Published online Mar 7, 2022. doi: 10.3748/wjg.v28.i9.881
Peer-review started: February 4, 2021
First decision: July 27, 2021
Revised: August 9, 2021
Accepted: January 29, 2022
Article in press: January 29, 2022
Published online: March 7, 2022
Processing time: 391 Days and 12.9 Hours
Chronic hepatitis B virus (HBV) infection is an international health problem with extremely high mortality and morbidity rates. Although current clinical chronic hepatitis B (CHB) treatment strategies can partly inhibit and eliminate HBV, viral breakthrough may result due to non-adherence to treatment, the emergence of viral resistance, and a long treatment cycle. Persistent CHB infection arises as a consequence of complex interactions between the virus and the host innate and adaptive immune systems. Therefore, understanding the immune escape mechanisms involved in persistent HBV infection is important for designing novel CHB treatment strategies to clear HBV and achieve long-lasting immune control. This review details the immunological and biological characteristics and escape mechanisms of HBV and the novel immune-based therapies that are currently used for treating HBV.
Core Tip: Chronic hepatitis B (CHB) infection is an international health problem. Current clinical CHB treatment strategies can partly inhibit and eliminate hepatitis B virus (HBV), but cannot achieve long-lasting immune control of the virus. Persistent CHB infection arises as a consequence of the complex interactions between HBV and the host innate and adaptive immune systems. Therefore, it is important to understand the immunological mechanisms involved in CHB infection. In this review, we detail the immune biological characteristics and escape mechanisms of HBV and discuss novel immune-based therapies.
- Citation: Zhao HJ, Hu YF, Han QJ, Zhang J. Innate and adaptive immune escape mechanisms of hepatitis B virus. World J Gastroenterol 2022; 28(9): 881-896
- URL: https://www.wjgnet.com/1007-9327/full/v28/i9/881.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i9.881
Hepatitis B virus (HBV) is a small hepatotropic, enveloped DNA virus. Hepatitis B is an international health problem caused by HBV infection. Currently, approximately 257 million people worldwide are chronic HBV carriers with a high risk of developing chronic liver diseases such as liver cirrhosis and hepatocellular cell carcinoma (HCC). Each year, approximately 1 million patients die of HBV-related liver diseases[1,2]. Current clinical treatment strategies for chronic hepatitis B (CHB) mainly include pegylated interferon-α (PEG-IFN-α) and nucleos(t)ide analogues (NAs). Unfortunately, current treatments have limitations and often fail to achieve long-term virologic control.
Generally, the host innate and adaptive immune systems play critical roles in eliminating HBV upon infection. However, HBV has evolved and developed efficient strategies for escaping host immune surveillance, which results in persistent infections. The majority of HBV infections occur in newborn infants with the presence of immunological defects, characterized by a lower quality and quantity of HBV-specific T cells and B cells. In addition, maternal hepatitis B e antigen (HBeAg) can induce the Kupffer cells (KCs) of the offspring by upregulating programmed death-ligand 1 (PD-L1) to suppress the HBV-specific CD8+ T cells response to support HBV persistence after birth[3]. In addition, HBV circumvents endogenous type I interferon (IFN-I) responses[4] and inhibits the function of innate and adaptive immune cells[5]. Prolonged exposure of T cells to large quantities of viral antigens, such as hepatitis B surface antigen (HBsAg) and HBeAg, induces a defective T-cell response with the loss of effector functions and increased inhibitory receptor expression, facilitating viral persistence. Moreover, HBV infection affects the expression of human leukocyte antigen (HLA)-II alleles, including HLA-DP, HLA-DQ, and HLA-DR, on antigen-presenting cells (APCs)[6], which in turn impairs antigen presentation capacity with induction of an inefficient T-cell response, leading to persistent HBV infection.
The clinical outcomes of patients with CHB are highly based on the complex interactions between HBV and the host innate and adaptive immune systems. In this review, we detail the interaction between HBV and the host immune system to understand the immunological and biological characteristics of and escape mechanisms involved in CHB infection, and present the current immune-based therapies for CHB treatment.
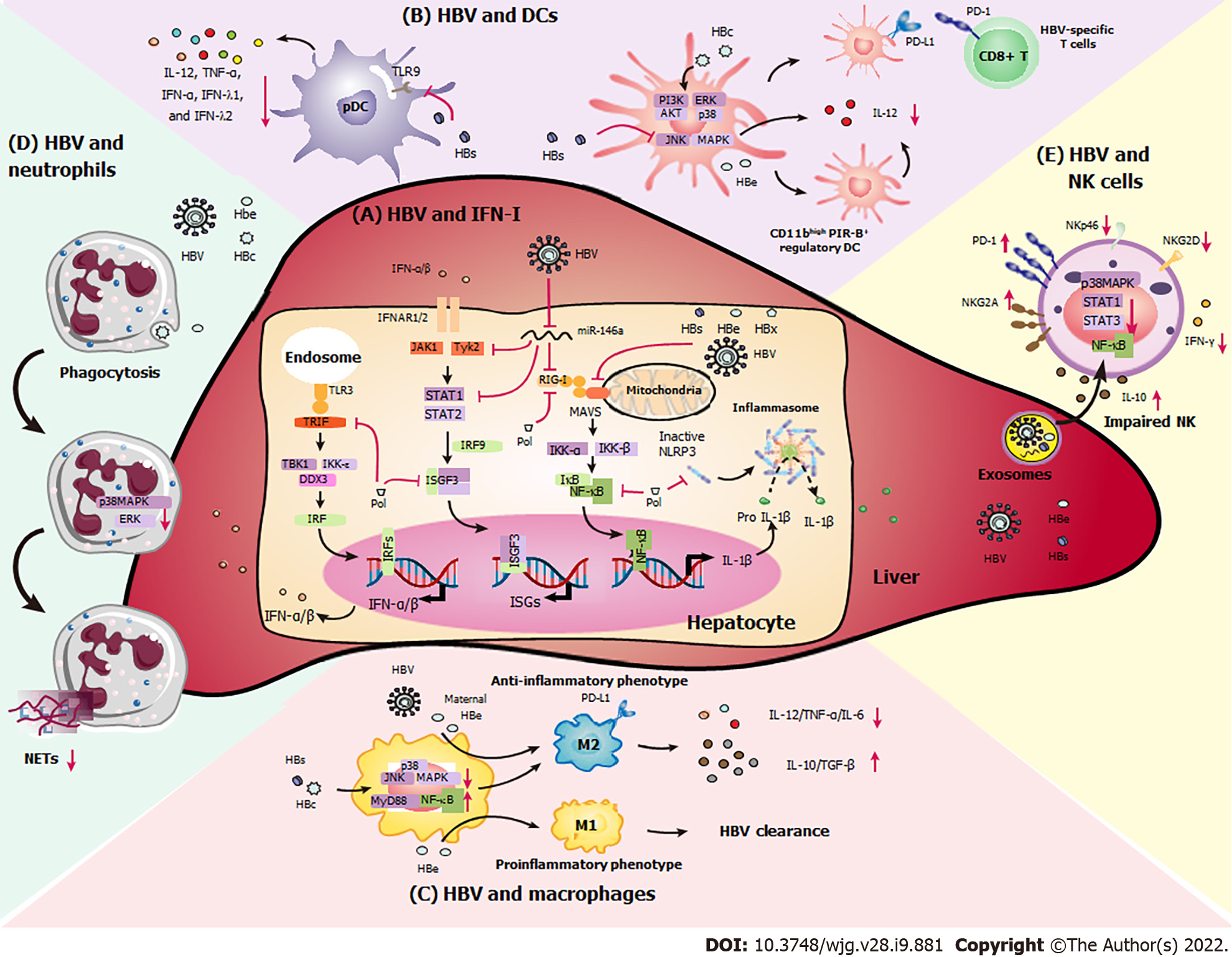
Innate immune responses act as the first line of immune defense against viruses, bacteria, and tumors. Complement components, chemokines, and cytokines are soluble factors that form parts of the innate system. Granulocytes, dendritic cells (DCs), macrophages, mast cells, and natural killer (NK) cells are important effector cells[7,8]. Commonly, an effective innate immune response is initiated when pathogen-associated molecular pattern (PAMP) molecules bind pattern recognition receptors (PRRs), which stimulates chemokine and proinflammatory cytokine production, and innate immune cell activation, resulting in the elimination of viruses[9]. Here, we described the interaction between HBV and innate immunity (Figure 1).
PRRs, which are widely expressed by KCs, hepatic DCs, liver sinusoidal endothelial cells (LSECs), and hepatocytes, can recognize PAMPs from HBV and induce antiviral immune responses, resulting in the secretion and IFN-I and other inflammatory cytokines. IFN-I, as a major component of the innate immune response, is critical for HBV clearance. However, HBV circumvents endogenous IFN-I responses through multiple pathways to sustain persistent HBV infection.
Chronic HBV infection downregulates the expression of Toll-like receptor 3 (TLR3), retinoic acid-inducible gene I (RIG-I), and melanoma differentiation-associated protein 5 (MDA-5) in DCs and hepatocytes, leading to the reduction of responsiveness to PAMPs and impairment of IFN-I synthesis[10]. A previous study found that HBV infection upregulates microRNA-146a (miR-146a) expression in hepatocytes, inhibiting the expression of RIG-I-like receptors and in turn suppressing IFN-I transcription[11]. Additionally, HBsAg, HBeAg, HBx, and HBV virions themselves can inhibit IFN-β synthesis by downregulating mitochondrial antiviral signaling (MAVS) and interfering with the interaction between MAVS and RIG-I[12].
Binding of IFN-I to the IFN receptor can induce the activation of IFN-stimulated genes (ISGs), thereby directly inhibiting HBV infection. However, HBV can extensively impair IFN-I-induced signal transduction and dampen IFN-I-mediated immune responses[4]. HBx is able to reduce transcription of the IFN-α receptor (IFNAR1) and downregulate tyrosine kinase 2, which is essential for cell surface IFNAR1 expression[13]. Additionally, matrix metalloproteinase 9, which is increased in the peripheral blood mononuclear cells of patients with CHB, binds to IFNAR1 and facilitates its phosphorylation, ubiquitination, subcellular distribution, and degradation[14]. HBV can inhibit the activities of IFN-stimulated response elements with lower ISG expression by disrupting the intracellular Janus kinase–signal transducer and activator of transcription 1 (STAT1) signaling pathway. HBV-induced miR-146a downregulates cellular STAT1 levels and blocks STAT1-Tyr701 phosphorylation in hepatocytes[11]. HBV polymerase interferes with the binding of DEAD-box helicase 3 X-linked (DDX3) to the TANK-binding kinase 1/IκB kinase epsilon complex and the induction of IFN-stimulated gene factor 3 (ISGF3) to inhibit IFN-β induction[10]. In addition, HBV polymerase suppresses interleukin 1 beta (IL-1β) production by inhibiting nuclear factor kappa B (NF-κB) signaling and the inflammasome-caspase-1 pathway, resulting in IFN-α resistance and persistent HBV infection[15].
Based on these findings, IFN-I is used for the treatment of CHB. IFN-α-mediated HBV suppression is correlated with the levels of apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like (known as APOBEC) and the base excision repair gene Nei endonuclease VIII-like 3[16]. IFN-α can also epigenetically regulate the HBV covalently closed circular DNA (cccDNA) minichromosome by disrupting general control non-depressible 5-mediated histone H3 lysine 79 methylation succinylation, resulting in the clearance of HBV cccDNA[17]. Imam et al[18] identified sterile alpha motif domain containing 4A as an important anti-HBV ISG that binds to and triggers degradation of the unidentified Smaug-recognition region sequence in viral RNA. ISG20 can induce the degradation of HBV RNA by selectively recognizing and binding N6-methyladenosine. In addition, tripartite motif containing 5 gamma suppresses HBV replication by interacting with the HBx protein, which promotes HBx ubiquitination at residue K48 and its subsequent degradation[19]. MX dynamin like GTPase 2 reduces HBV cccDNA by indirectly impairing the conversion of relaxed circular DNA to cccDNA[20]. Interestingly, IFN-α can also induce soluble factors that compete with HBV for binding to heparin glycosaminoglycans, thereby inhibiting HBV infection[21]. In addition to its direct antiviral effects on HBV, IFN-I indirectly exerts antiviral functions by activating immune cells. IFN-I can quickly recruit and activate NK cells and DCs, promoting the initiation of adaptive immunity[5,22], which in turn contributes to the elimination of HBV.
As professional APCs, DCs serve as a bridge between the innate and adaptive immune responses[23]. Recent data have shown that functional impairment of DCs by HBV infection fails to induce efficient anti-HBV immunity, leading to CHB infection and the progression of liver disease[24]. Previous data have demonstrated that patients with CHB have significantly fewer peripheral blood DCs than control subjects, accompanied by a functional decline and directly causing HBV-specific T cell dysfunction[24]. Compared to those of healthy donors, myeloid DCs (mDCs) isolated from patients with CHB display limited antigen-presenting capacity and migration capacity, features that are accompanied by the decreased expression of interleukin 6 cytokine family signal transducer (also known as gp130)[24]. Persistent HBV infection downregulates cluster of differentiation 80 (CD80), CD83, CD86, and CD40 expression in DCs, which suppresses the transduction of costimulatory signals to T cells. In addition, HBsAg reduces IL-12 production by mDCs by disrupting the c-Jun N-terminal kinase (JNK)–mitogen-activated protein kinase (MAPK) pathway, resulting in a markedly tolerogenic phenotype[25]. HBcAg upregulates programmed death-ligand 1 (PD-L1) by activating the phosphoinositide 3-kinase (PI3K)–AKT, extracellular-regulated kinase (ERK), and p38 signal transduction pathways, which suppresses HBV-specific T cell immune function[26]. HBeAg induces the conversion of bone marrow-derived DCs into CD11bhi PIR-B+ regulatory DCs, which exhibit extremely low T-cell stimulatory capacity and IL-12 production[27]. Furthermore, HBV particles, especially HBsAg, downregulate TLR expression and abrogate TLR9-triggered maturation of plasmacytoid DCs, resulting in the significantly decreased secretion of certain cytokines such as IL-12, tumor necrosis factor alpha (TNF-α), IFN-α, IFN-λ1, and IFN-λ2[28-30]. In addition, chronic HBV infection impairs IFN-α secretion and pDC maturation in response to TLR7 ligands[28].
Macrophages are important innate immune cells that fight against pathogen infection and can interact with lymphocytes by activating and inhibitory surface molecules. HBV can affect the functions of monocytes and macrophages, thereby contributing to persistent HBV infection. HBV infection promotes the activation of anti-inflammatory macrophages with increased IL-10 production, which support the functional inactivation of CD8+ T cells.
KCs, which are localized in liver sinusoids, are the largest population of innate immune cells in the liver[31]. They are stationary and able to effectively phagocytose cellular debris, foreign material, or pathogens, acting as critical sentinels for liver homeostasis[32]. Chronic HBV infection induces the production of immunomodulatory mediators such as IL-10 and transforming growth factor beta (TGF-β), and the expression of PD-L1 and PD-L2 by KCs, suppressing anti-HBV T cell responses. Furthermore, upon HBV infection, elderly mice have a significantly higher number of TNF-α-producing Ly6C+ monocytes and a much lower number of IL-10-secreting KCs than younger mice, facilitating HBV clearance[33]. However, KCs can play different roles in the presence of different HBV antigens[34]. Boltjes et al[35] found that KCs could interact with HBsAg, which induced secretion of the proinflammatory cytokines IL-6 and TNF that was substantially increased compared with that seen in healthy controls. In vivo experiments have demonstrated that HBcAg interacts with KCs upon TLR2 activation, mediating humoral and cellular tolerance via IL-10 production during CHB infection, and TLR2 knockout or KC depletion leads to an accelerated HBV clearance and improved HBV-specific CD8+ T cell responses[36]. HBeAg suppresses lipopolysaccharide-induced NOD-, LRR- and pyrin domain-containing protein 3 activation and IL-1β maturation in KCs by inhibiting NF-κB phosphorylation and reactive oxygen species production[37]. Nonetheless, HBeAg can play two distinct roles in macrophage function. Upon HBV infection, maternal HBeAg enhances PD-L1 expression in KCs with an M2-like anti-inflammatory phenotype, which suppresses the HBV-specific cytotoxic T lymphocyte (CTL) response and leads to HBV persistence; however, in control mice born to HBeAg-negative mothers, HBeAg promotes the M1 proinflammatory phenotype, contributing to HBV clearance[3].
Except for KCs, monocyte-derived macrophages are critical for regulating the anti-HBV immune response and disease pathogenesis during HBV infection. Intrahepatic macrophages that had phagocytosed HBcAg show anti-inflammatory over proinflammatory functions and favor the maintenance of infection. In addition, HBsAg inhibits TLR2-induced phosphorylation of p38 MAPK and JNK MAPK with reduced production of IL-6, TNF-α, and IL-12 in human monocytes[38]. Previous findings have also shown that HBsAg can interact with monocytes and induce the MyD88–NF-κB-signaling pathway with high expression of the inhibitory molecules PD-L1, IL-10, and TGF-β, thereby initiating an immunosuppressive cascade[39]. In contrast to HBeAg and HBsAg, HBcAg from HBV-infected hepatocytes upregulates IL-23 secretion in monocyte-derived macrophages and enhances macrophage-mediated angiogenesis[40]. In addition, HBV-induced M2-like macrophages promote the immunosuppressive activity of regulatory T (Treg) cells by enhancing cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), inducible T cell costimulator, and CD39 expression levels in an amphiregulin-dependent manner[41], impairing T helper type 1 cell immune responses and accelerating liver fibrosis and pathology.
Neutrophils exhibit protective functions against microbial infections via phagocytosis, degranulation, and the formation of neutrophil extracellular traps (NETs). Recent results have shown that HBV might suppress the neutrophil response. For example, neutrophils from patients with liver cirrhosis show a decreased capability for NET release, accompanied by the reduced expression of CD69 and CD80[42]. Additionally, HBV-related antigens, such as HBeAg and HBcAg, decrease NET release by decreasing p38 MAPK and ERK phosphorylation and autophagy, which facilitates HBV immune escape, replication, and persistence[43].
As the main effector cells of the innate immune system, NK cells constitute up to 40%–50% of human liver lymphocytes, and serve as the first line of defense against pathogens. Activated cytolytic CD56dim NK cells expressing NKp46, NKp30, and perforin are associated with efficient HBV containment[44,45]. Additionally, antibody-mediated activation of NK cells plays a vital role in resolving HBV infection[45]. Abnormal NK cell receptor expression and hepatic NK cell dysfunction contribute to persistent CHB infection and HCC progression, which are related to the poor prognosis and survival of patients with liver cancer[46]. The levels of activating receptors (e.g., NKp30, NKp46, and natural killer group 2 member D [NKG2D]) and cytokines (e.g., IFN-γ and TNF-α) are significantly decreased in patients with CHB, which is accompanied by the higher secretion of inhibitory NK cell receptors such as T cell immunoglobulin and mucin domain-containing protein 3 (Tim-3), NKG2A, and IL-10[47]. In addition, the decreased expression of CD122, the common β chain of the IL-2 receptor on CD56dim NK cells, is associated with NK cell dysfunction during CHB infection[48].
The roles of circulating HBV-related antigens (e.g., HBsAg and HBeAg) in mediating NK cell inhibition remain unclear. Recently, we found that HBsAg and HBeAg directly interact with NK cells and act as inhibitory mediators by interfering with the activation of STAT1, NF-κB, and p38 MAPK, which in turn impairs NK cell cytotoxicity and cytokine production[49]. HBsAg downregulates STAT3 expression, which is partially correlated with degranulation and cytokine production in patients with HBeAg-negative CHB[50]. Additionally, HBsAg-treated monocytes promote the conversion of NK cells into IL-10-producing regulatory NK cells via PD-L1 and major histocompatibility complex, class I, E signals, which contribute to persistent CHB infection[39]. Importantly, exosomes derived from patients with CHB shuttle HBV nucleic acids into NK cells and then dampen the RIG-I, NF-κB, and p38 MAPK signaling pathways, resulting in the functional suppression of NK cells during CHB infection[51]. miR-146a is significantly elevated in patients with CHB and modulate both NK and T-cell responses[52]. Interestingly, we found that miR-146a in NK cells can be induced by exogenous IL-10 and TGF-β, which in turn leads to NK cell dysfunction by directly targeting STAT1, accompanied by weakened IFN-γ and TNF-α secretion in patients with CHB[53].
Adaptive immunity plays a critical role in HBV infection, accompanied by antigen specificity and sustained memory responses. Under the stimulation of APCs, HBV-specific CD4+ T cells and CD8+ T cells are activated and then secrete antiviral cytokines such as IL-12, IFN-γ, and TNF-α, and induce CTL responses to kill HBV-infected hepatocytes[54]. In addition, follicular helper T (Tfh) cells promote B cell differentiation into plasma cells, which are capable of producing HBV-specific antibodies[55]. Recent studies have demonstrated that newborn infants have immunological defects, and the inability to induce HBV-specific T- and B-cell responses have been found in neonatal animals, which develop chronic HBV infection[3,56]. Furthermore, chronic HBV infection can suppress the adaptive immune system and dampen HBV clearance, leading to persistent HBV infection in patients with CHB.
HLA in APCs plays a critical role in initiating the host antiviral immune response against HBV infection due to its capacity to attract and bind peptides. HLA-I molecules can present HBV peptides to CD8+ cytotoxic T lymphocytes, resulting in the direct cytolysis of HBV-infected hepatocytes. HLA-II alleles, including HLA-DP, HLA-DQ, and HLA-DR, encode MHC-II molecules that present exogenous antigens to CD4+ T cells[57,58]. Different HLA-II alleles with particular amino acid polymorphisms determine which peptides can be presented to T cells. Moreover, these HLA alleles expressed on APCs contribute to presenting a broad range of peptides, thus determining the variability in the host immune response to HBV. Compared to HLA-DP and HLA-DQ, HLA-DR allele containing an extra β-chain gene whose product can pair with the DRα chain on APCs, is more important for the induction of sustained HBV-specific immune response. Upon HBV infection, single nucleotide polymorphisms of HLA-II antigens may also contribute to the induction of immune tolerance, leading to persistent HBV infection[57,59-62]. HBV infection reduces the expression of HLA-DP and HLA-DQ molecules on APCs, which in turn results in impaired antigen presentation capacity and an inefficient T-cell response[12,13]. Thus, polymorphisms of HLA-II genes during HBV infection can alter the antigen-binding properties of HLA-II and affect the HBV-specific immune response, partly promoting the persistent HBV infection.
HBV-specific CD8+ T cells function as key cellular effectors against HBV infection[63]. During CHB infection, CD8+ T cells encounter HBV antigens presented by intrahepatic APCs, such as DCs, KCs, or LSECs with weakened costimulatory signals, resulting in immune tolerance[64]. In addition, HBV infection can cause deficient secretion of inflammatory cytokines such as IL-12 and IFN-α/β in response to PAMP stimulation, further dampening the third signal required for CD8+ T-cell activation. Additionally, sustained exposure to high doses of HBV antigens (e.g., HBsAg and HBeAg) leads to exhausted T cells and impaired effector functions during CHB infection. Microarray analyses have revealed that HBV significantly upregulates the expression of the proapoptotic molecule Bcl-2-like protein 11 among HBV-specific CD8+ T cells, suggesting a key mechanism related to the depletion of CD8+ T cells during CHB infection[65]. Exhausted CD8+ T cells show reduced IL-2, IFN-γ, and TNF-α secretion and lost cytotoxic and proliferative capacities[66,67]. Moreover, exhausted HBV-specific CD8+ T cells display multiple inhibitory receptors such as PD-1, CTLA-4, CD244 (2B4), Tim-3, and lymphocyte activation gene 3[68], closely mimicking the transcriptional profiles of CD8+ T cells[67,69,70].
T-bet is essential for successful CD8+ T cell responses against HBV, whereas eomesodermin (EOMES) is a key driver of T cell exhaustion during chronic HBV infection[67,69,71]. Schurich et al[71] observed that reduced PD-1 expression on HBV-specific CD8+ T cells is accompanied by high levels of T-bet, which can increase CD8+ T cell functions[71], whereas EOMES might compensate for the lack of T-bet expression during HBV infection[67]. Data from a recent study showed that exhausted CD8+ T cells express elevated levels of the transcription factors interferon regulatory factor 4, basic leucine zipper ATF-like transcription factor, and nuclear factor of activated T cells 1, which in turn promote the expression of multiple inhibitory receptors (such as PD-1) and is accompanied by impaired antiviral function and cellular metabolism. However, transcription factor T cell factor 1 (TCF1) expression is repressed in exhausted CD8+ T cells, which is important considering that TCF1 is essential for memory T cell differentiation[72]. Moreover, when compared with HBV core-specific CD8+ T cells, HBV polymerase-specific CD8+ T cells show higher expression of CD38 and EOMES, accompanied by lower T-bet expression and a reduced expansion capacity[70]. Recently, transcriptome analysis of exhausted HBV-specific CD8+ T cells in patients with CHB revealed a lower mitochondrial potential and substantial mitochondrial dysfunction[73,74], whereas exposure to antioxidants or IL-12 partially reinvigorated the antiviral activity of HBV-specific CD8+ T cells[73,74].
CD4+ T cells are important in regulating CD8+ T cell activation, proliferation, and memory responses during HBV infection[75]. Generally, a loss help of CD4+ T cells is considered the major factor involved in HBV-specific CTL cell failure[76]. Increasing attention has been given to the exhaustion of CD4+ T cells during CHB infection. Previous results have demonstrated that HBV-related antigens, such as HBcAg and HBsAg, can upregulate the expression of inhibitory molecules on CD4+ T cells. For example, Li et al[77] found that HBcAg increased PD-1 expression on CD4+ T cells via the JNK, ERK, and PI3K/AKT signaling pathways disrupt the function of CD4+ T cells. HBsAg increased the expression of human protein inhibitor of activated STAT1 expression (which is dependent on activation of the ERK and p38 MAPK signaling pathways), thereby contributing to the ineffectiveness of traditional treatments for CHB patients[78]. Data from a recent study showed that CD4+ T cells in patients with CHB expressed high levels of TRAIL receptors, and these T cells could be targeted by TRAIL+ NK cells, leading to a reduction in the number of CD4+ T cells[79]. Additionally, the decreased secretion of proinflammatory cytokines (such as IL-2, IFN-γ, and IL-21) by HBV-specific CD4+ T cells contribute to the exhaustion of CD8+ T cell responses during chronic HBV infection[80]. CD4+ T cells also differentiate into CD4+ CD25+ Foxp3+ Treg cells, which secrete the suppressive cytokines IL-10 and TGF-β, resulting in a progressive loss of HBV-specific CD8+ T cells[81]. Thus, CD4+ T cells can directly influence HBV clearance by regulating CD8+ T cells.
Tfh cells express C-X-C chemokine receptor type 5 (CXCR5) and can specifically recognize and bind to follicular B cells expressing CXCL13, which promote the formation of affinity-matured, long-lived plasma cells and antibody secretion[82]. A deficiency of Tfh cells can inhibit the formation of germinal centers (GCs) in the spleen[83]. Thus, Tfh cells play important roles in orchestrating the humoral immune response and HBsAg seroconversion in patients with CHB[84]. Previous results have shown that the frequency of Tfh cells correlates negatively with HBsAg levels in patients with CHB after PEG-IFN-α therapy[85]. The recovery of Tfh cell responses induces the production of anti-HBs antibodies and accelerates HBV clearance[55]. Additionally, Wang et al[86] found that HBV infection significantly increases the proportion of CD4+ CXCR5+ CD25+ Foxp3+ follicular regulatory T (Tfr) cells. Compared to CD25- Tfh cells, CD25+ Tfh cells express high levels of inhibitory receptors, such as PD-1 and CTLA-4, with lower levels of IFN-γ and IL-17 and higher TGF-β secretion. Importantly, Tfr cells can suppress the GC reaction of B cells and the antiviral effect of CD8+ T cells. Therefore, Tfh cell dysfunction might disrupt humoral immune responses during CHB infection.
Anti-HBs antibodies are protective antibodies that can prevent HBV from entering into host hepatocytes and can clear infectious HBV particles from the body[87,88], and B cells are essential for effective HBV control. Although the total number of B cells is enriched during CHB infection[89], previous data revealed that patients with CHB showed a decreased frequency of HBsAg-specific B cells[90]. Furthermore, the production of anti-HBs was defective in patients with CHB[91]. Recent findings indicate that hyperactivated B cells with increased expression of CD69 and CD71, have deficient proliferative capacity and are unable to achieve HBsAg seroconversion in CHB patients.
Burton et al[92] found that HBsAg-specific B cells in patients with CHB exhibited a CD21- CD27- atypical memory B cell (atMBC) phenotype, which was accompanied by high levels of inhibitory receptors such as PD-1, BTLA, and CD22. atMBCs exhibit impaired survival, proliferation, and cytokine production and cannot normally differentiate into antibody-producing plasma cells, which dampens humoral immune responses in patients with CHB. Poonia et al[93] found that HBcAg binding to B cells can induce high expression of the inhibitory receptors Fc receptor-like 5 (FcRL4) and FcRL5 on B cells, as well as dysfunctional phenotypes, and can also suppress the proliferation and activation of B cells mediated by the B cell receptor and TLR signaling. Additionally, HBcAg drives B cell differentiation into IL-10-producing regulatory B (Breg) cells characterized as CD19+ CD24hi CD38hi, which suppresses CD8+ T cell responses[89]. Moreover, Breg cells have also been found to promote the conversion of CD4+ CD25− effector T cells into CD4+ CD25+ Treg cells, thereby participating in the maintenance of immune tolerance during chronic HBV infection[89].
In addition to antibody production, B cells are also considered professional APCs during CHB infection. However, the levels of costimulatory molecules (CD80 and CD40) are significantly decreased in circulating B cells, which might impair the interactions between B cells and effector T cells, thus inducing T cell exhaustion[94]. B cells can also regulate the immune response by secreting cytokines during CHB infection. HBeAg can stimulate B cell activation by promoting B-cell activating factor production via IL-6 and IFN-γ secretion[95,96], where IL-6 can play a non-cytolytic antiviral role against HBV by inducing cccDNA decay, reducing HBV transcription, and downregulating the NTPC receptor[97].
Current CHB treatments fail to cure HBV and are often accompanied by serious side effects. The long-term use of HBV drugs may even lead to mutations in HBV polymerase and cause drug resistance[98,99]. Therefore, to overcome the limitations of clinical CHB therapy, researchers are developing new immune strategies to achieve sustained virologic remission (Table 1).
| Target | Drug name | Sponsor | Phase | Notes | Ref. |
| HBsAg inhibitor | REP-2139 | Replicor | II | Reduce the level of HBsAg | [101,102] |
| HBsAg inhibitor | REP-2165 | Replicor | II | Reduce the level of HBsAg (similar to REP-2139) | [102] |
| RIG and NOD agonist | SB9200 | Spring Bank | IIb/III | Prolonged IFN-α and IFN-β secretion; Reduce hepatitis virus antigen and DNA | [107] |
| TLR7 agonist | RO7020531 | Roche | Ⅰ | Activate HBV-specific CD8+ T and Tfh cells; Reduce the frequency of Tregs and MDSCs | [108] |
| TCR-T cells | HBsAg-TCR-T cells | Lion TCR Pte | Ⅰ | Safely and efficiently reduced HBsAg levels; Reduced level of HBV DNA and HBsAg | [115] |
| Therapeutic vaccine | TG1050 | Transgene | Ⅰ | Reduced level of HBV DNA and HBsAg; Long-lasting HBV-specific T cell responses | [125] |
| Therapeutic vaccine | HBsAg-HBIG (YIC) | National Vaccine and Serum Institute | III | Increase the level of IL-2; Long-lasting HBV-specific T-cell responses | [126] |
| Therapeutic vaccine | Nasvac | CIGB | III | Sustained control of HBV DNA; Clearance of HBeAg | [127] |
| Therapeutic vaccine | GS-4774 | Gilead | III | Strong immune stimulatory effect on T cells | [128] |
Sustained exposure to a high load of viral antigens leads to T cell exhaustion in patients with CHB. Serum HBsAg levels can be as high as 400 μg/mL in patients with CHB; thus, this phenomenon can play a key role in inhibiting HBV-specific immune responses. Neutralizing antibodies against HBsAg or preS1 eliminated HBV and restored HBV-specific immune responses to preventative HBV vaccines in HBV carrier mice. Gao et al[100] identified a novel monoclonal antibody (E6F6) that targets the HBsAg-aa119-125 peptide, which can mediate long-lasting HBsAg clearance and facilitates the HBV-specific T cell response via Fc-gamma receptor-mediated phagocytosis. Multiple release inhibitors and monoclonal antibodies against HBsAg have been tested in clinical trials, such as GC1102 (a recombinant human monoclonal anti-HBs antibody, Green Cross, phase II/III), EYP001 (farnesoid X receptor agonist, Enyo Pharma, phase II), REP-2139 (Replicor, phase II)[101,102], REP-2165 (Replicor, phase II)[102], and RG7834 (Roche, pre-clinical)[103].
Cytokines have been widely used as immunomodulatory agents to regulate immune responses during CHB treatment. For example, IL-12 administration alone can induce IFN-γ secretion and the recovery of exhausted CD8+ T cells in patients with CHB[71]. Moreover, employing IL-12 as an adjuvant combined with the recombinant HBV vaccine (rHBVvac) elicits systemic HBV-specific CD4+ and CD8+ T cell responses and restores HBsAg-specific humoral immunity, thereby overcoming immune tolerance in patients with CHB[104]. Recent findings have shown that co-administering GM-CSF with the rHBVvac induces the secretion of HBsAg-specific IFN-γ and CTL responses to clear HBV in vivo[105]. In addition, IL-2, IL-15, IL-21, and IL-33 also efficiently clear persistent HBV infection and produce a long-term immune response against HBV reinfection[106].
Currently, various TLR ligands are used as drugs for clinical CHB therapy, such as SB9200 (Spring Bank, phase IIb/III)[107], RG-7795 (Roche, phase II), RO7020531 (Roche, phase I)[108], GS-9620 (Gilead, phase II), GS-9688 (Gilead, phase II), RG-7854 (Roche, phase I), and JNJ-4964 (Janssen, preclinical). Data from previous studies have shown that GS9620 (a TLR7 agonist) can upregulate IFN-α production, restore the effector functions of CD8+ T cells and NK cells, and decrease HBsAg and HBeAg titers. Single-stranded RNA40 (a TLR8 agonist) can selectively induce IL-12, IL-18, and IFN-γ secretion by monocytes in patients with CHB, which is beneficial for HBV clearance[109]. SB9200, an agonist of RIG-I and nucleotide binding oligomerization domain containing 2, can stimulate prolonged IFN-α and IFN-β secretion and ISG activation and efficiently reduce hepatic woodchuck hepatitis virus antigen and DNA levels in infected woodchucks[107]. Interestingly, compared with entecavir administration, SB9200 pretreatment better reduces HBV virion production[110]. Additionally, we found that a small interfering RNA targeting HBx (3p-siHBx) induced RIG-I activation, which improved the immune microenvironment and triggered the activation of NK cells and CD8+ T cells in HBV-carrier mice[111].
Prolonged exposure to HBV leads to NK cell dysfunction and hyperexpression of immune checkpoint proteins in T cells. As an alternative approach, blocking the inhibitory receptor NKG2A increases the activity of human NK cells to promote HBsAg clearance[112]. In addition to direct anti-HBV effects, recent data have shown that NK cells in patients with CHB selectively inhibit HBV-specific T cell responses via an IL-10-dependent pathway[39]. Furthermore, NK cells in NA-treated CHB patients expressing high levels of death receptor ligands, such as TNF-related apoptosis inducing ligand (TRAIL) or NKG2D, can mediate the lysis of activated T cells, thereby contributing to the development of chronic HBV infection[46,49]. Depleting inhibitory NK cells and blocking the NKG2D and TRAIL pathways during NA treatment further induces significant improvements in terms of HBV-specific T cell functions to achieve an HBV cure[79]. Additionally, blocking inhibitory receptors, such as PD-1, 2B4, and Tim-3, can restore exhausted HBV-specific CD8+ T cells by promoting the recovery of cytotoxicity, cytokine production, and proliferation, offering an excellent opportunity to achieve sustained virologic control[113].
The adoptive transfer of autologous T cells, such as chimeric antigen receptor (CAR) T cells, is another promising immunotherapeutic option for HBV therapy. These CAR T cells can directly recognize HBV antigens on infected hepatocytes and HBV-related HCC cells independently of HLA, without any need for antigen processing and presentation[114]. Qasim et al[115] found that genetically modified T cell receptor (TCR) T cells safely and effectively targeted HBsAg and reduced HBsAg levels in a patient with HBV-related HCC who had undergone liver transplantation. As an alternative strategy, CAR T cells expressing a recombinant HBsAg-specific antibody together with CD28 and CD3 zeta could also recognize and eliminate HBsAg-positive hepatocytes in vitro. Moreover, HBsAg-CAR-CD8+ T cells localized to the liver and rapidly reduced HBV replication without significant liver damage after adoptive transfer in a transgenic mouse model of HBV[116]. Additionally, HBsAg-CAR T cells specifically decreased plasma HBsAg, HBV-DNA, and HBV core-positive hepatocytes in persistently HBV-infected chimeric mice with humanized livers after adoptive transfer of HBsAg-CAR-T cells[117], thereby providing a potential therapeutic approach for HBV.
However, specific challenges related to TCR or CAR T-cell therapy remain for HBV treatment, including the risk of developing severe liver damage and the suppressive effects of transferred TCR/CAR T cells due to the immune tolerance of the liver. To prevent severe side effects, such as liver damage and uncontrolled proliferation, Kah et al[118] developed a method for transient mRNA electroporation into engineered HBV-TCR T cells. In a separate study, PD-1 knockdown in HBV-TCR-T cells increased their effector functions and ability to kill tumor cells in the PD-L1hi liver microenvironment[119]. In addition, engineered CAR T cells to overexpress c-Jun, an AP-1 transcription factor, showed enhanced expansion and functional capacity with improved antitumor efficiency[120]. These findings have facilitated the development of TCR/CAR T cells for clinical CHB treatment.
Therapeutic vaccination presents an attractive strategy for HBV eradication. A novel hepatitis B therapeutic vaccine could overcome immune tolerance; effectively induce powerful CD4+ T cells, CD8+ T cells, and humoral immune responses; and ultimately achieve sustained control of CHB infection. Kosinska et al[121] proposed approaches for developing safe and effective therapeutic vaccines, including: Designing potent vaccine components or schemes to prime antigen-specific immune responses; combining checkpoint inhibitors with other strategies to restore exhausted T cells; and Reducing HBV-related antigen levels to prevent T cell attrition and exhaustion. Based on this guidance, we prepared HBsAg nanogels (Ng) with chitosan (CS) and poly γ-glutamic acid (γ-PGA). Interestingly, we found that single-dose HBsAg CS-γ-PGA Ng immunization, especially HBsAg Ng (+), enhanced DC maturation and induced HBV-specific cellular and humoral immunity, and promoted the generation of effector memory T cells for HBV clearance[122]. Recent data also showed that a ferritin NP-preS1 vaccine manifested an efficient antibody response, resulting in efficient viral clearance by delivering preS1 to SIGNR1+ DC[123]. Additionally, we demonstrated that employing poly I:C as an adjuvant combined with rHBVvac also efficiently and safely decreased HBV DNA, HBV RNA, and HBsAg in an HBV carrier mouse model. Importantly, we found that the therapeutic vaccine partially reversed immune tolerance and promoted HBV-specific CD8+ T cell terminal differentiation into KLRG1+ effector T cells, thereby playing a crucial role in HBV clearance[124].
Multiple therapeutic vaccines have been developed and administered for CHB treatment with different clinical outcomes. TG1050, a novel HBV-targeted immunotherapeutic vaccine based on a non-replicative adenovirus vector encoding multiple HBV antigens (S, core, and polymerase), effectively induced polyfunctional and long-lasting HBV-specific T cell responses and reduced HBV DNA and HBsAg levels in vivo. The results of a phase Ib trial showed that TG1050 induced the production of IFN-γ-producing HBV-specific T cells and safely achieved effective viral suppression[125]. Currently, TG1050 is being investigated in phase II clinical trials and may be a very promising therapeutic vaccine for HBV, especially in combination with TLR9 agonists. Additional therapeutic HBV vaccines under investigation in clinical trials worldwide including HBsAg-HBIG ("YIC", National Vaccine and Serum Institute, phase III)[126], Nasvac (CIGB, phase II/III)[127], GS-4774 (Gilead, phase II)[128], HepTcell (Altimmune, phase Ib), AIC 649 (AiCuris, phase I), INO-1800 (Inovio, phase I), HB-110 (Ichor, phase I), JNJ-64300535 (Janssen, phase I), TomegaVax HBV (TomegaVax, preclinical), and VR-CHB01 (Vical, preclinical).
PEG-IFN-α and NAs are the current major treatment strategies for CHB. Although these antiviral drugs can partly inhibit and eliminate HBV, viral breakthroughs may result from non-adherence due to limitations such as the high cost, the emergence of viral resistance, a long treatment cycle, and adverse side effects. Ideal anti-HBV strategies should meet the following criteria: the strong ability to inhibit virus replication with low drug resistance; stimulation of antiviral immune responses; Long-lasting effects without recurrence; and eventual removal of the virus. Therefore, the design of novel strategies to clear HBV and achieve long-lasting immune control remains a challenging task. Persistent CHB infection arises as a consequence of the complex interactions between HBV and the host innate and adaptive immune systems. Therefore, understanding the immunological mechanisms involved in CHB infection is important for designing potential therapeutic strategies for clinical CHB treatment. In this review, we detailed the immune biological characteristics and escape mechanisms of HBV and discussed novel immune-based therapies. Targeting a combination of viral and host factors provides the best possible chance for achieving a functional cure for CHB.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tai DI S-Editor: Vasudevan A L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Tang LSY, Covert E, Wilson E, Kottilil S. Chronic Hepatitis B Infection: A Review. JAMA. 2018;319:1802-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 487] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 2. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 2000] [Article Influence: 200.0] [Reference Citation Analysis (4)] |
| 3. | Tian Y, Kuo CF, Akbari O, Ou JH. Maternal-Derived Hepatitis B Virus e Antigen Alters Macrophage Function in Offspring to Drive Viral Persistence after Vertical Transmission. Immunity. 2016;44:1204-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 4. | Mani SKK, Andrisani O. Interferon signaling during Hepatitis B Virus (HBV) infection and HBV-associated hepatocellular carcinoma. Cytokine. 2019;124:154518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Bonjardim CA, Ferreira PC, Kroon EG. Interferons: signaling, antiviral and viral evasion. Immunol Lett. 2009;122:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | O'Brien TR, Kohaar I, Pfeiffer RM, Maeder D, Yeager M, Schadt EE, Prokunina-Olsson L. Risk alleles for chronic hepatitis B are associated with decreased mRNA expression of HLA-DPA1 and HLA-DPB1 in normal human liver. Genes Immun. 2011;12:428-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Janeway CA Jr. How the immune system works to protect the host from infection: a personal view. Proc Natl Acad Sci U S A. 2001;98:7461-7468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 208] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Riera Romo M, Pérez-Martínez D, Castillo Ferrer C. Innate immunity in vertebrates: an overview. Immunology. 2016;148:125-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 267] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 9. | Hopcraft SE, Damania B. Tumour viruses and innate immunity. Philos Trans R Soc Lond B Biol Sci. 2017;372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Yu S, Chen J, Wu M, Chen H, Kato N, Yuan Z. Hepatitis B virus polymerase inhibits RIG-I- and Toll-like receptor 3-mediated beta interferon induction in human hepatocytes through interference with interferon regulatory factor 3 activation and dampening of the interaction between TBK1/IKKepsilon and DDX3. J Gen Virol. 2010;91:2080-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (3)] |
| 11. | Hou ZH, Han QJ, Zhang C, Tian ZG, Zhang J. miR146a impairs the IFN-induced anti-HBV immune response by downregulating STAT1 in hepatocytes. Liver Int. 2014;34:58-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Jiang J, Tang H. Mechanism of inhibiting type I interferon induction by hepatitis B virus X protein. Protein Cell. 2010;1:1106-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (4)] |
| 13. | Cho IR, Oh M, Koh SS, Malilas W, Srisuttee R, Jhun BH, Pellegrini S, Fuchs SY, Chung YH. Hepatitis B virus X protein inhibits extracellular IFN-α-mediated signal transduction by downregulation of type I IFN receptor. Int J Mol Med. 2012;29:581-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Chen J, Xu W, Chen Y, Xie X, Zhang Y, Ma C, Yang Q, Han Y, Zhu C, Xiong Y, Wu K, Liu F, Liu Y, Wu J. Matrix Metalloproteinase 9 Facilitates Hepatitis B Virus Replication through Binding with Type I Interferon (IFN) Receptor 1 To Repress IFN/JAK/STAT Signaling. J Virol. 2017;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Lei Q, Li T, Kong L, Li L, Ding X, Wang X, Zhang X, Qin B. HBV-Pol is crucial for HBV-mediated inhibition of inflammasome activation and IL-1β production. Liver Int. 2019;39:2273-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Li Y, Xia Y, Han M, Chen G, Zhang D, Thasler WE, Protzer U, Ning Q. IFN-α-mediated Base Excision Repair Pathway Correlates with Antiviral Response Against Hepatitis B Virus Infection. Sci Rep. 2017;7:12715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Yuan Y, Yuan H, Yang G, Yun H, Zhao M, Liu Z, Zhao L, Geng Y, Liu L, Wang J, Zhang H, Wang Y, Zhang XD. IFN-α confers epigenetic regulation of HBV cccDNA minichromosome by modulating GCN5-mediated succinylation of histone H3K79 to clear HBV cccDNA. Clin Epigenetics. 2020;12:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Imam H, Kim GW, Mir SA, Khan M, Siddiqui A. Interferon-stimulated gene 20 (ISG20) selectively degrades N6-methyladenosine modified Hepatitis B Virus transcripts. PLoS Pathog. 2020;16:e1008338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 19. | Tan G, Yi Z, Song H, Xu F, Li F, Aliyari R, Zhang H, Du P, Ding Y, Niu J, Wang X, Su L, Qin FX, Cheng G. Type-I-IFN-Stimulated Gene TRIM5γ Inhibits HBV Replication by Promoting HBx Degradation. Cell Rep. 2019;29:3551-3563.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Wang YX, Niklasch M, Liu T, Wang Y, Shi B, Yuan W, Baumert TF, Yuan Z, Tong S, Nassal M, Wen YM. Interferon-inducible MX2 is a host restriction factor of hepatitis B virus replication. J Hepatol. 2020;72:865-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 21. | Xia Y, Cheng X, Blossey CK, Wisskirchen K, Esser K, Protzer U. Secreted Interferon-Inducible Factors Restrict Hepatitis B and C Virus Entry In Vitro. J Immunol Res. 2017;2017:4828936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Madera S, Rapp M, Firth MA, Beilke JN, Lanier LL, Sun JC. Type I IFN promotes NK cell expansion during viral infection by protecting NK cells against fratricide. J Exp Med. 2016;213:225-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 23. | Novak N, Koch S, Allam JP, Bieber T. Dendritic cells: bridging innate and adaptive immunity in atopic dermatitis. J Allergy Clin Immunol. 2010;125:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Yonejima A, Mizukoshi E, Tamai T, Nakagawa H, Kitahara M, Yamashita T, Arai K, Terashima T, Iida N, Fushimi K, Okada H, Sakai Y, Honda M, Kaneko S. Characteristics of Impaired Dendritic Cell Function in Patients With Hepatitis B Virus Infection. Hepatology. 2019;70:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Op den Brouw ML, Binda RS, van Roosmalen MH, Protzer U, Janssen HL, van der Molen RG, Woltman AM. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (2)] |
| 26. | Li M, Zhou ZH, Sun XH, Zhang X, Zhu XJ, Jin SG, Gao YT, Jiang Y, Gao YQ. Hepatitis B core antigen upregulates B7-H1 on dendritic cells by activating the AKT/ERK/P38 pathway: a possible mechanism of hepatitis B virus persistence. Lab Invest. 2016;96:1156-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Lan S, Wu L, Wang X, Wu J, Lin X, Wu W, Huang Z. Impact of HBeAg on the maturation and function of dendritic cells. Int J Infect Dis. 2016;46:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Buschow SI, Biesta PJ, Groothuismink ZMA, Erler NS, Vanwolleghem T, Ho E, Najera I, Ait-Goughoulte M, de Knegt RJ, Boonstra A, Woltman AM. TLR7 polymorphism, sex and chronic HBV infection influence plasmacytoid DC maturation by TLR7 ligands. Antiviral Res. 2018;157:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 29. | Ouaguia L, Leroy V, Dufeu-Duchesne T, Durantel D, Decaens T, Hubert M, Valladeau-Guilemond J, Bendriss-Vermare N, Chaperot L, Aspord C. Circulating and Hepatic BDCA1+, BDCA2+, and BDCA3+ Dendritic Cells Are Differentially Subverted in Patients With Chronic HBV Infection. Front Immunol. 2019;10:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Xu Y, Hu Y, Shi B, Zhang X, Wang J, Zhang Z, Shen F, Zhang Q, Sun S, Yuan Z. HBsAg inhibits TLR9-mediated activation and IFN-alpha production in plasmacytoid dendritic cells. Mol Immunol. 2009;46:2640-2646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 780] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 32. | Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 704] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 33. | Wu LL, Peng WH, Wu HL, Miaw SC, Yeh SH, Yang HC, Liao PH, Lin JS, Chen YR, Hong YT, Wang HY, Chen PJ, Chen DS. Lymphocyte Antigen 6 Complex, Locus C+ Monocytes and Kupffer Cells Orchestrate Liver Immune Responses Against Hepatitis B Virus in Mice. Hepatology. 2019;69:2364-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Li H, Zheng HW, Chen H, Xing ZZ, You H, Cong M, Jia JD. Hepatitis B virus particles preferably induce Kupffer cells to produce TGF-β1 over pro-inflammatory cytokines. Dig Liver Dis. 2012;44:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 35. | Boltjes A, van Montfoort N, Biesta PJ, Op den Brouw ML, Kwekkeboom J, van der Laan LJ, Janssen HL, Boonstra A, Woltman AM. Kupffer cells interact with hepatitis B surface antigen in vivo and in vitro, leading to proinflammatory cytokine production and natural killer cell function. J Infect Dis. 2015;211:1268-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 36. | Li M, Sun R, Xu L, Yin W, Chen Y, Zheng X, Lian Z, Wei H, Tian Z. Kupffer Cells Support Hepatitis B Virus-Mediated CD8+ T Cell Exhaustion via Hepatitis B Core Antigen-TLR2 Interactions in Mice. J Immunol. 2015;195:3100-3109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 37. | Yu X, Lan P, Hou X, Han Q, Lu N, Li T, Jiao C, Zhang J, Zhang C, Tian Z. HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1β production via suppressing the NF-κB pathway and ROS production. J Hepatol. 2017;66:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 262] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 38. | Wang S, Chen Z, Hu C, Qian F, Cheng Y, Wu M, Shi B, Chen J, Hu Y, Yuan Z. Hepatitis B virus surface antigen selectively inhibits TLR2 ligand-induced IL-12 production in monocytes/macrophages by interfering with JNK activation. J Immunol. 2013;190:5142-5151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (2)] |
| 39. | Li H, Zhai N, Wang Z, Song H, Yang Y, Cui A, Li T, Wang G, Niu J, Crispe IN, Su L, Tu Z. Regulatory NK cells mediated between immunosuppressive monocytes and dysfunctional T cells in chronic HBV infection. Gut. 2018;67:2035-2044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 40. | Zang M, Li Y, He H, Ding H, Chen K, Du J, Chen T, Wu Z, Liu H, Wang D, Cai J, Qu C. IL-23 production of liver inflammatory macrophages to damaged hepatocytes promotes hepatocellular carcinoma development after chronic hepatitis B virus infection. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3759-3770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Dai K, Huang L, Sun X, Yang L, Gong Z. Hepatic CD206-positive macrophages express amphiregulin to promote the immunosuppressive activity of regulatory T cells in HBV infection. J Leukoc Biol. 2015;98:1071-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Agraz-Cibrian JM, Segura-Ortega JE, Delgado-Rizo V, Fafutis-Morris M. Alterations in neutrophil extracellular traps is associated with the degree of decompensation of liver cirrhosis. J Infect Dev Ctries. 2016;10:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Hu S, Liu X, Gao Y, Zhou R, Wei M, Dong J, Yan H, Zhao Y. Hepatitis B Virus Inhibits Neutrophil Extracellular Trap Release by Modulating Reactive Oxygen Species Production and Autophagy. J Immunol. 2019;202:805-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Stelma F, Willemse SB, Erken R, de Niet A, Sinnige MJ, van Dort K, Zaaijer HL, van Leeuwen EMM, Kootstra NA, Reesink HW. Dynamics of the Immune Response in Acute Hepatitis B Infection. Open Forum Infect Dis. 2017;4:ofx231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Yu WH, Cosgrove C, Berger CT, Cheney PC, Krykbaeva M, Kim AY, Lewis-Ximenez L, Lauer GM, Alter G. ADCC-Mediated CD56DIM NK Cell Responses Are Associated with Early HBsAg Clearance in Acute HBV Infection. Pathog Immun. 2018;3:2-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Ghosh S, Nandi M, Pal S, Mukhopadhyay D, Chakraborty BC, Khatun M, Bhowmick D, Mondal RK, Das S, Das K, Ghosh R, Banerjee S, Santra A, Chatterjee M, Chowdhury A, Datta S. Natural killer cells contribute to hepatic injury and help in viral persistence during progression of hepatitis B e-antigen-negative chronic hepatitis B virus infection. Clin Microbiol Infect. 2016;22:733.e9-733.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Fisicaro P, Rossi M, Vecchi A, Acerbi G, Barili V, Laccabue D, Montali I, Zecca A, Penna A, Missale G, Ferrari C, Boni C. The Good and the Bad of Natural Killer Cells in Virus Control: Perspective for Anti-HBV Therapy. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 48. | Han W, Ni Q, Liu K, Yao Y, Zhao D, Liu X, Chen Y. Decreased CD122 on CD56dim NK associated with its impairment in asymptomatic chronic HBV carriers with high levels of HBV DNA, HBsAg and HBeAg. Life Sci. 2018;195:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Yang Y, Han Q, Zhang C, Xiao M, Zhang J. Hepatitis B virus antigens impair NK cell function. Int Immunopharmacol. 2016;38:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Zheng B, Yang Y, Han Q, Yin C, Pan Z, Zhang J. STAT3 directly regulates NKp46 transcription in NK cells of HBeAg-negative CHB patients. J Leukoc Biol. 2019;106:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Yang Y, Han Q, Hou Z, Zhang C, Tian Z, Zhang J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol Immunol. 2017;14:465-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 52. | Li JF, Dai XP, Zhang W, Sun SH, Zeng Y, Zhao GY, Kou ZH, Guo Y, Yu H, Du LY, Jiang SB, Zhou YS. Upregulation of microRNA-146a by hepatitis B virus X protein contributes to hepatitis development by downregulating complement factor H. mBio. 2015;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 53. | Xu D, Han Q, Hou Z, Zhang C, Zhang J. miR-146a negatively regulates NK cell functions via STAT1 signaling. Cell Mol Immunol. 2017;14:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 54. | You CR, Lee SW, Jang JW, Yoon SK. Update on hepatitis B virus infection. World J Gastroenterol. 2014;20:13293-13305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (2)] |
| 55. | Wang X, Dong Q, Li Q, Li Y, Zhao D, Sun J, Fu J, Meng F, Lin H, Luan J, Liu B, Wang M, Wang FS, He F, Tang L. Dysregulated Response of Follicular Helper T Cells to Hepatitis B Surface Antigen Promotes HBV Persistence in Mice and Associates With Outcomes of Patients. Gastroenterology. 2018;154:2222-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 56. | Terrault NA, Levy MT, Cheung KW, Jourdain G. Viral hepatitis and pregnancy. Nat Rev Gastroenterol Hepatol. 2021;18:117-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 57. | Matei HV, Vica ML, Siserman CV. Association between HLA class II alleles and hepatitis B virus infection in Transylvania, Romania. Immunol Invest. 2018;47:735-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Zhang Z, Wang C, Liu Z, Zou G, Li J, Lu M. Host Genetic Determinants of Hepatitis B Virus Infection. Front Genet. 2019;10:696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 59. | Kamatani Y, Wattanapokayakit S, Ochi H, Kawaguchi T, Takahashi A, Hosono N, Kubo M, Tsunoda T, Kamatani N, Kumada H, Puseenam A, Sura T, Daigo Y, Chayama K, Chantratita W, Nakamura Y, Matsuda K. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet. 2009;41:591-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 430] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 60. | Li Y, Si L, Zhai Y, Hu Y, Hu Z, Bei JX, Xie B, Ren Q, Cao P, Yang F, Song Q, Bao Z, Zhang H, Han Y, Wang Z, Chen X, Xia X, Yan H, Wang R, Zhang Y, Gao C, Meng J, Tu X, Liang X, Cui Y, Liu Y, Wu X, Li Z, Wang H, Hu B, He M, Gao Z, Xu X, Ji H, Yu C, Sun Y, Xing B, Yang X, Tan A, Wu C, Jia W, Li S, Zeng YX, Shen H, He F, Mo Z, Zhou G. Genome-wide association study identifies 8p21.3 associated with persistent hepatitis B virus infection among Chinese. Nat Commun. 2016;7:11664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Huang YH, Liao SF, Khor SS, Lin YJ, Chen HY, Chang YH, Huang YH, Lu SN, Lee HW, Ko WY, Huang C, Liu PC, Chen YJ, Wu PF, Chu HW, Wu PE, Tokunaga K, Shen CY, Lee MH. Large-scale genome-wide association study identifies HLA class II variants associated with chronic HBV infection: a study from Taiwan Biobank. Aliment Pharmacol Ther. 2020;52:682-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Choga WT, Anderson M, Zumbika E, Phinius BB, Mbangiwa T, Bhebhe LN, Baruti K, Kimathi PO, Seatla KK, Musonda RM, Bell TG, Moyo S, Blackard JT, Gaseitsiwe S. In Silico Prediction of Human Leukocytes Antigen (HLA) Class II Binding Hepatitis B Virus (HBV) Peptides in Botswana. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Murata Y, Kawashima K, Sheikh K, Tanaka Y, Isogawa M. Intrahepatic Cross-Presentation and Hepatocellular Antigen Presentation Play Distinct Roles in the Induction of Hepatitis B Virus-Specific CD8+ T Cell Responses. J Virol. 2018;92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Li TY, Yang Y, Zhou G, Tu ZK. Immune suppression in chronic hepatitis B infection associated liver disease: A review. World J Gastroenterol. 2019;25:3527-3537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 65. | Lopes AR, Kellam P, Das A, Dunn C, Kwan A, Turner J, Peppa D, Gilson RJ, Gehring A, Bertoletti A, Maini MK. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J Clin Invest. 2008;118:1835-1845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 66. | Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215-4225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 738] [Article Influence: 41.0] [Reference Citation Analysis (1)] |
| 67. | Kurktschiev PD, Raziorrouh B, Schraut W, Backmund M, Wächtler M, Wendtner CM, Bengsch B, Thimme R, Denk G, Zachoval R, Dick A, Spannagl M, Haas J, Diepolder HM, Jung MC, Gruener NH. Dysfunctional CD8+ T cells in hepatitis B and C are characterized by a lack of antigen-specific T-bet induction. J Exp Med. 2014;211:2047-2059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 68. | Heim K, Neumann-Haefelin C, Thimme R, Hofmann M. Heterogeneity of HBV-Specific CD8+ T-Cell Failure: Implications for Immunotherapy. Front Immunol. 2019;10:2240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 69. | Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 284] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 70. | Schuch A, Salimi Alizei E, Heim K, Wieland D, Kiraithe MM, Kemming J, Llewellyn-Lacey S, Sogukpinar Ö, Ni Y, Urban S, Zimmermann P, Nassal M, Emmerich F, Price DA, Bengsch B, Luxenburger H, Neumann-Haefelin C, Hofmann M, Thimme R. Phenotypic and functional differences of HBV core-specific versus HBV polymerase-specific CD8+ T cells in chronically HBV-infected patients with low viral load. Gut. 2019;68:905-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 71. | Schurich A, Pallett LJ, Lubowiecki M, Singh HD, Gill US, Kennedy PT, Nastouli E, Tanwar S, Rosenberg W, Maini MK. The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLoS Pathog. 2013;9:e1003208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 72. | Man K, Gabriel SS, Liao Y, Gloury R, Preston S, Henstridge DC, Pellegrini M, Zehn D, Berberich-Siebelt F, Febbraio MA, Shi W, Kallies A. Transcription Factor IRF4 Promotes CD8+ T Cell Exhaustion and Limits the Development of Memory-like T Cells during Chronic Infection. Immunity. 2017;47:1129-1141.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 350] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 73. | Fisicaro P, Barili V, Montanini B, Acerbi G, Ferracin M, Guerrieri F, Salerno D, Boni C, Massari M, Cavallo MC, Grossi G, Giuberti T, Lampertico P, Missale G, Levrero M, Ottonello S, Ferrari C. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat Med. 2017;23:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 273] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 74. | Schurich A, Pallett LJ, Jajbhay D, Wijngaarden J, Otano I, Gill US, Hansi N, Kennedy PT, Nastouli E, Gilson R, Frezza C, Henson SM, Maini MK. Distinct Metabolic Requirements of Exhausted and Functional Virus-Specific CD8 T Cells in the Same Host. Cell Rep. 2016;16:1243-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 75. | Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 723] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 76. | Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 481] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 77. | Li M, Sun XH, Zhu XJ, Jin SG, Zeng ZJ, Zhou ZH, Yu Z, Gao YQ. HBcAg induces PD-1 upregulation on CD4+T cells through activation of JNK, ERK and PI3K/AKT pathways in chronic hepatitis-B-infected patients. Lab Invest. 2012;92:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Wang H, Wu D, Wang X, Chen G, Zhang Y, Yan W, Luo X, Han M, Ning Q. Hepatitis B virus surface protein-induced hPIAS1 transcription requires TAL1, E47, MYOG, NFI, and MAPK signal pathways. Biol Chem. 2016;397:1173-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Boni C, Lampertico P, Talamona L, Giuberti T, Invernizzi F, Barili V, Fisicaro P, Rossi M, Cavallo MC, Vecchi A, Pedrazzi G, Alfieri A, Colombo M, Missale G, Ferrari C. Natural killer cell phenotype modulation and natural killer/T-cell interplay in nucleos(t)ide analogue-treated hepatitis e antigen-negative patients with chronic hepatitis B. Hepatology. 2015;62:1697-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 80. | Zimmerli SC, Harari A, Cellerai C, Vallelian F, Bart PA, Pantaleo G. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc Natl Acad Sci U S A. 2005;102:7239-7244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 81. | Kondo Y, Shimosegawa T. Significant roles of regulatory T cells and myeloid derived suppressor cells in hepatitis B virus persistent infection and hepatitis B virus-related HCCs. Int J Mol Sci. 2015;16:3307-3322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 82. | Qi H. T follicular helper cells in space-time. Nat Rev Immunol. 2016;16:612-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 216] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 83. | Ryg-Cornejo V, Ioannidis LJ, Ly A, Chiu CY, Tellier J, Hill DL, Preston SP, Pellegrini M, Yu D, Nutt SL, Kallies A, Hansen DS. Severe Malaria Infections Impair Germinal Center Responses by Inhibiting T Follicular Helper Cell Differentiation. Cell Rep. 2016;14:68-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 84. | Vyas AK, Sharma BC, Sarin SK, Trehanpati N. Immune correlates of hepatitis B surface antigen spontaneous seroconversion in hepatitis B e antigen negative chronic hepatitis B patients. Liver Int. 2018;38:38-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Zhang L, Li H, Ren H, Hu P. Circulating PD-1hiCXCR5+CD4+ T cells are associated with a decrease in hepatitis B surface antigen levels in patients with chronic hepatitis B who are receiving peginterferon-α therapy. Mol Immunol. 2018;103:270-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Wang R, Xie R, Song Z. Circulating regulatory Tfh cells are enriched in patients with chronic hepatitis B infection and induce the differentiation of regulatory B cells. Exp Cell Res. 2018;365:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Corti D, Benigni F, Shouval D. Viral envelope-specific antibodies in chronic hepatitis B virus infection. Curr Opin Virol. 2018;30:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Ma Z, Zhang E, Gao S, Xiong Y, Lu M. Toward a Functional Cure for Hepatitis B: The Rationale and Challenges for Therapeutic Targeting of the B Cell Immune Response. Front Immunol. 2019;10:2308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 89. | Liu Y, Cheng LS, Wu SD, Wang SQ, Li L, She WM, Li J, Wang JY, Jiang W. IL-10-producing regulatory B-cells suppressed effector T-cells but enhanced regulatory T-cells in chronic HBV infection. Clin Sci (Lond). 2016;130:907-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 90. | Tian C, Chen Y, Liu Y, Wang S, Li Y, Wang G, Xia J, Zhao XA, Huang R, Lu S, Wu C. Use of ELISpot assay to study HBs-specific B cell responses in vaccinated and HBV infected humans. Emerg Microbes Infect. 2018;7:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 91. | Loggi E, Gamal N, Bihl F, Bernardi M, Andreone P. Adaptive response in hepatitis B virus infection. J Viral Hepat. 2014;21:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Burton AR, Pallett LJ, McCoy LE, Suveizdyte K, Amin OE, Swadling L, Alberts E, Davidson BR, Kennedy PT, Gill US, Mauri C, Blair PA, Pelletier N, Maini MK. Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J Clin Invest. 2018;128:4588-4603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 232] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 93. | Poonia B, Ayithan N, Nandi M, Masur H, Kottilil S. HBV induces inhibitory FcRL receptor on B cells and dysregulates B cell-T follicular helper cell axis. Sci Rep. 2018;8:15296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 94. | Xing T, Xu H, Yu W. Role of T follicular helper cells and their associated molecules in the pathogenesis of chronic hepatitis B virus infection. Exp Ther Med. 2013;5:885-889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 95. | Lu B, Zhang B, Wang L, Ma C, Liu X, Zhao Y, Jiao Y. Hepatitis B Virus e Antigen Regulates Monocyte Function and Promotes B Lymphocyte Activation. Viral Immunol. 2017;30:35-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 372] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 97. | Bouezzedine F, Fardel O, Gripon P. Interleukin 6 inhibits HBV entry through NTCP down regulation. Virology. 2015;481:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 98. | Boni C, Barili V, Acerbi G, Rossi M, Vecchi A, Laccabue D, Penna A, Missale G, Ferrari C, Fisicaro P. HBV Immune-Therapy: From Molecular Mechanisms to Clinical Applications. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 99. | Devi U, Locarnini S. Hepatitis B antivirals and resistance. Curr Opin Virol. 2013;3:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Gao Y, Zhang TY, Yuan Q, Xia NS. Antibody-mediated immunotherapy against chronic hepatitis B virus infection. Hum Vaccin Immunother. 2017;13:1768-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 101. | Bazinet M, Pântea V, Cebotarescu V, Cojuhari L, Jimbei P, Albrecht J, Schmid P, Le Gal F, Gordien E, Krawczyk A, Mijočević H, Karimzadeh H, Roggendorf M, Vaillant A. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2017;2:877-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 194] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 102. | Roehl I, Seiffert S, Brikh C, Quinet J, Jamard C, Dorfler N, Lockridge JA, Cova L, Vaillant A. Nucleic Acid Polymers with Accelerated Plasma and Tissue Clearance for Chronic Hepatitis B Therapy. Mol Ther Nucleic Acids. 2017;8:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 103. | Han X, Zhou C, Jiang M, Wang Y, Wang J, Cheng Z, Wang M, Liu Y, Liang C, Wang Z, Weikert R, Lv W, Xie J, Yu X, Zhou X, Luangsay S, Shen HC, Mayweg AV, Javanbakht H, Yang S. Discovery of RG7834: The First-in-Class Selective and Orally Available Small Molecule Hepatitis B Virus Expression Inhibitor with Novel Mechanism of Action. J Med Chem. 2018;61:10619-10634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 104. | Zhao H, Wang H, Hu Y, Xu D, Yin C, Han Q, Zhang J. Chitosan Nanovaccines as Efficient Carrier Adjuvant System for IL-12 with Enhanced Protection Against HBV. Int J Nanomedicine. 2021;16:4913-4928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 105. | Wang X, Dong A, Xiao J, Zhou X, Mi H, Xu H, Zhang J, Wang B. Overcoming HBV immune tolerance to eliminate HBsAg-positive hepatocytes via pre-administration of GM-CSF as a novel adjuvant for a hepatitis B vaccine in HBV transgenic mice. Cell Mol Immunol. 2016;13:850-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 106. | Shen Z, Yang H, Yang S, Wang W, Cui X, Zhou X, Liu W, Pan S, Liu Y, Zhang J, Xie Y, Liu J. Hepatitis B virus persistence in mice reveals IL-21 and IL-33 as regulators of viral clearance. Nat Commun. 2017;8:2119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 107. | Korolowicz KE, Iyer RP, Czerwinski S, Suresh M, Yang J, Padmanabhan S, Sheri A, Pandey RK, Skell J, Marquis JK, Kallakury BV, Tucker RD, Menne S. Antiviral Efficacy and Host Innate Immunity Associated with SB 9200 Treatment in the Woodchuck Model of Chronic Hepatitis B. PLoS One. 2016;11:e0161313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 108. | Luk A, Jiang Q, Glavini K, Triyatni M, Zhao N, Racek T, Zhu Y, Grippo JF. A Single and Multiple Ascending Dose Study of Toll-Like Receptor 7 Agonist (RO7020531) in Chinese Healthy Volunteers. Clin Transl Sci. 2020;13:985-993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 109. | Jo J, Tan AT, Ussher JE, Sandalova E, Tang XZ, Tan-Garcia A, To N, Hong M, Chia A, Gill US, Kennedy PT, Tan KC, Lee KH, De Libero G, Gehring AJ, Willberg CB, Klenerman P, Bertoletti A. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog. 2014;10:e1004210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 197] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 110. | Suresh M, Korolowicz KE, Balarezo M, Iyer RP, Padmanabhan S, Cleary D, Gimi R, Sheri A, Yon C, Kallakury BV, Tucker RD, Afdhal N, Menne S. Antiviral Efficacy and Host Immune Response Induction during Sequential Treatment with SB 9200 Followed by Entecavir in Woodchucks. PLoS One. 2017;12:e0169631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 111. | Han Q, Hou Z, Yin C, Zhang C, Zhang J. 5'-triphosphate siRNA targeting HBx elicits a potent anti-HBV immune response in pAAV-HBV transfected mice. Antiviral Res. 2019;161:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 112. | Li F, Wei H, Gao Y, Xu L, Yin W, Sun R, Tian Z. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology. 2013;144:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 113. | Sun C, Lan P, Han Q, Huang M, Zhang Z, Xu G, Song J, Wang J, Wei H, Zhang J, Sun R, Zhang C, Tian Z. Oncofetal gene SALL4 reactivation by hepatitis B virus counteracts miR-200c in PD-L1-induced T cell exhaustion. Nat Commun. 2018;9:1241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 114. | Festag MM, Festag J, Fräßle SP, Asen T, Sacherl J, Schreiber S, Mück-Häusl MA, Busch DH, Wisskirchen K, Protzer U. Evaluation of a Fully Human, Hepatitis B Virus-Specific Chimeric Antigen Receptor in an Immunocompetent Mouse Model. Mol Ther. 2019;27:947-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 115. | Qasim W, Brunetto M, Gehring AJ, Xue SA, Schurich A, Khakpoor A, Zhan H, Ciccorossi P, Gilmour K, Cavallone D, Moriconi F, Farzhenah F, Mazzoni A, Chan L, Morris E, Thrasher A, Maini MK, Bonino F, Stauss H, Bertoletti A. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol. 2015;62:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 116. | Krebs K, Böttinger N, Huang LR, Chmielewski M, Arzberger S, Gasteiger G, Jäger C, Schmitt E, Bohne F, Aichler M, Uckert W, Abken H, Heikenwalder M, Knolle P, Protzer U. T cells expressing a chimeric antigen receptor that binds hepatitis B virus envelope proteins control virus replication in mice. Gastroenterology. 2013;145:456-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 117. | Kruse RL, Shum T, Tashiro H, Barzi M, Yi Z, Whitten-Bauer C, Legras X, Bissig-Choisat B, Garaigorta U, Gottschalk S, Bissig KD. HBsAg-redirected T cells exhibit antiviral activity in HBV-infected human liver chimeric mice. Cytotherapy. 2018;20:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 118. | Kah J, Koh S, Volz T, Ceccarello E, Allweiss L, Lütgehetmann M, Bertoletti A, Dandri M. Lymphocytes transiently expressing virus-specific T cell receptors reduce hepatitis B virus infection. J Clin Invest. 2017;127:3177-3188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 119. | Otano I, Escors D, Schurich A, Singh H, Robertson F, Davidson BR, Fusai G, Vargas FA, Tan ZMD, Aw JYJ, Hansi N, Kennedy PTF, Xue SA, Stauss HJ, Bertoletti A, Pavesi A, Maini MK. Molecular Recalibration of PD-1+ Antigen-Specific T Cells from Blood and Liver. Mol Ther. 2018;26:2553-2566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 120. | Lynn RC, Weber EW, Sotillo E, Gennert D, Xu P, Good Z, Anbunathan H, Lattin J, Jones R, Tieu V, Nagaraja S, Granja J, de Bourcy CFA, Majzner R, Satpathy AT, Quake SR, Monje M, Chang HY, Mackall CL. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature. 2019;576:293-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 588] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 121. | Kosinska AD, Bauer T, Protzer U. Therapeutic vaccination for chronic hepatitis B. Curr Opin Virol. 2017;23:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 122. | Wang H, Han Q, Zhao H, Xu D, Zhang J. Single dose HBsAg CS-γ-PGA nanogels induce potent protective immune responses against HBV infection. Eur J Pharm Biopharm. 2018;124:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 123. | Wang W, Zhou X, Bian Y, Wang S, Chai Q, Guo Z, Wang Z, Zhu P, Peng H, Yan X, Li W, Fu YX, Zhu M. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat Nanotechnol. 2020;15:406-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 124. | Zhao HJ, Han QJ, Wang G, Lin A, Xu DQ, Wang YQ, Zhao LH, Tian ZG, Zhang J. Poly I:C-based rHBVvac therapeutic vaccine eliminates HBV via generation of HBV-specific CD8+ effector memory T cells. Gut. 2019;68:2032-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 125. | Zoulim F, Fournier C, Habersetzer F, Sprinzl M, Pol S, Coffin CS, Leroy V, Ma M, Wedemeyer H, Lohse AW, Thimme R, Lugardon K, Martin P, Bastien B, Sansas B, Adda N, Halluard C, Bendjama K, Brandely M, Inchauspé G. Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: a phase 1b placebo-controlled trial. Hum Vaccin Immunother. 2020;16:388-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 126. | Zhou C, Li C, Gong GZ, Wang S, Zhang JM, Xu DZ, Guo LM, Ren H, Xu M, Xie Q, Pan C, Xu J, Hu Z, Geng S, Zhou X, Wang X, Mi H, Zhao G, Yu W, Wen YM, Huang L, Wang XY, Wang B. Analysis of immunological mechanisms exerted by HBsAg-HBIG therapeutic vaccine combined with Adefovir in chronic hepatitis B patients. Hum Vaccin Immunother. 2017;13:1989-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 127. | Al Mahtab M, Akbar SMF, Aguilar JC, Guillen G, Penton E, Tuero A, Yoshida O, Hiasa Y, Onji M. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment controlled phase III clinical trial). PLoS One. 2018;13:e0201236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 128. | Gaggar A, Coeshott C, Apelian D, Rodell T, Armstrong BR, Shen G, Subramanian GM, McHutchison JG. Safety, tolerability and immunogenicity of GS-4774, a hepatitis B virus-specific therapeutic vaccine, in healthy subjects: a randomized study. Vaccine. 2014;32:4925-4931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |