4%, 48.6% and 98.5%, respectively. The sensitivity, specificity, positive predictive value and negative predictive value for LCE were 90.7%, 87.0%, 37.5% and 99.1%, respectively. No significant differences in sensitivity, positive predictive value and negative predictive value were noted between LCI and LCE. However, the specificity of LCI was significantly higher than that of LCE (92.4% vs 87.0%, P = 0.0023).
The characteristics of the neoplastic lesions detected by LCI and LCE of the respective comparison groups are shown in Table 3. No significant differences in lesion location, size, morphologic type or pathology were noted between the two modality groups. However, the procedure time was significantly shorter for the LCI group compared with the LCE group [42 (34, 50) s vs 160 (130, 189) s, P < 0.001].
| Item | Detected by LCI, n = 36 patients/38 lesions | Detected by LCE, n = 39 patients/41 lesions | P value | |
| Lesion location | 0.969 | |||
| Upper | 3 (7.9) | 3 (7.3) | ||
| Middle | 11 (28.9) | 11 (26.8) | ||
| Lower | 24 (63.2) | 27 (65.9) | ||
| Morphologic type | 0.902 | |||
| I | 1 (2.6) | 1 (2.4) | ||
| IIa | 9 (23.7) | 8 (19.5) | ||
| IIb | 18 (47.4) | 24 (58.5) | ||
| IIc | 9 (23.7) | 7 (17.1) | ||
| III | 1 (2.6) | 1 (2.4) | ||
| Size in mm | 0.814 | |||
| ≤ 10 | 14 (36.8) | 18 (43.9) | ||
| 10-20 | 3 (7.9) | 3 (7.3) | ||
| > 20 | 21 (55.3) | 20 (48.8) | ||
| Pathology | 0.998 | |||
| LGIN | 18 (47.4) | 21 (51.2) | ||
| HGIN | 3 (7.9) | 3 (7.3) | ||
| ESCC | EP/LPM | 3 (7.9) | 3 (7.3) | |
| MM/SM1 | 1 (2.6) | 1 (2.4) | ||
| ≥ SM2 | 13 (34.2) | 13 (31.7) | ||
| Procedure time (s), median (IQR) | 42 (34, 50) | 160 (130, 189) | < 0.0011 | |
All detected neoplastic lesions were further divided into three subgroups according to the different diagnosis modalities: LCI only group (detected by LCI only, n = 4 lesions), LCE only group (detected by LCE only, n = 7 lesions), and LCI + LCE group (detected by both LCI and LCE, n = 34 lesions). We compared the clinicopathological features of the three groups in Supplementary Table 1. The lesion location, morphologic type and pathology were comparable among the three groups. However, the lesion sizes of the three groups were significantly different: 20 (55.8%) lesions in the LCI + LCE group were > 20 mm; 3 (75%) lesions in the LCI only group were ≤ 10 mm; and 7 (100%) lesions in the LCE only group were ≤ 10 mm (P = 0.016). We further compared the lesion size in the LCI only group and LCE only group, and the difference was not statistically significant (P = 0.364).
Of the 45 neoplastic lesions, 30 lesions had endoscopic images with clear visibility. Color analysis was conducted in the 30 lesions. Supplementary Table 2 compares the color difference between the lesion and surrounding mucosa in WLI and LCI. We found that the ΔE between the lesions and the surrounding mucosa was significantly higher in LCI compared with WLI (21.20 ± 9.79 vs 15.92 ± 7.50, respectively, P = 0.023). For the three-dimensional color parameters of L*a*b*, Δa* was higher in LCI than in WLI (12.43 ± 10.00 vs 4.97 ± 6.96, respectively, P = 0.001), and ΔL* and Δb* were similar in LCI and WLI. We further analyzed the color difference between the lesion and surrounding mucosa in different neoplastic pathologies (LGIN, HGIN and ESCC) in LCI mode, as shown in Supple
In this prospective study, we demonstrated that the sensitivity and specificity of LCI to detect ESCC and/or precancerous lesions (LGIN or/and HGIN) are acceptable. The specificity of LCI is higher than that of LCE, and the differences in sensitivity between the two modes were not obvious.
Previous studies have reported the utility of LCE in the detection of esophageal cancer[7,10-12]. LCE has served as the reference technique in patients at high risk for esophageal cancer[8,9]. Nevertheless, LCE is not suitable for general population screening given its side effects[16,17]. As a type of enhanced endoscopy, some studies have shown that narrow-band imaging is comparable to LCE in detecting esophageal cancer due to its excellent sensitivity and specificity[13-15]. Narrow-band imaging is typically combined with magnifying endoscopy, and the requirements for hospitals and operators are relatively high. LCI is a novel enhanced endoscopic technique, and Ono et al[20] reported that the detection rate of LCI in the diagnosis of neoplastic lesions in the upper gastrointestinal tract is 1.67 times higher than that of WLI. Yi et al[25] compared LCI and LCE in the detection of multiple primary esophageal cancers in primary ESCC patients and found that both modalities exhibited great value for multiple primary esophageal cancers. However, no study has compared their screening ability. To the best of our knowledge, this was the first prospective study to compare the effectiveness of LCI and LCE in screening esophageal cancer and precancerous lesions.
The present results showed that LCI is significantly more specific than LCE (92.4% vs 87.0%, P = 0.0023), and both modes exhibited high sensitivity for detecting neoplastic lesions (LGIN/HGIN/ESCC) (83.7% vs 90.7%, P = 0.520). The lesions were all pathologically diagnosed as LGIN (7 lesions in LCI and 4 in LCE). Therefore, our study results were consistent with previously published data reporting that the sensitivity of narrow-band imaging and Lugol for the diagnosis of ESCC and/or HGIN was approximately 100%[13,14]. The LCI only group included a lesion approximately 4 cm in length, and no unstained areas were observed. Based on the endoscopic findings, we thought that it was an erosive mucosal lesion caused by gastric acid reflux. Our results indicate that the capability of LCI to detect neoplastic lesions is acceptable.
The LCI technique is convenient for clinical endoscopists to use with the Fujifilm system because the imaging modes can easily be switched during the examination. Thus, the process is not as time-consuming as iodine staining. In our research, the median LCI procedure time was significantly shorter than for LCE (42 s vs 160 s, P < 0.001). In addition, Lugol solution can irritate the mucosa and may cause many side effects (chemical esophagitis, laryngitis, bronchopneumonia, chest pain, esophagospasm, gastritis and hypersensitivity)[16,17]. In clinical practice, the upper end of the Lugol staining site is generally less than 20 cm from the incisor given the high risk of solution aspiration. Therefore, it is difficult to detect cervical esophageal lesions, which reduces the detection of synchronous or metachronous neoplastic lesions. Therefore, the LCI technique is more useful for the diagnosis of ESCC or precancerous lesions given the mild mucosal irritation.
After color analysis, we found that the color difference between the lesion and the surrounding mucosa was greater for LCI compared with WLI. The results demonstrated that the lesion was more visible in LCI mode, which is consistent with previous studies[18,19,21,22]. Kobayashi et al[21] investigated the relationship between color information and the invasion depth of ESCC in LCI mode. They found that the color difference was greater in muscularis mucosa/submucosa invading ≤ 200 μm below the inferior margin of the muscularis mucosa or deeper lesions compared with epithelium and lamina propria mucosa lesions using LCI. However, they did not compare the color differences between ESCC and precancerous lesions. In our study, we further compared the color difference of different neoplastic pathologies in LCI mode, and the differences among LGIN, HGIN and ESCC were not significant. Notably, Tsunoda et al[35] reported a case using LCI and blue laser imaging with Lugol staining to provide an accurate diagnosis of ESCC and squamous intraepithelial neoplasia. Therefore, whether LCI and blue laser imaging combined with Lugol staining can be used to evaluate esophageal neoplastic lesions before endoscopic treatment deserves further study.
There are some limitations in our study. First, the number of lesions, especially neoplastic lesions, was low. Second, LCI and LCE can be performed sequentially during the same endoscopy procedure, but it is impossible to perform them in the reverse order. Thus, we were unable to perform a random crossover trial. Third, the images obtained in this study included two arbitrary regions of interest in the lesion mucosa and surrounding mucosa, which may have sampling errors and affected the measurement results. However, for the WLI and LCI modes, we choose similar distance and angle images to calculate color values to reduce the influence of color differences based on various conditions. In the future, we need to conduct a multicenter study and collect more neoplastic lesions to further evaluate the usefulness of LCI. Further evaluation of the validity of LCI in diagnosing the depth of invasion of ESCC is also warranted.
Our study confirmed that LCI is efficient and specific for the surveillance of ESCC without causing discomfort. In the future, LCI, as a promising screening strategy, could replace LCE in the screening of esophageal neoplastic lesions in the general population.
Lugol chromoendoscopy (LCE) has served as a standard screening technique in high-risk patients with esophageal cancer. Nevertheless, LCE is not suitable for the general population screening given its side effects. Linked color imaging (LCI) is a novel image-enhanced endoscopic technique that can distinguish subtle differences in mucosal color. It would be beneficial for the general population if LCE can provide similar diagnostic performance to LCI.
We compared the diagnostic performance of LCI with LCE in detecting esophageal squamous cell carcinoma (ESCC) and precancerous lesions. If LCI can replace LCE in detecting esophageal neoplastic lesions, it would be useful for esophageal screening in the general population.
As a novel image-enhanced endoscopic technique, LCI has been confirmed to be superior to white light imaging (WLI) in detecting neoplastic lesions in the upper gastrointestinal tract. We aimed to confirm that the diagnostic performance of LCI is comparable to LCE for the surveillance of ESCC.
This was a single-center, prospective, registered clinical study. In this noninferiority study, we prospectively enrolled 543 patients who underwent WLI, LCI and LCE successively. We compared the sensitivity and specificity of LCI and LCE in the detection of esophageal neoplastic lesions. We further used L*a*b* color space to evaluate the color differences of LCI.
In total, 43 patients were analyzed. The sensitivity of LCI was similar to that of LCE, whereas the specificity of LCI was greater than that of LCE. The LCI procedure time in the esophageal examination was significantly shorter than that of LCE. However, the color difference in LCI was similar in different pathological types.
Our study showed that LCI is efficient and specific for the surveillance of ESCC without causing discomfort. In the future, LCI, as a promising screening strategy, could replace LCE in the screening of esophageal neoplastic lesions in the general population.
Because of the low detection rate of esophageal cancer, we were only able to enroll a limited number of neoplastic lesions. In the future, we need to conduct a multicenter study and collect more neoplastic lesions to further evaluate the usefulness of LCI. Further evaluation of the validity of LCI in diagnosing the depth of invasion of ESCC is also warranted.
We thank our peer reviewers for the insightful and thorough suggestions on this manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abe Y, Japan; Tyakht AV, United Kingdom; Woromogo SH, Congo S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70:313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 454] [Article Influence: 90.8] [Reference Citation Analysis (1)] |
| 2. | Ciocirlan M, Lapalus MG, Hervieu V, Souquet JC, Napoléon B, Scoazec JY, Lefort C, Saurin JC, Ponchon T. Endoscopic mucosal resection for squamous premalignant and early malignant lesions of the esophagus. Endoscopy. 2007;39:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Merkow RP, Bilimoria KY, Keswani RN, Chung J, Sherman KL, Knab LM, Posner MC, Bentrem DJ. Treatment trends, risk of lymph node metastasis, and outcomes for localized esophageal cancer. J Natl Cancer Inst. 2014;106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J, Arnold M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates From GLOBOCAN 2020. Gastroenterology. 2022;163:649-658.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 554] [Article Influence: 184.7] [Reference Citation Analysis (0)] |
| 5. | Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology. 2020;159:335-349.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1229] [Article Influence: 245.8] [Reference Citation Analysis (0)] |
| 6. | Obermannová R, Alsina M, Cervantes A, Leong T, Lordick F, Nilsson M, van Grieken NCT, Vogel A, Smyth EC; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:992-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 332] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 7. | Hashimoto CL, Iriya K, Baba ER, Navarro-Rodriguez T, Zerbini MC, Eisig JN, Barbuti R, Chinzon D, Moraes-Filho JP. Lugol's dye spray chromoendoscopy establishes early diagnosis of esophageal cancer in patients with primary head and neck cancer. Am J Gastroenterol. 2005;100:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Bisschops R, Areia M, Coron E, Dobru D, Kaskas B, Kuvaev R, Pech O, Ragunath K, Weusten B, Familiari P, Domagk D, Valori R, Kaminski MF, Spada C, Bretthauer M, Bennett C, Senore C, Dinis-Ribeiro M, Rutter MD. Performance measures for upper gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2016;48:843-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 9. | Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, Leighton JA, Qureshi WA, Rajan E, Fanelli R, Wheeler-Harbaugh J, Baron TH, Faigel DO; Standards of Practice Committee, American Society for Gastrointestinal Endoscopy. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 315] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 10. | Shiozaki H, Tahara H, Kobayashi K, Yano H, Tamura S, Imamoto H, Yano T, Oku K, Miyata M, Nishiyama K. Endoscopic screening of early esophageal cancer with the Lugol dye method in patients with head and neck cancers. Cancer. 1990;66:2068-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Muto M, Hironaka S, Nakane M, Boku N, Ohtsu A, Yoshida S. Association of multiple Lugol-voiding lesions with synchronous and metachronous esophageal squamous cell carcinoma in patients with head and neck cancer. Gastrointest Endosc. 2002;56:517-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Shimizu Y, Tukagoshi H, Fujita M, Hosokawa M, Kato M, Asaka M. Endoscopic screening for early esophageal cancer by iodine staining in patients with other current or prior primary cancers. Gastrointest Endosc. 2001;53:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Morita FH, Bernardo WM, Ide E, Rocha RS, Aquino JC, Minata MK, Yamazaki K, Marques SB, Sakai P, de Moura EG. Narrow band imaging versus lugol chromoendoscopy to diagnose squamous cell carcinoma of the esophagus: a systematic review and meta-analysis. BMC Cancer. 2017;17:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Gruner M, Denis A, Masliah C, Amil M, Metivier-Cesbron E, Luet D, Kaasis M, Coron E, Le Rhun M, Lecleire S, Antonietti M, Legoux JL, Lefrou L, Renkes P, Tarreirias AL, Balian P, Rey P, Prost B, Cellier C, Rahmi G, Samaha E, Fratte S, Guerrier B, Landel V, Touzet S, Ponchon T, Pioche M. Narrow-band imaging versus Lugol chromoendoscopy for esophageal squamous cell cancer screening in normal endoscopic practice: randomized controlled trial. Endoscopy. 2021;53:674-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Nagami Y, Tominaga K, Machida H, Nakatani M, Kameda N, Sugimori S, Okazaki H, Tanigawa T, Yamagami H, Kubo N, Shiba M, Watanabe K, Watanabe T, Iguchi H, Fujiwara Y, Ohira M, Hirakawa K, Arakawa T. Usefulness of non-magnifying narrow-band imaging in screening of early esophageal squamous cell carcinoma: a prospective comparative study using propensity score matching. Am J Gastroenterol. 2014;109:845-854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Sreedharan A, Rembacken BJ, Rotimi O. Acute toxic gastric mucosal damage induced by Lugol's iodine spray during chromoendoscopy. Gut. 2005;54:886-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Park JM, Seok Lee I, Young Kang J, Nyol Paik C, Kyung Cho Y, Woo Kim S, Choi MG, Chung IS. Acute esophageal and gastric injury: complication of Lugol's solution. Scand J Gastroenterol. 2007;42:135-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Kanzaki H, Takenaka R, Kawahara Y, Kawai D, Obayashi Y, Baba Y, Sakae H, Gotoda T, Kono Y, Miura K, Iwamuro M, Kawano S, Tanaka T, Okada H. Linked color imaging (LCI), a novel image-enhanced endoscopy technology, emphasizes the color of early gastric cancer. Endosc Int Open. 2017;5:E1005-E1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Khurelbaatar T, Miura Y, Osawa H, Nomoto Y, Tokoro S, Tsunoda M, Sekiguchi H, Kobayashi T, Funayama Y, Nagayama M, Takezawa T, Mieno M, Ueno T, Fukuda H, Iwashita C, Takahashi H, Ino Y, Kawarai Lefor A, Yamamoto H. Usefulness of linked color imaging for the detection of obscure early gastric cancer: Multivariate analysis of 508 lesions. Dig Endosc. 2022;34:1012-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Ono S, Kawada K, Dohi O, Kitamura S, Koike T, Hori S, Kanzaki H, Murao T, Yagi N, Sasaki F, Hashiguchi K, Oka S, Katada K, Shimoda R, Mizukami K, Suehiro M, Takeuchi T, Katsuki S, Tsuda M, Naito Y, Kawano T, Haruma K, Ishikawa H, Mori K, Kato M; LCI-FIND Trial Group. Linked Color Imaging Focused on Neoplasm Detection in the Upper Gastrointestinal Tract : A Randomized Trial. Ann Intern Med. 2021;174:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 21. | Kobayashi K, Miyahara R, Funasaka K, Furukawa K, Sawada T, Maeda K, Yamamura T, Ishikawa T, Ohno E, Nakamura M, Kawashima H, Nakaguro M, Okumura Y, Hirooka Y, Fujishiro M. Color information from linked color imaging is associated with invasion depth and vascular diameter in superficial esophageal squamous cell carcinoma. Dig Endosc. 2020;32:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Nakamura K, Urabe Y, Oka S, Nagasaki N, Yorita N, Hata K, Masuda K, Kurihara M, Kotachi T, Boda T, Tanaka S, Chayama K. Usefulness of linked color imaging in the early detection of superficial esophageal squamous cell carcinomas. Esophagus. 2021;18:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | de Groof AJ, Fockens KN, Struyvenberg MR, Pouw RE, Weusten BLAM, Schoon EJ, Mostafavi N, Bisschops R, Curvers WL, Bergman JJ. Blue-light imaging and linked-color imaging improve visualization of Barrett's neoplasia by nonexpert endoscopists. Gastrointest Endosc. 2020;91:1050-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Diao W, Huang X, Shen L, Zeng Z. Diagnostic ability of blue laser imaging combined with magnifying endoscopy for early esophageal cancer. Dig Liver Dis. 2018;50:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Yi N, Huang P, Zhang W, Jiang C, Liang L. [A comparative study of endoscopic linked color imaging and iodine staining in the diagnosis of multiple primary esophageal carcinoma]. Chin J Clin Gastroenterol. 2019;31:139-144. |
| 26. | Wei WQ, Chen ZF, He YT, Feng H, Hou J, Lin DM, Li XQ, Guo CL, Li SS, Wang GQ, Dong ZW, Abnet CC, Qiao YL. Long-Term Follow-Up of a Community Assignment, One-Time Endoscopic Screening Study of Esophageal Cancer in China. J Clin Oncol. 2015;33:1951-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 27. | Zhang N, Li Y, Chang X, Lei F, Ma H, Liu J, Yang J, Su M, Sun X, Zhao D, Sun Q, Wei W, Wang G, Wang J. Long-term effectiveness of one-time endoscopic screening for esophageal cancer: A community-based study in rural China. Cancer. 2020;126:4511-4520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | National Quality Control Center of Digestive Endoscopy NCRCfDDS, National Early Gastrointestinal-Cancer Prevention & Treatment Center Alliance (GECA); Digestive Endoscopy Professional Committee of Chinese Endoscopist Association; Chinese Society of Digestive Endoscopy, Chinese Society of Health Management; Cancer Endoscopy Professional Committee of China Anti-Cancer. [China experts consensus on the protocal of early esophageal cancer and pre-cancerous lesion screening (2019, Xinxiang)]. Chin J Dig Endosc. 2019;36:793-801. |
| 29. | Jin D, Wang J, Zhan Q, Huang K, Wang H, Zhang G, Xu Y, Yao J, Sun R, Huang Q, Ye F. The safety and efficacy of 2% vitamin C solution spray for relief of mucosal irritation caused by Lugol chromoendoscopy: a multicenter, randomized, double-blind, parallel trial. Gastrointest Endosc. 2020;92:554-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1325] [Article Influence: 60.2] [Reference Citation Analysis (4)] |
| 31. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, Hattori T, Hirota T, Itabashi M, Iwafuchi M, Iwashita A, Kim YI, Kirchner T, Klimpfinger M, Koike M, Lauwers GY, Lewin KJ, Oberhuber G, Offner F, Price AB, Rubio CA, Shimizu M, Shimoda T, Sipponen P, Solcia E, Stolte M, Watanabe H, Yamabe H. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1548] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 32. | Yokoyama A, Ohmori T, Makuuchi H, Maruyama K, Okuyama K, Takahashi H, Yokoyama T, Yoshino K, Hayashida M, Ishii H. Successful screening for early esophageal cancer in alcoholics using endoscopy and mucosa iodine staining. Cancer. 1995;76:928-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Liu M, Zhou R, Guo C, Xu R, Liu A, Yang H, Li F, Duan L, Shen L, Wu Q, Liu Z, Liu F, Liu Y, Pan Y, Cai H, Weiss NS, He Z, Ke Y. Size of Lugol-unstained lesions as a predictor for risk of progression in premalignant lesions of the esophagus. Gastrointest Endosc. 2021;93:1065-1073.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Kuehni RG. Color-tolerance data and the tentative CIE 1976 L a b formula. J Opt Soc Am. 1976;66:497-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Tsunoda M, Miura Y, Osawa H, Khurelbaatar T, Sakaguchi M, Fukuda H, Lefor AK, Yamamoto H. New Diagnostic Approach for Esophageal Squamous Cell Neoplasms Using Linked Color Imaging and Blue Laser Imaging Combined with Iodine Staining. Clin Endosc. 2019;52:497-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |