INTRODUCTION
Frailty is one of the most complicated clinical syndromes and is defined as a decrease in the reserve and restoring capacity of the body[1]. For frail people, a slight irritant can result in strong responses, which require a longer period to recover. Thus, frailty can also be regarded as a decline in the ability to maintain homeostasis. Multiple organs and systems, such as the skeletal muscle, immune, endocrine, hematopoiesis, and cardiovascular systems, are involved in the process of frailty[2]. Patients with frailty have a high risk of developing age-related diseases, including neurodegenerative diseases (such as dementia), type II diabetes, atherosclerosis, and chronic heart failure[3]. Frailty places a heavy burden on modern society, which has an extensive elderly population.
Although there are a few hypotheses at present, the mechanisms involved in frailty remain unknown. Researchers have different opinions about the origin of frailty. It is generally accepted that frailty is related to aging. According to Jayanama et al’s survey[4], among 9030 volunteers over the age of 20, the average age with a frailty index score of less than 0.1 was 39.7 years old. Moreover, those who had an average age of 65.3 years had a frailty index score of more than 0.3. Miller et al[5] reported that 75.4% of 18- to 64-year-old people have an simplified, 5-item frailty index (sFI) = 0, which means they are considered nonfrail. In contrast, 20.1% of the 80 to 89-year-old age group had an sFI ≥ 3, and thus, this age group can be referred to as a frail population. There were 9252 patients included in this analysis.
The incidence of frailty increases with age. However, frailty is not necessarily related to aging. According to an investigation by Xing and Guo[6], 43.2% of 683 older people (≥ 60 years old) from Beijing did not have frailty, while the incidence of prefrailty was 45.7%, and 11.1% of the cohort was frail. These data suggest that healthy aging could be achieved by preventing and improving frailty status.
With the increasing focus on frailty, emerging evidence has increased our understanding of this syndrome. Findings from centenarians suggest that specific gut microbiota (GM) constituents may contribute to healthy aging. For example, Escherichia, Ruminococcus[7], and Clostridioides leptum are increased, whereas Faecalibacterium prausnitzii and other species are decreased in centenarians[8]. Biagi et al[8] also showed that the proportion of Clostridioides cluster XIVa was significantly lower in centenarians than in elderly and younger adults. Furthermore, the diversity and abundance of the GM vary between elderly adults and centenarians. However, the bridge between the GM and the occurrence of frailty remains unclear. In this review, we proposed the possible mechanisms involved in frailty from the perspective of the GM and oxidative stress (OS). The correlations and potential causality among these factors are discussed. The idea of using GM biomarkers to predict frailty is then proposed prospectively. Notably, frailty is not an irreversible status[9]. Timely interventions have the potential to revert the prefrailty or frailty state to a nonfrailty state. According to existing research, dietary interventions are the most commonly used treatment for frailty[10]. Moreover, traditional Chinese medicine (TCM) is a unique application for the treatment of frailty (Figure 1). Achieving healthy aging is becoming a core goal of future research.
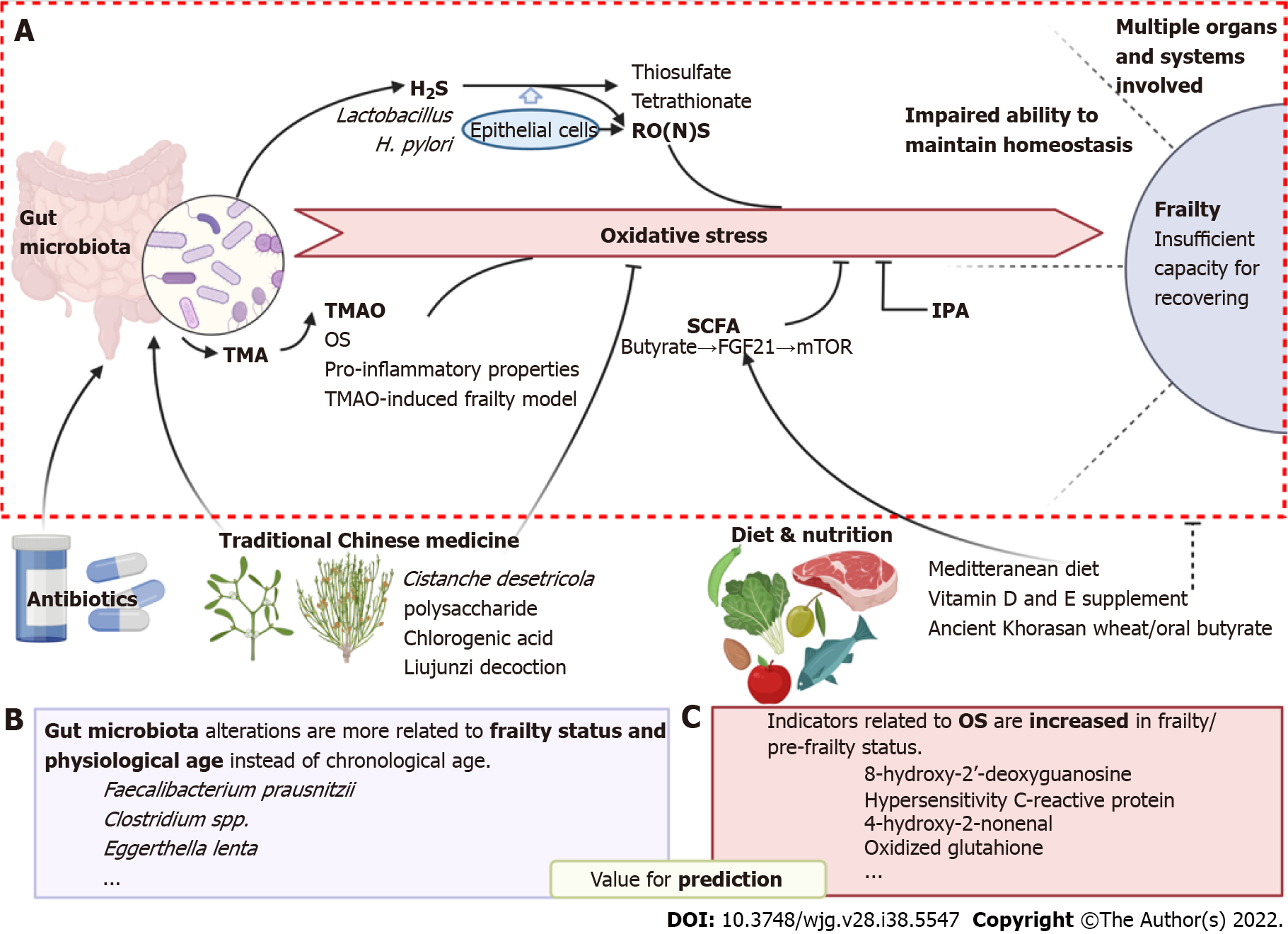
Figure 1 Oxidative stress is involved in the association between the gut microbiota and frailty syndrome.
A: Involving molecular and pathways of how oxidative stress bridges gut microbiota and frailty; B and C: Potential bacterial species and oxidative markers which are valuable for prediction of frailty are listed in B and C, respectively. In addition, possible intervene strategies including traditional Chinese medicine and diet & nutrition are shown at the bottom of the figure. Created with BioRender.com. OS: Oxidative stress; TMA: Trimethylamine; SCFA: Short-chain fatty acid; FGF21: Fibroblast growth factor 21; IPA: Indole-3-propionic acid; RONS: Reactive oxygen and nitrogen species; 8-OdG: 8-hydroxy-2’-deoxyguanosine; 4-HNE: 4-hydroxy-2-nonenal; GSSG: Glutathione.
THE GM AND FRAILTY
The abundance and diversity of the GM changes with aging. Alterations in the GM partially lead to individual differences in the health status of elderly people by interacting with the host immune system[11]. Evidence has shown that the elderly population has higher abundances of Clostridioides cluster XIVa, Faecalibacterium prauanitzii, Actinomycetes (mainly Bifidobacterium) and Proteobacteria than those of young adults[12]. Biagi et al[8] showed that the abundance of Clostridioides cluster XIVa was significantly higher in the elderly population (49%) than in the centenarians (34%) and the young population (44%). There was no significant difference in the abundance of Clostridioides cluster IV among these three age groups, but the subgroups had a different tendency to change. The abundance of Faecalibacterium prausnitzii et rel. decreased in centenarians, and the abundance of Clostridioides leptum et rel. was increased. Centenarians had the highest abundance of Bacilli (12%) and Proteobacteria (2.6%), and the young population and the old population had similar proportions of Bacilli (5%) and Proteobacteria (1.2%). Jackson et al[13] studied the relationship between frailty and the composition of the GM. They found that frailty was negatively correlated with the abundance of Clostridioides (in which it accounted for the largest proportion of the GM). Moreover, frailty was positively correlated with the abundance of Eggerthella lenta. Centenarians, who are regarded as the “healthy aging” population, have a different GM composition than the aged population. This finding indicates that the GM is not entirely chronological age-correlated but that it is more closely related to the frailty status of the body.
In addition to chronological age, physiological age may better reflect the correlation between frailty and the GM (Figure 1B). In the study by Maffei et al[14], 85 volunteers aged 43-79 years were divided into low, medium and upper groups according to either physiological age or chronological age. The chronological age is the number of years from birth to the present. Physiological age was assessed by 34 items of the frailty index (frailty index 34, FI34). According to the FI34 value, there were significant differences in the abundance and diversity of the GM among the three age groups. Excluding the effect of sex, body mass index, antibiotic usage, and other confounders, Eggerthella, Rumen coccus and Coprobacillus were the three main species with the greatest influence. According to the Rockwood FI value, Faecalibacterium prausnitzii was negatively correlated with age, while Eubacterium dolichum and Eggerthella lenta were positively correlated with age[15]. However, there was no significant difference in the GM among the three groups according to chronological age. Although there are contradictory changes in certain strains in the gut, it can be concluded that the change in Clostridioides abundance with age is the most remarkable. All the experiments mentioned above suggest that the GM changes with frailty status and that there is a potential link between the GM and frailty syndrome.
In frailty, the most significant change in the GM is a decrease in diversity. In the frail aged population, the microbiota shifts toward a predomination by Bacteroidetes, especially in the genus Prevotella and its subspecies. Changes in microbiota diversity affect the influence of the metabolites and pathways related to the microbiota. For instance, the GM regulates estrogen levels by secreting β-glucuronidase. β-glucuronidase is an enzyme that can deconjugate estrogen into the active form. Low levels of estrogen downregulate physiological functions that affect neural development, cardiovascular health, bone density and neoplastic diseases[16]. Scientific evidence suggests that estrogen supplementation could improve physiological functions that prevent or reverse frailty[17]. Said et al[18] found that diarylpropionitrile, an estrogen receptor β agonist, had longevity-promoting properties and could reverse frailty.
THE ROLE OF OS IN FRAILTY
OS was regarded as one of the main causes of frailty as early as the 1950s. Harman[19] first proposed the free radical theory of aging; according to this theory, oxygen free radicals damage cell components and intercellular substances, causing the deterioration of body functions and age-related diseases. In the 1980s, OS was discovered. The advent of OS indicates that the balance between oxidation and antioxidation is inclined toward oxidation. This leads to neutrophil inflammatory infiltration, an increase in protease secretion, and the generation of a large number of oxidative intermediates. The study by Viña et al[20] suggested that OS was an important negative factor that led to aging and diseases, such as cardiovascular disease, neurodegenerative disease, chronic obstructive pulmonary disease or cancer and other illnesses in aged individuals. Some researchers have thought that oxidative damage may be due to the imbalance between antioxidation defenses and reactive oxygen and nitrogen species (RONS), thus inducing cell apoptosis. This imbalance would lead to alterations in the expression of a number of transcription factors and then cause chemical modifications in macromolecular substances, such as lipids, proteins and DNA[21,22].
Since the free radical theory of aging was proposed, increasing concern has been given to the influence of OS on frailty (Figure 1C). Wu et al[23] screened 90 volunteers who were older than 65 years old, dividing them into a frailty group, a prefrailty group and a nonfrailty group according to Fried’s phenotype. The serum levels of OS biomarkers such as 8-hydroxy-2’-deoxyguanosine (8-OdG), metabolic markers such as albumin, and inflammatory markers such as hypersensitivity C-reactive protein (hs-CRP) were measured by competitive enzyme-linked immunosorbent assay. The results demonstrated that the serum albumin and hs-CRP levels in the frail group were higher than those in the other two groups. Furthermore, the levels of 8-OdG and hs-CRP increased with the progression of frailty. Serviddio et al[24] compared the levels of oxidized glutathione (GSSG), tumor necrosis factor-α, malondialdehyde (MDA), 4-hydroxy-2-nonenal (4-HNE), and other OS-related indicators in patients with frailty syndrome with those in nonfrail individuals. The data suggested that these indicators of OS were all significantly increased in frail individuals.
In addition to the direct evaluation of the frailty index, OS was also found to be related to sarcopenia. Severe sarcopenia in the elderly was defined as the prophase of frailty by the European Working Group on Sarcopenia. Bernabeu-Wittel et al[25] found that in 444 polypathological patients, 97 patients had sarcopenia. The number of patients with a combination of sarcopenia and frailty was 80. The authors also reported that OS markers were significantly increased in patients with sarcopenia, frailty or both. OS was evaluated by the levels of catalase, GSSG reductase, total antioxidant capacity to reactive oxygen species (ROS), and superoxide dismutase (SOD). Coto Montes et al[26] found that sarcopenia was closely related to lipid peroxidation. Significant increases in biomarkers such as MDA and 4-HNE were observed in 200 independent 80-year-old persons who had been diagnosed with sarcopenia. Liu et al[27] also discovered that the incidence of frailty increased with a rise in the levels of interleukin-6 (IL-6), isoprostaglandin and lipoprotein phosphorylation A2. These studies suggested a correlation between frailty and OS. OS-related biomarkers in serum may be potential biomarkers for predicting frailty. This possibility needs further study.
THE UNDERLYING MECHANISM OF THE INFLUENCE OF THE GM ON FRAILTY SYNDROME WAS ANALYZED BY OS
OS is affected by many factors in vivo and in vitro. The GM plays a role as “environmental factors” which regulates ROS levels in plasma and maintaining the functions of the digestive, endocrine, immune, skeletal, cardiovascular, and nervous systems. The stability of the GM is fragile and easily affected by external factors, including diet, heavy use of drugs, geographic position, individual lifestyle, and the genetic background of the host[28]. Once the GM is disturbed (“dysbiosis”), it may cause a series of related illnesses, such as inflammatory bowel diseases, obesity, and type II diabetes. These diseases can also affect the oxidative balance, thus forming a vicious cycle.
For example, a variety of colon bacteria, such as Fusobacterium, Clostridioides, Escherichia, Salmonella, Klebsiella, Streptococcus, Desulfovibrio and Enterobacter, can convert sulfide to hydrogen sulfide (H2S)[29]. Intestinal epithelial cells protect themselves by converting H2S into thiosulfate (S2O32-). Thiosulfate can be oxidized to tetrathionate ions (S4O62-) in the case of intestinal inflammation (Figure 1A). This process is accompanied by the formation of RONS. In both anaerobic and microaerobic environments, tetrathionate can specifically promote the proliferation of Salmonella enterica Typhimurium model strains[30]. It has also been reported that certain GM can cause a sharp increase in ROS. Lactobacillus is a strong inducer of ROS. It can stimulate the production of ROS by enhancing the ability to pass through the mucous membrane or increasing the secretion of mucus. Additionally, it can interact with certain cellular receptors, such as formylated peptide receptors or Toll-like receptors[31,32]. In addition, Lactobacilli promotes the oxidation of soluble redox proteins, such as GSSG and thioredoxin, and it facilitates the transcription of redox regulatory factors by activating the nuclear factor erythroid 2-related factor 2 pathway[32]. The pathogenic mechanisms of action of Helicobacter pylori (H. pylori) may also be related to its promotion of ROS production. H. pylori can induce severe white blood cell tissue infiltration and release virulence factors that stimulate epithelial cells to produce ROS and secrete myeloperoxidase, chemokines, and proinflammatory cytokines. Consequently, H. pylori results in gastrointestinal microbiome dysbiosis and induces nonalcoholic fatty liver disease (NAFLD)[33]. The antioxidant enzyme SOD can be used to treat NAFLD. SOD can convert superoxide anion into hydrogen peroxide and molecular oxygen, thus preventing the accumulation of ROS[34]. In addition to H. pylori, the GM has been proposed to be strongly associated with the development and progression of metabolic diseases, including NAFLD[33]. NAFLD patients showed lower fecal microbial diversity than that of healthy controls in one study[35]. At the taxonomic and functional levels, another recent study showed that Lactobacilli inhibited the incidence and development of NAFLD by improving hepatic mitochondria and lipid metabolism by promoting the production of the antioxidant GSSG[36].
These studies indicate that the activation of OS by the GM is a possible mechanism of frailty. For example, gut microbes metabolize choline, L-carnitine and betaine in the daily diet to trimethylamine (TMA). These substances are mainly found in red meat, meat products, eggs, and shellfish[37,38]. Nine GM strains can convert choline to TMA: Anaerococcus hydrogenalis, Clostridioides asparagiforme, Clostridioides hathewayi, Clostridioides sporogenes, Escherichia fergusonii, Proteus penneri, Providencia rettgeri, Edwardsiella tarda and Providencia rustiganii. These species belong to Firmicutes and Proteobacteria[39]. Liver enzymes further convert TMA to TMAO through the flavin-containing monooxygenase (FMO) family members FMO1 and FMO3[37]. Researchers have found that the level of TMAO was increased in the aged population and that TMAO was independently associated with frailty. Nam et al[40] reported that the average levels of TMAO were 3.21 μM and 4.04 μM in nonfrail and frail participants, respectively. Moreover, participants who had the highest plasma TMAO levels showed a significant 3.7-fold increase in the incidence of frailty syndrome. A high level of plasma TMAO can increase the risk of stroke, atrial fibrillation, diabetes, congestive heart failure, chronic kidney disease, coronary artery disease, peripheral artery disease and other cardiovascular diseases, which are associated with frailty[40]. Moreover, TMAO significantly increases OS, inflammatory conditions and endothelial dysfunction. TMAO stimulates the TXNIP-NLRP3 inflammasome and activates the release of the inflammatory cytokines IL-1β and IL-18. However, it inhibits the production of endothelial nitric oxide (NO) synthase and NO[41]. It has also been reported that TMAO can promote inflammatory hepatocellular carcinoma by inducing ROS and activating ILK/AKT/mammalian target of rapamycin (mTOR) signaling via POSTN[42]. Our previous studies showed that administration of TMAO produced a novel model of frailty in mice[43]. In this TMAO-induced frailty model, the abundance of Firmicutes was decreased slightly, while the abundance of Bacteroidetes was significantly increased in the gut. The ratio of Firmicutes to Bacteroidetes (F/B) was significantly decreased. Relevantly, the F/B ratio is reported to be a potential marker of obesity and GM disorder[44], both of which are potential factors of frailty[45,46]. According to an investigation, obese females have higher FI values than those of nonobese females. In addition to TMAO, short-chain fatty acids (SCFAs) are also common metabolites of the GM that are converted from undigested carbohydrates, such as starch and pectin (Figure 1A). Butyrate is a SCFA product. Studies have shown that there is a significant association between SCFAs/butyrate and gut/celiac diseases[47,48]. It is transcriptionally activated by peroxisome proliferator-activated receptor α by replacing histone deacetylase 3 on the fibroblast growth factor 21 (FGF21) promoter. This reaction actives FGF21 and induces the expression and secretion of FGF21[46]. FGF21 regulates the adenosine monophosphate-activated protein kinase-Sirtuin1-mTOR pathway, which is associated with longevity. FGF21 can also alleviate various diseases related to aging[49,50]. In addition, the GM can affect the levels of inflammation by regulating the immune system and can affect insulin resistance by regulating the metabolomic system[51].
In addition to these metabolites, indole-3-propionic acid (IPA), another microbiota-derived metabolite of tryptophan, has been proven to have a positive impact at the cellular level by preventing OS injury and lipoperoxidation and inhibiting the synthesis of proinflammatory cytokines[52], suggesting that IPA may be a promising new target for improving frailty.
The GM and metabolites changes with frailty. Scientific evidence also shows that some antibiotics can reverse frailty. This action may be due to the effect of antibiotics changing the composition of the GM. For example, rapamycin is a broad-spectrum antibiotic. It also acts as an immunosuppressor. Rapamycin regulates protein synthesis and redox reactions. Animal studies have shown that rapamycin prolongs lifespan and improves frailty[50]. These results indicate that the GM plays an important role in the process of frailty via OS.
POTENTIAL INTERVENTIONS FOR FRAILTY
By understanding the role of the GM and OS in frailty, several interventions have been proposed to improve this syndrome and to achieve the goal of healthy aging.
Effect of diet on frailty
One possible intervention is to regulate the GM via the daily diet. Data from short-lived organisms, such as yeast, worms and fruit flies, showed that diet had an anti-senescence effect and prolonged the lives of these organisms by enhancing stress resistance, reliance on lipid fuel use, and the activation of proteostatic mechanisms[53]. Such effects of diet and nutrition on aging can also be reproduced in humans[54]. For example, the Mediterranean diet can improve frailty. The Mediterranean diet is characterized by an increase in the consumption of vegetables, legumes, fruits, nuts, olive oil and fish[55]. At the same time, the consumption of meat and unsaturated fatty acids is reduced. The Mediterranean diet provides a higher intake of micronutrients, such as antioxidant nutrients, polyphenols and plant bioactive compounds[56]. Ghosh et al[57] found that one year of Mediterranean diet intervention can change the composition of the GM and alleviate frailty. Some of the GM that belong to the “diet positive” taxa increased, including Faecalibacterium prausnitzii, Roseburia, Eubacterium (E. rectale, E. eligens, and E. xylanophilum), Bacteroides thetaiotaomicron, Prevotella copri and Anaerostipes hadrus. An increase in these microbiotas facilitates the production of SCFAs and the enhancement of the anti-inflammatory capacity. On the other hand, the microbiota deemed to be “diet negative” taxa, such as Ruminococcus torques, Collinsella aerofaciens, Coprococcus comes, Dorea formicigenerans, Clostridioides ramosum, Veillonella dispar, Flavonifractor plautii and Actinomyces lingnae, were reduced. These “diet negative” taxa are associated with type II diabetes, colorectal cancer, atherosclerosis, cirrhosis and inflammatory bowel disease. With the progressive aggravation of frailty symptoms, the “diet-positive” taxa decreased, and the “diet-negative” taxa increased. Proper nutritional supplements can improve frailty. Bo conducted a double-blind experiment and found that mixed supplementation with whey protein, vitamin D and vitamin E can significantly improve muscle mass and strength (handgrip strength)[58]. It was also used to treat elderly individuals with sarcopenia, and the supplementation improved frailty and quality of life. Moreover, the Mediterranean diet was found to tend to increase the plasma concentration of IPA[52]. IPA could reduce the level of OS in the body and help to improve frailty.
In addition to the Mediterranean diet, some other diet regimens can also improve GM and OS. For example, the replacement diet based on ancient Khorasan wheat results in beneficial GM compositional and functional modifications that positively correlate with an improvement in fibromyalgia symptomatology[59]. A habitual diet supplemented with oral butyrate could reduce the production of ROS in leukocytes[60]. Whether these diet regimens could improve frailty remains to be further investigated.
Effect of TCM on frailty
With an increasing number of studies on TCM, many effective monomers and prescription preparations of TCM have been found to improve frailty by regulating the GM and OS. For example, Cistanche deserticola and Eucommia ulmoides are common TCM with a tonifying effect on the kidneys, which are important organs for aging[61-63]. Modern pharmacological investigations have demonstrated that both TCMs can regulate the GM and OS. Cistanche deserticola polysaccharide is one of the main active components of Cistanche deserticola. It can produce antiaging effects by scavenging free radicals, reducing telomerase activity, improving mitochondrial antioxidant capacity and improving mitochondrial energy metabolism[64]. Moreover, Cistanche deserticola polysaccharide can inhibit the growth of a variety of gut pathogenic bacteria and can promote the growth of probiotics. These effects help to maintain the health of the GM[65]. Chlorogenic acid is one of the main effective components of Eucommia ulmoides, and it has strong anti-inflammatory and antioxidant effects. It can reduce OS and improve mitochondrial dysfunction[66]. Furthermore, chlorogenic acid and its hydrolytic form, which is called caffeic acid, have been demonstrated to alleviate inflammation and OS by improving the GM[67]. This effect is manifested by an increase in the content of Ackermann bacteria and the restoration of the abundance of GM[68]. It can increase the expression of tight junction proteins in intestinal epithelial cells to repair the intestinal barrier and reduce permeability[69]. These actions may contribute to the antiaging effect of chlorogenic acid[69] and further prolong the lifespan in experiments with Caenorhabditis elegans[70]. Liujunzi decoction, a TCM prescription consisting of Dangshen, Tuckahoe, Atracylodes macrocephala, Licorice, Pinellia ternata and Orange peel, was found to significantly improve the frailty state of elderly patients with stable chronic obstructive pulmonary disease. This effect was significantly better than that of tiotropium bromide[71]. In addition, administration of Liujunzi decoction for eight weeks increased the muscle weight and exercise endurance of the frail patients. Supplementation with Chinese medicinal plant extracts from Lonicera hypoglauca and Scutellaria baicalensis, as well as Dihydroquercetin supplementation, could mitigate colonic inflammation by regulating OS and the GM[72,73].
Since TCM is popular in China, Japan and other East Asian regions, most of the available literature to date is limited to the data obtained from Asian populations. However, considering that most modern pharmacological studies are performed with standard experimental animals, the anti-frailty effect of these TCMs may potentially be extended to Western populations based on these basic research data. However, these medicines still need to be more widely investigated in studies involving a larger population.
CONCLUSION
In summary, the abundance and diversity of the GM were found to change with age. Specific GMs and their metabolites stimulate the production of ROS and affect OS in the body, leading to damage to multiple biological macromolecules. The occurrence of OS may be the intermediate process of the GM that leads to frailty, producing a direct action on the body. This may be one of the precipitating factors of frailty syndrome. Thus, the GM and its metabolites can be used as biomarkers of frailty syndrome. Regulating the GM and OS by diet or TCM could help to improve frailty. Additionally, there is increasing evidence indicating that circadian rhythms may also play important roles in mediating the impact of the GM on chronic diseases[74]. Clarifying the mechanisms involved in frailty from the perspective of the GM may be important to achieving the goal of healthy aging.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Almeida C, Portugal; Amedei A, Italy; Konopelski P, Poland; Yi B, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ