Published online Sep 21, 2022. doi: 10.3748/wjg.v28.i35.5154
Peer-review started: May 25, 2022
First decision: July 12, 2022
Revised: July 23, 2022
Accepted: August 22, 2022
Article in press: August 22, 2022
Published online: September 21, 2022
Processing time: 112 Days and 23.3 Hours
Colorectal cancer (CRC) is a common malignant tumor. Alcohol consumption is positively correlated with CRC malignant metastasis; however, the mechanism is unclear. The interaction between laminin-γ2 (LAMC2) and integrin-β1 (ITGB1) plays a role in premetastatic niche signaling, which may induce epithelial mesen
To investigate the effects of alcohol on CRC metastasis from the molecular mecha
The interaction between LAMC2 and ITGB1 was measured by Duolink assay, and the expression levels of LAMC2, ITGB1 and focal adhesion kinase (FAK), snail, fibronectin, N-cadherin and special AT-rich sequence binding protein 1 (SATB1) were measured by quantitative real-time polymerase chain reaction, immunohistochemistry and western blotting. Interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and IL-6 levels were measured via enzyme-linked immunosorbent assay, histopathological assessment via hematoxylin eosin staining, and determination of aberrant crypt foci via methylene blue.
The lymph node metastasis rate was higher in the alcohol group than non-alcohol group. There was a significant increase in interaction signals between LAMC2 and ITGB1, and an increase in phosphorylate-FAK/FAK, snail, fibronectin, N-cadherin and SATB1, whereas E-cadherin was reduced in the alcohol group compared to the non-alcohol group in both animal and clinical samples. Serum IL-1β, TNF-α and IL-6 were higher in alcohol group than in non-alcohol group. Alcohol may promote CRC metastasis by influencing the molecular mechanism of the premetastatic niche.
Our study suggests that alcohol promotes EMT-mediated premetastatic niche formation of CRC by activating the early interaction between LAMC2 and ITGB1 and lead to CRC metastasis.
Core Tip: Our study indicated that alcohol promotes epithelial mesenchymal transformation-mediated premetastatic niche formation of colorectal cancer (CRC) by activating the early interaction between laminin-γ2 and integrin-β1 and lead to CRC metastasis.
- Citation: Nong FF, Liang YQ, Xing SP, Xiao YF, Chen HH, Wen B. Alcohol promotes epithelial mesenchymal transformation-mediated premetastatic niche formation of colorectal cancer by activating interaction between laminin-γ2 and integrin-β1. World J Gastroenterol 2022; 28(35): 5154-5174
- URL: https://www.wjgnet.com/1007-9327/full/v28/i35/5154.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i35.5154
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy worldwide[1]. A majority of colorectal cancers are associated with the environmental factors such as lifestyle changes including diet, obesity, smoking and alcohol consumption[2,3]. It is reported that long-term alcohol consumption is associated with an increased risk of CRC metastasis[3]. In the presence of concomitant metastasis, the maximum survival rates after surgical intervention do not exceed 20%[4,5]. Therefore, the relationship between alcohol consumption and CRC metastasis should be investigated. However, the mechanism that the alcohol promotes CRC metastasis has not been clearly explained[6,7].
Accumulating evidence suggests that metastasis develops from disseminated cancer cells that can remain dormant, and the dissemination may occur during the early stage of tumor evolution[7,8]. The microenvironment around the early disseminated cancer cells, also known as the premetastatic niche[9,10], may play an important role during the proliferation and migration of disseminated cancer cells in the early stage of tumors[11-13]. The extracellular matrix (ECM) is an important part of the premetastatic niche[14-17].
As an important component of the ECM, laminins are important for adhesion, differentiation, migration, and resistance to apoptosis of various cells, including cancer cells[18-21]. Reports have indicated that laminins are involved in the formation of the premetastatic niche[22,23]. Laminin-γ2 (LAMC2), a subunit of laminin-332, has been reported to be an important component in the survival dependent environment of epithelial cells in CRC and plays a key role in the regulation of tumor cell motility in premetastatic niche formation[24-27]. The interaction between LAMC2 and integrin-β1 (ITGB1) may stimulate the formation of premetastatic focal contacts by activating the premetastatic niche signaling, which promotes the migration of cancer cells[28]. Focal adhesion kinase (FAK) is a premetastatic focal contact involved in LAMC2 and β1 integrin signal transduction[29]. Signaling cascades triggered by integrin-laminin interactions in cells phosphorylate FAK, which induced epithelial mesenchymal transformation (EMT). EMT is a key initiating step during the metastasis and invasion of cancer[30,31] and involved in the formation of the premetastatic niche[32,33].
Alcohol consumption may influence the ECM remodeling and thus induce EMT, which may be closely related to the premetastatic niche[34]. Therefore, we explore its effect on CRC metastasis from the view of the premetastatic niche.
The following antibodies were used: LAMC2 monoclonal antibody (YT5379, Immunoway, United States), ITGB1 monoclonal antibody (sc-9970, Santa Cruz, United States), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody (ab125247, ABCAM, United Kingdom), phosphorylate-FAK (p-FAK)-Y397 (AP0302, ABclonal, Wuhan, China), FAK monoclonal antibody (A11131, ABclonal, Wuhan, China), snail monoclonal antibody (ab53519, ABCAM, United Kingdom), E-cadherin polyclonal antibody (ab1416, ABCAM, United Kingdom), N-cadherin polyclonal antibody (22018-1-AP, Proteintech, United States), fibronectin polyclonal antibody (A16678, ABclonal, Wuhan, China) and special AT-rich sequence binding protein 1 (SATB1) polyclonal antibody (A5800, ABclonal, Wuhan, China). Secondary antibodies were ab288151 and ab150113 (ABCAM, United Kingdom). Polyvinylidene difluoride membranes with a pore size of 0.45 mm (Millipore, United States) were used. RNAEX reagent (Accurate Biotechnology, Beijing, China), Evo M-MLV RT Premix (Accurate Biotechnology, Beijing, China), SYBR Green Premix Pro Taq HS qPCR Kit (Accurate Biotechnology, China). Interleukin-6 (IL-6), IL-1β and tumor necrosis factor-α (TNF-α) enzyme-linked immunosorbent assay (ELISA) kits (Enzyme-linked Biotechnology Co., LTD, Shanghai, China) were used. Duolink proximity ligation assay (PLA) kits (DUO92012, Merck, United States), Duolink PLA Rabbit PLUS (DUO92002, Merck, United States), and PLA Mouse MINUS (DUO92004, Merck, United States) proximity probes were purchased from Sigma-Aldrich. All above the mentioned antibodies can be used in both in rats and in humans.
A total of 63 patients (26-86 years old) at The First Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangzhou, China) from July 2019 to February 2020 was enrolled in this study. The baseline characteristics were collected, and data on alcohol consumption were collected at baseline using a direct interview method[35]. Participants were asked to answer whether they drank alcoholic beverages (“Yes”, “Yes, but not now”, or “No” were the answer options to the question, “Have you ever drunk alcohol?”), and for “ever drinkers”, consumption frequency and average quantity of one serving over the past year by beverage type was recorded. CRC patients who never drank alcohol were added into the non-alcohol group, and CRC patients who drank alcohol over 3 times a week for more than 5 years were added into the alcohol group. And patients with smoking, family history, blood lipids, obesity, and chronic diseases were excluded. The present study was approved by the Ethics Committee of First Affiliated Hospital of Guangzhou University of Chinese Medicine [No. Y (2019) 172] and carried out in accordance with the “Helsinki Declaration”. All CRC patients have sighed informed consent. Patients who received neoadjuvant chemotherapy or radiotherapy or had a history of malignancy were excluded. Clinicopathological data including pathological report, sex, age, macroscopic classification, tumor location, tumor size, tumor differentiation, lymphatic infiltration and invasion depth were collected via medical record. The American Joint Council on Cancer tumor, node and metastasis (TNM) staging system is used for the clinical staging of tumors[36].
A total of 32 male Wistar rats, 7 wk old and weighing 200 ± 20 g, were obtained from the Experimental Animal Center of Southern Medical University [Certificate of quality: SCXK (Yue) 2016-0041]. All rats were housed in cages (25 cm × 30 cm × 30 cm, four rats per cage) under specified-pathogens free conditions at 25℃, 40%-60% relative humidity, and a 12-h light/dark cycle in the experimental animal center of Guangzhou University of Chinese Medicine. All rats had ad libitum access to standard rodent chow and filtered water and were acclimatized for 1 wk prior to the initiation of the experiment. The use of laboratory animals was checked by the “Institutional Animal Ethical Committee” and all procedures were approved by the Ethics Committee of Guangzhou University of Chinese Medicine, and performed according to the “Principles of Laboratory Animal Care” as well as specific national laws where applicable. All experimental protocols and handling of the animals followed the guide for the care and use of laboratory animals[37].
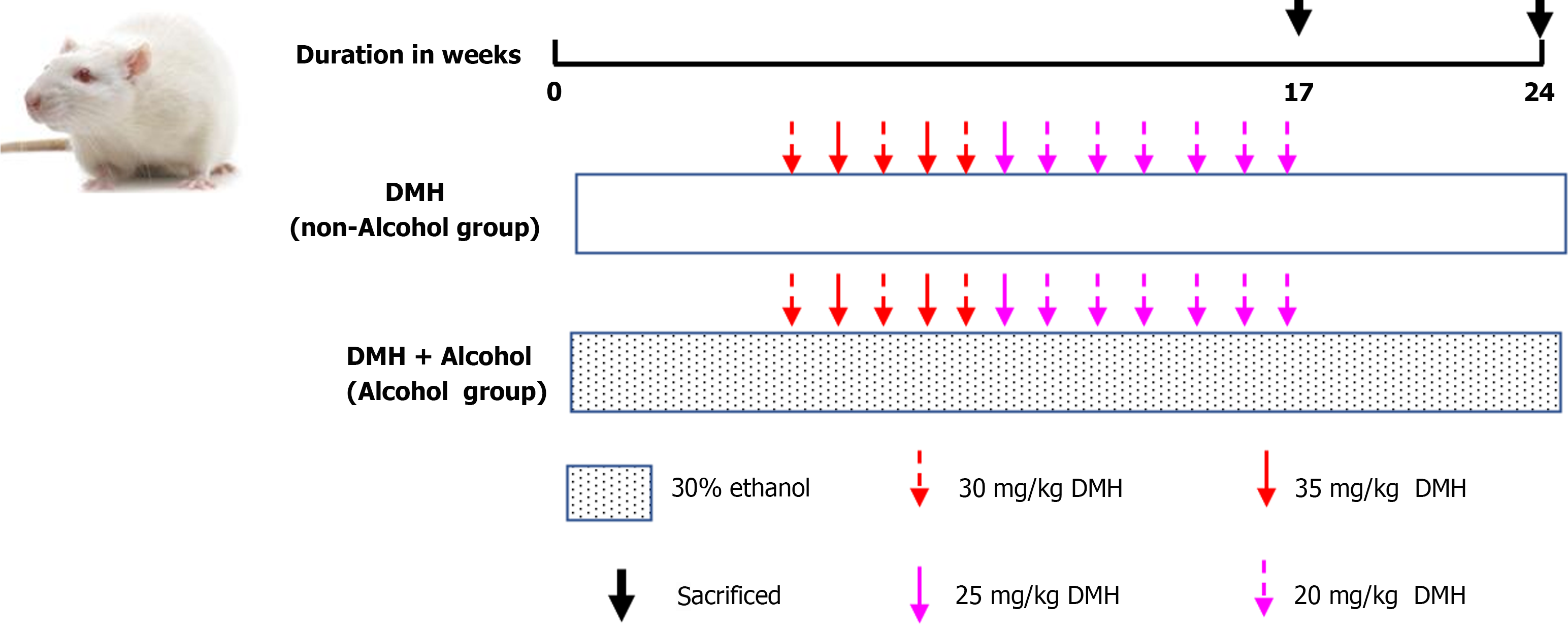
A total of 32 rats were randomly divided into two groups: the non-alcohol group and the alcohol group, with 16 rats in each group. Non-alcohol group: The rats were fed water (10 mL/kg b.wt.) every day and from the 6th week on, the rats were administered a subcutaneous injection of 1,2-dimethylhydrazine hydrochloride (DMH) at a dose of 30, 35, 25 or 20 mg/kg b.wt. once a week for 12 wk in order to induce CRC. Alcohol group (DMH + ethanol): The rats were fed 30% ethanol (10 mL/kg b.wt.) every day, and from the 6th week on, they were administered a subcutaneous injection of DMH at a dose of 30, 35, 25 or 20 mg/kg b.wt. once a week for 12 wk in order to induce CRC. The details of the specific modeling method of each group were shown in Figure 1. The blood samples were collected through the posterior orbital venous plexus. And the rats were sacrificed by cervical dislocation, then the colons were removed and used in further experiments.
All specimens were fixed in 4% paraformaldehyde solution for 24 h and embedded in paraffin and processed by standard histological processing techniques. Tissue sections (4-µm thick) were obtained from each sample. The hematoxylin and eosin results were observed under a light microscope at 40× magnifications (Olympus, BX51) to observe the colonic histology differences by the method of Martin B[38].
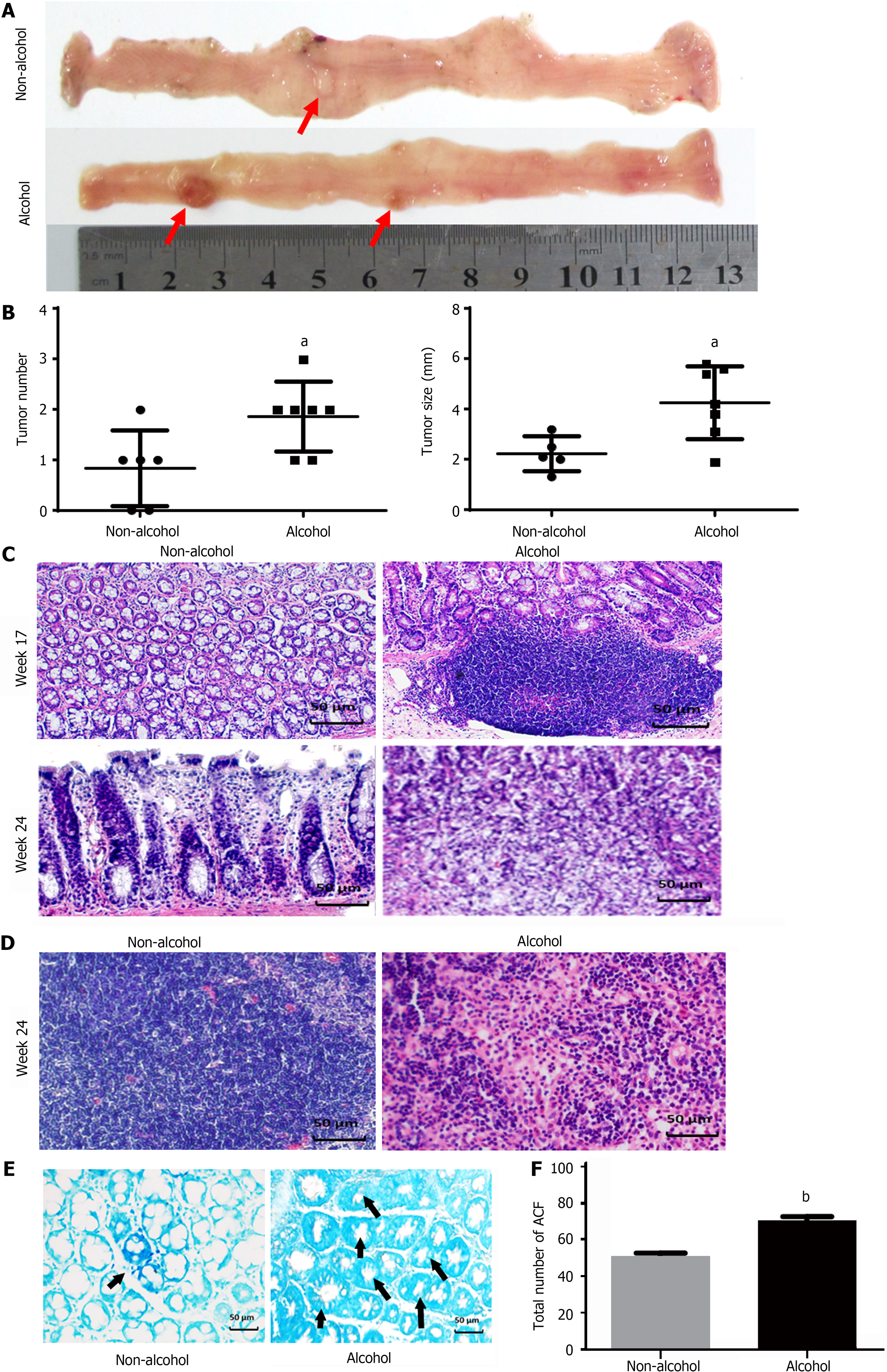
Aberrant crypt foci (ACF) were determined according to the method described by Bird et al[39]. Then, the formalin-fixed colon was stained with 0.2% methylene blue for 3 min and observed under light microscope. The diagnosis of colonic pathology and ACF was performed by two pathologists. The number of ACF was counted as described by Sivaranjani et al[40].
Serum samples of rats were collected, and the inflammatory factors (TNF-α, IL-6 and IL-1β) were determined using ELISA kits according to the manufacturers’ instructions. The optical density of the colorimetric reaction was measured at 450 nm with a flat microplate reader.
For western blotting, 50 µg of extracted protein was fractionated on a 10% SDS-PAGE gel and then transferred to a polyvinylidene fluoride membrane with a 0.45-mm pore size (Millipore, MA, United States). The membrane was blocked in 5% milk for 3 h at room temperature and probed with antibodies against acetylated LAMC2 (1:1000), integrinβ1 (1:1000), p-FAK (1:1000), FAK (1:1000), snail (1:1000), E-cadherin (1:1000), fibronectin (1:1000) and N-cadherin (1:1000) at 4℃ overnight. Horseradish peroxide-conjugated goat anti-rabbit and rabbit anti-mouse was used as the secondary antibodies. FAK and GAPDH (1:8000) were used as loading controls. Band intensity was visualized by ChemiDoc TMXRS+ (Bio-Rad, United States).
The immunohistochemical (IHC) was performed by the method described in previous studies[41,42], all colonic tissue were fixed with 4% paraformaldehyde and paraffin embedded, then thin 4μm sections were obtained and deparaffinized in xylene and rehydrated through decreasing grades of alcohol. The sections were placed in a 95℃ antigen retrieval solution for 15 min. After cooling the retrieval solutions for 20 min at room temperature, the slides were treated with hydrogen peroxide (H2O2) for 10 min to block endogenous peroxidase activity. Then, slides were incubated with primary antibodies against ITGB1 (dilution 1:100), LAMC2 (dilution 1:500), p-FAK (dilution 1:500), snail (dilution 1:500), E-cadherin (dilution 1:100), N-cadherin (dilution 1:800), fibronectin (dilution 1:200), and SATB1 (dilution 1:200) overnight at 4℃. The next day, biotin-conjugated secondary antibody and streptavidin-biotin peroxidase were applied each for 20 min. 3,3'-Diaminobenzidine tetrahydrochloride was used as the substrate, and nuclear contrast was performed using hematoxylin counterstaining. The negative controls for IHC are performed with PBS instead of primary antibody. The results were observed under a light microscope at 40× magnifications (Olympus, BX51). For quantitative analyses, the IHC images were analyzed by Image-Pro Plus 6.0 to calculate the average optical density (AOD), and the following equation: AOD = integrated optical density/area.
The Duolink PLA in situ was used in this study to detect the interaction between LAMC2 and ITGB1 in tissue samples, and it was performed as previously described by Bai et al[43]. Briefly, after washing, permeabilizing, and blocking as histological analysis, colon sections were incubated with primary antibodies against LAMC2 (1:200) and ITGB1 (1:400) overnight at 4℃. Then the slides were incubated with Duolink PLA Rabbit MINUS and PLA Mouse PLUS proximity probes for 1 h at 37℃. Ligation and amplification were conducted using the Duolink in situ detection reagent kit according to the protocol. Images were captured under the light microscope (Olympus, BX51).
The total RNA was extracted from tissues using RNAEX reagent (Accurate Biotechnology, China). It was reverse transcribed into cDNA by using Evo M-MLV RT premix (Accurate Biotechnology, China) and quantitative real-time polymerase chain reaction (RT-PCR) system (TaKaRa, Japan). Then a SYBR Green Premix Pro Taq HS qPCR Kit (Accurate Biotechnology, China) was used for PCR. The PCR liquid volume was 20 μL. Quantitative PCR was performed using an RT-PCR system (BioRad, Singapore). At 95℃, 40 cycles were amplified after 90 s of initial denaturation, 10 s desaturation at 95℃, and 34 s of extension at 60℃. GAPDH was used as the reference gene for calculation. And the primer sequences are shown in Table 1.
| Gene name | Forward (5’>3’) | Reverse (5’>3’) |
| For human | ||
| LAMC2 | TTCTACAACGATCCGCACGAC | GAGTTAAAAGCAGCCCTGGT |
| ITGB1 | CCTACTTCTGCACGATGTGATG | CCTTTGCTACGGTTGGTTACATT |
| FAK | GTCTGCCTTCGCTTCACG | GAATTTGTAACTGGAAGATGCAAG |
| Snail | CTAGCGAGTGGTTCTTCTGC | GTAGTTAGGCTTCCGATTGGG |
| E-cadherin | GACCGGTGCAATCTTCAAA | TTGACGCCGAGAGCTACAC |
| N-cadherin | AGCTTCTCACGGCATACACC | GTGCATGAAGGACAGCCTCT |
| Fibronectin | GCCTGGTACAGAATA TGTAGTG | ATCCCAGCTGATC AGTAGGCTGGTG |
| SATB1 | GTGGAAGCCTTGGGAATCC | CTGACAGCTCTTCTTCTAGTT |
| GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |
| For rats | ||
| LAMC2 | GTGAAGCTTCCCTGCAAAAC | CATTTGGCCCCACGTAGT |
| ITGB1 | TCGAAGGACCACTAGACCTGA | TTCCATGGGAACAAAAGGTAA |
| FAK | TTGGGTTGCAAACTATCTCTAGAAC | TGGTACAAAACTGGCACAGAA |
| Snail | TTCACATCCGAGTGGGTCTG | ACCCACACTGGTGAGAAGCC |
| E-cadherin | AAAGCAGGAAGAAAACACCACTC | AAAGGGCACGCTATCAACATTAG |
| N-cadherin | TCAGTGGCGGAGATCCTAC | GTGCTGAATTCCCTTGGCTA |
| Fibronectin | TGTCACCCACCACCTTGA | CTGATTGTTCTTCAGTGCGA |
| SATB1 | AGATGCAGGGAGTGCCTTTA | TGCTCCTCCTTGCAATCATA |
| GAPDH | TGCCCTCATGTTCCTGATAAAT | CATTACATCACAGCTTTCCAGG |
The data were described using the mean (standard deviation), median (range), or frequency (percentage). An independent-samples t test or the Mann-Whitney U test was applied for the comparison between two groups (non-alcohol group and alcohol group). The associations between the clinical parameters and immunohistochemical results were analyzed using the χ2 test. Statistical analyses were conducted using SPSS version 25.0 for Windows (SPSS, Inc.). Statistical analysis was performed: bP < 0.01; aP < 0.05; nsP > 0.05.
The total number of ACFs detected in the alcohol group was 70.33 ± 5.16 and 51.6 ± 4.5 in the non-alcohol group (Figure 2E and F), which implied that alcohol consumption may significantly increase considerably raise the risk of precancerous lesions of colorectal cancer (P < 0.05). Furthermore, CRC rats in alcohol group had more tumors and larger tumor size was observed in the alcohol group than those in the non-alcohol group (Figure 2A and B). Histopathological analysis revealed that at 17th week, 62.5% of rats in the non-alcohol group showed atypical hyperplasia, and the other 37.5% showed normal colonic tissues. Atypical hyperplasia was seen in 75% of the rats in the alcohol group; however, 25% of the rats in the alcohol group had many heteroepithelial cells in the colonic epithelium and one or more large nucleoli in the pleomorphic cystic nucleus, indicating a carcinoma adenoma. At the 24th week, 25% of rats in the alcohol group showed intramucosal carcinoma (mucosal carcinoma), which was characterized by less mucus secretion of colonic epithelial cells, compact and irregular arrangement of glandular ducts, and clear invasion of cancer cells into lamina propria in colons. The other 75% of rats in the alcohol group had invasive mucosal infiltrating carcinoma (infiltrating carcinoma), which manifested as abnormal tubular or mucinous structures invading through the muscularis mucosa or deep intestinal wall. In total, 75% and 25% of rats in the non-alcohol group showed mucosal carcinoma and infiltrating carcinoma, respectively. We found no lymphatic metastasis in the non-alcohol group, while cancer cell infiltration was found in the lymph nodes of the alcohol group and the lymph node metastasis rate was 37.5% (Figure 2C and D; Table 2). The abovementioned results indicated that alcohol consumption promoted CRC metastasis.
| Wk | Group | n | Normal | Atypical hyperplasia | Adenoma | Adenocarcinoma | With lymphatic metastasis | ||
| Mucosal carcinoma | Infiltrating carcinoma | Metastasis | Tumor metastasis rate | ||||||
| 17 | Non-alcohol | 8 | 3 | 5 | 0 | 0 | 0 | 0 | 0.00% |
| Alcohol | 8 | 0 | 6 | 2 | 0 | 0 | 0 | 0.00% | |
| 24 | Non-alcohol | 8 | 0 | 0 | 0 | 6 | 2 | 0 | 0.00% |
| Alcohol | 8 | 0 | 0 | 0 | 2 | 6 | 3 | 37.5% | |
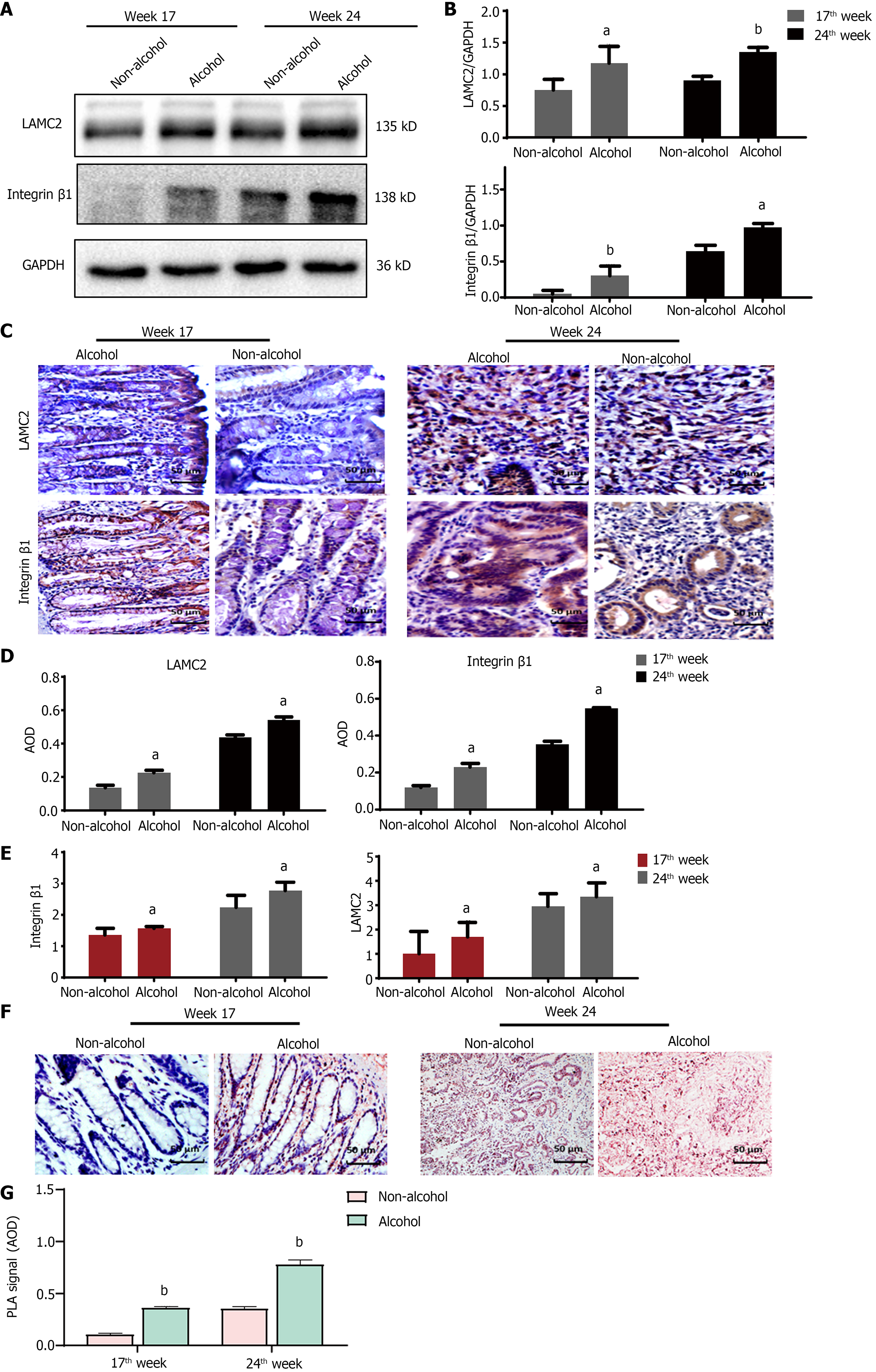
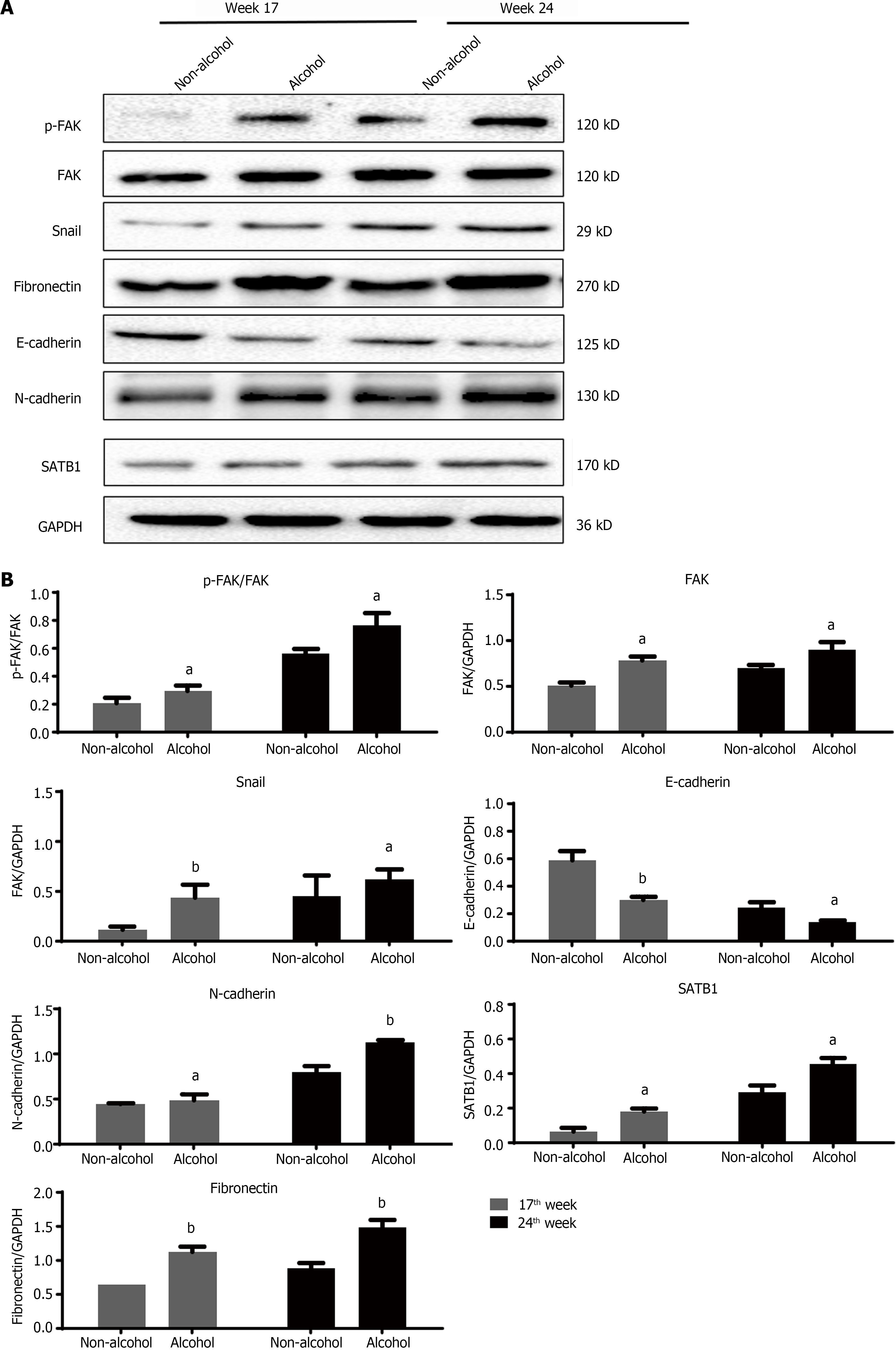
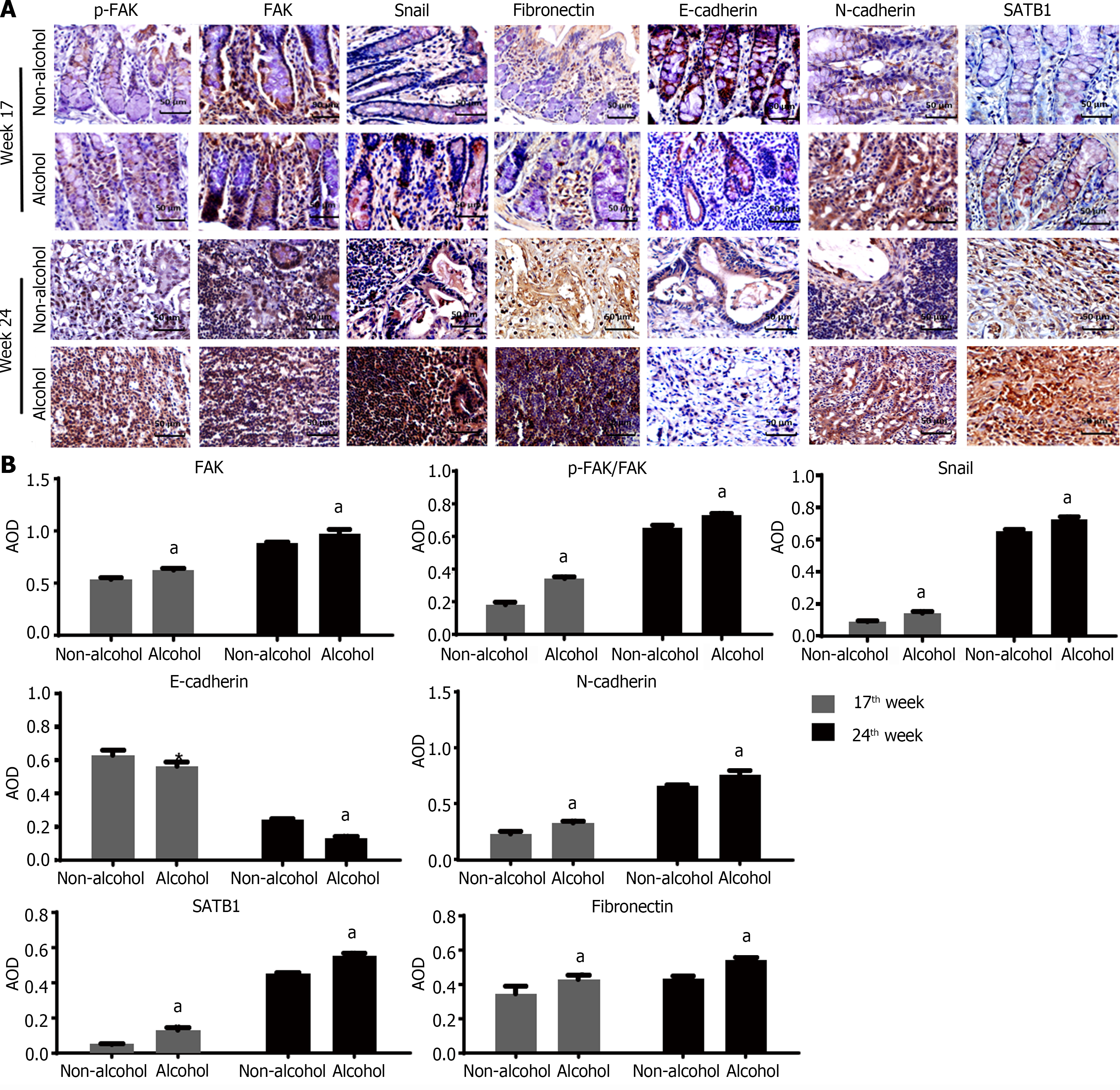
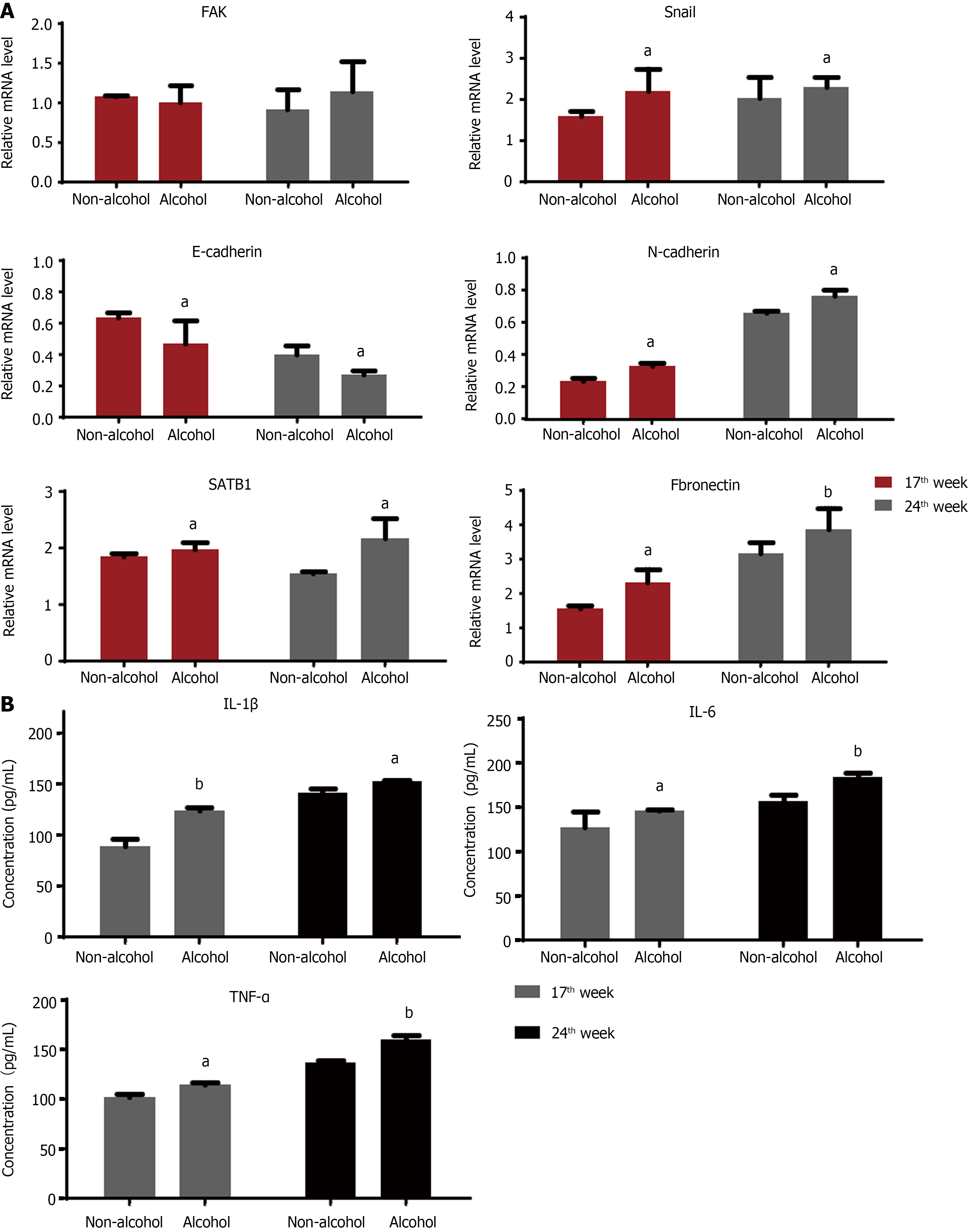
The protein levels of LAMC2 and ITGB1 measured by western blotting (Figure 3A and B) and IHC (Figure 3C and D). The result showed that at both of 17th week and 24th week, the protein levels of LAMC2 and ITGB1 in the alcohol group were higher than those in the non-alcohol group (P < 0.05). Furthermore, the mRNA levels of LAMC2 and ITGB1 in alcohol group were higher than those in non-alcohol group at both of 17th week and 24th week (P < 0.05; Figure 3E). In addition, the protein level of FAK in the alcohol group was higher than that in the non-alcohol group at the 17th and 24th weeks (Figures 4 and 5, P < 0.05). However, there was no significant difference in the mRNA level of FAK between the alcohol and non-alcohol group at both of the 17th and 24th week (P > 0.05; Figure 6A).
Duolink PLA were used to detect the interaction between ITGB1 and LAMC2, and the red spots represent the interactions between LAMC2 and ITGB1. The results revealed that (Figure 3F and G) at 17th week, more early interacting signals between LAMC2 and ITGB1 were found in the alcohol group than that in non-alcohol group. At week 24, the results were consistent with that of week 17. The present study suggested that alcohol consumption may enhance the early interaction between LAMC2 and ITGB1 in CRC rats.
High expression level of SATB1 significantly influenced the malignant phenotypic characteristics of CRC. As shown in Figures 4 and 5, the results of IHC and western blotting indicated that at 17th week, the protein level of SATB1 in the alcohol group was higher than that in non-alcohol group, and the results of qRT-PCR (Figure 6A) showed that the mRNA level of SATB1 was higher than that in non-alcohol group.
EMT is one of the key events that occur during the formation of premetastatic niche[44]. The results of western blotting (Figure 4A) and IHC (Figure 5) suggested that at the 17th week, long-term alcohol consumption resulted in an increase in the proteins involved in triggering the EMT process including p-FAK, N-cadherin, fibronectin and snail; however, there was a decrease in E-cadherin in the alcohol group. At week 24, the results were similar to those of week 17. In addition, the mRNA levels of FAK, E-cadherin, N-cadherin, fibronectin and snail were measured by qRT-PCR, and the results were consistent with those of protein levels (Figure 6A). The abovementioned results indicated that alcohol consumption may abnormally change the expression levels of EMT biomarkers in CRC rats in early stage.
The results of ELISA (Figure 6B) showed that IL-1β, IL-6 and TNF-α levels in the alcohol group were higher than those in the non-alcohol group at week 17. The results at week 24 were consistent with those at week 17, indicating that the concentrations of IL-1β, IL-6, and TNF-α were higher in the alcohol group than in the non-alcohol group during precancerous lesions, which may be one of the reasons for lymph node metastasis in CRC patients in the alcohol group.
The obtained results showed that in early stages of CRC, the contents of IL-6, IL-1β and TNF-α in the alcohol group were higher than those in the non-alcohol group. This might be one of the explanations for the higher metastatic rate in the alcohol group.
A total of 63 patients with a preliminary diagnosis of colorectal cancer participated in the trial. According to the baseline characteristics and data on alcohol consumption of 63 CRC patients, 29 CRC patients who had been drinking alcohol over 3 times each week for more than 5 years were classified in alcohol group, while the remaining 34 CRC patients who have never drunk alcohol before were classified in non-alcohol group. As shown in Table 3, there are significant differences in clinical stage and tumor metastasis (P < 0.05) between alcohol group and non-alcohol group. There were no significant differences in sex, age, degree of differentiation or T stage (P > 0.05). The experimental results were consistent with the results of animal experiments. The degree of tumor deterioration in CRC patients in the alcohol group was significantly higher than that in non-alcohol group.
| Baseline characteristics | Alcohol group (n = 29) | Non-alcohol group (n = 34) | P value |
| Gender | |||
| Male, n (%) | 18 (62.1) | 16 (47.1) | 0.233 |
| Female, n (%) | 11 (37.9) | 18 (52.9) | |
| Age (yr old) | |||
| < 55, n (%) | 10 (34.5) | 14 (41.2) | 0.586 |
| ≥ 55, n (%) | 19 (65.5) | 20 (58.8) | |
| Differentiation | |||
| Medium, n (%) | 24 (82.8) | 30 (88.2) | 0.536 |
| Poor, n (%) | 5 (17.2) | 4 (11.8) | |
| Clinical stage | |||
| Stage I-II, n (%) | 12 (41.4) | 24 (70.6) | 0.020a |
| Stage III-IV, n (%) | 17 (58.6) | 10 (29.4) | |
| T stage | |||
| T1-T2, n (%) | 6 (20.7) | 12 (35.3) | 0.201 |
| T3-T4, n (%) | 23 (79.3) | 22 (64.7) | |
| Tumor metastasis conditions | |||
| Distant metastasis | 12 (41.4) | 23 (70.6) | 0.046a |
| Lymphatic metastasis | 9 (31) | 8 (20.6) | |
| Non-metastasis | 8 (27.6) | 3 (8.8) | |
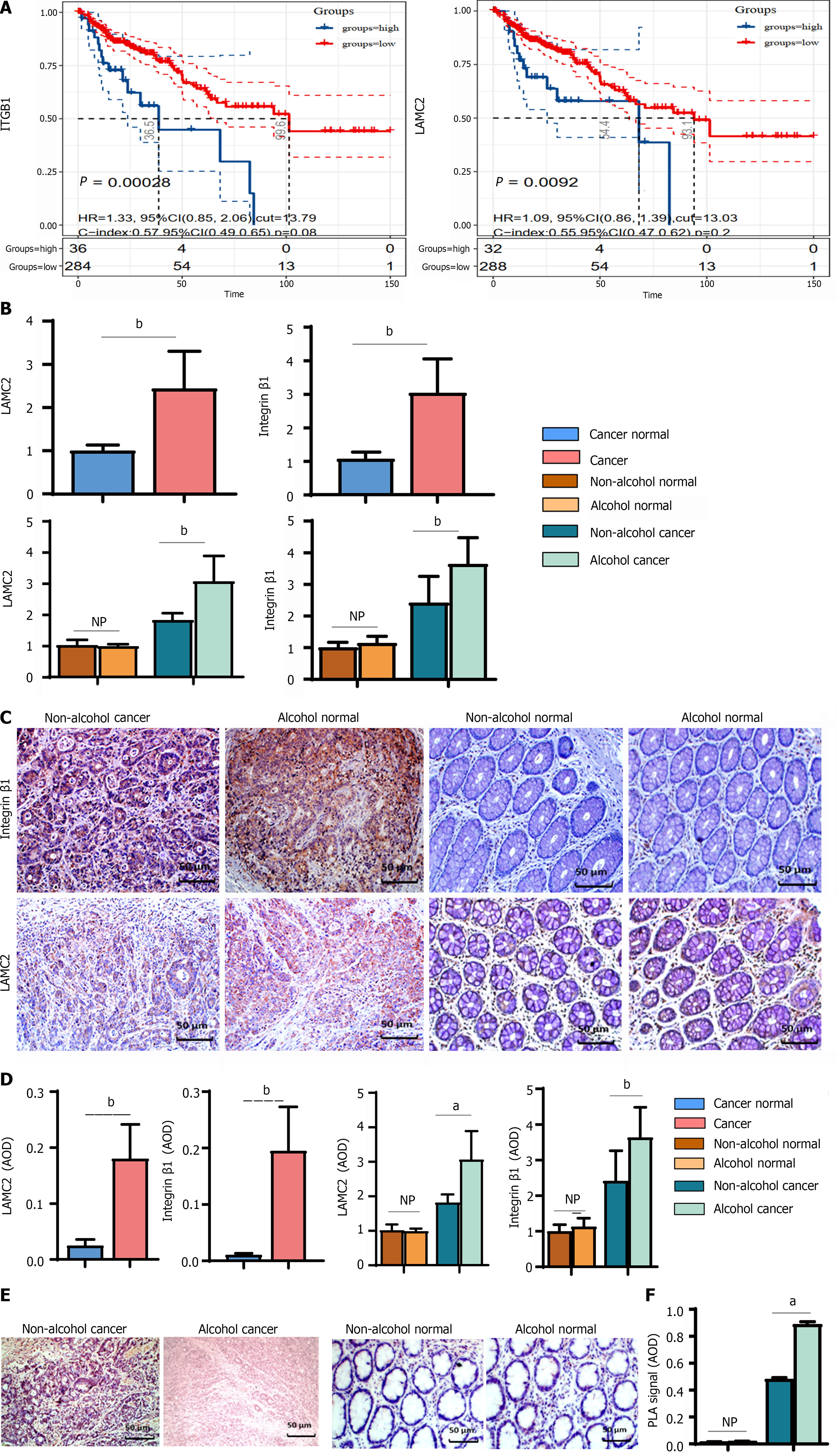
The expression of LAMC2 and ITGB1 in clinical samples determined by IHC (shown in Figure 7C and D). In cancer tissues, the mRNA and protein levels of LAMC2 and ITGB1 in alcohol group were significantly higher than those in non-alcohol group (Figure 7B). In addition, according to the survival analysis of 392 CRC patient samples from the GSE39582 dataset, we explored the prognostic significance of LAMC2 and ITGB1. As shown in Figure 7A, the overexpression of LAMC2 and ITGB1 was closely related to poor CRC prognosis. The abovementioned results were consistent with the results of animal experiments. Alcohol consumption may increase the expression levels and the interaction of LAMC2 and ITGB1 in CRC patients.
The interactions of LAMC2-ITGB1 are pivotal in CRC progression. Animal experiments revealed that more interactions between LAMC2 and ITGB1 were found in the alcohol group than in the non-alcohol group in the early stage of CRC. And it has been validated in clinical samples. According to the results of duolink PLA (Figure 7E and F), more interacting signals between LAMC2 and ITGB1 were found in alcohol group than those in non-alcohol group.
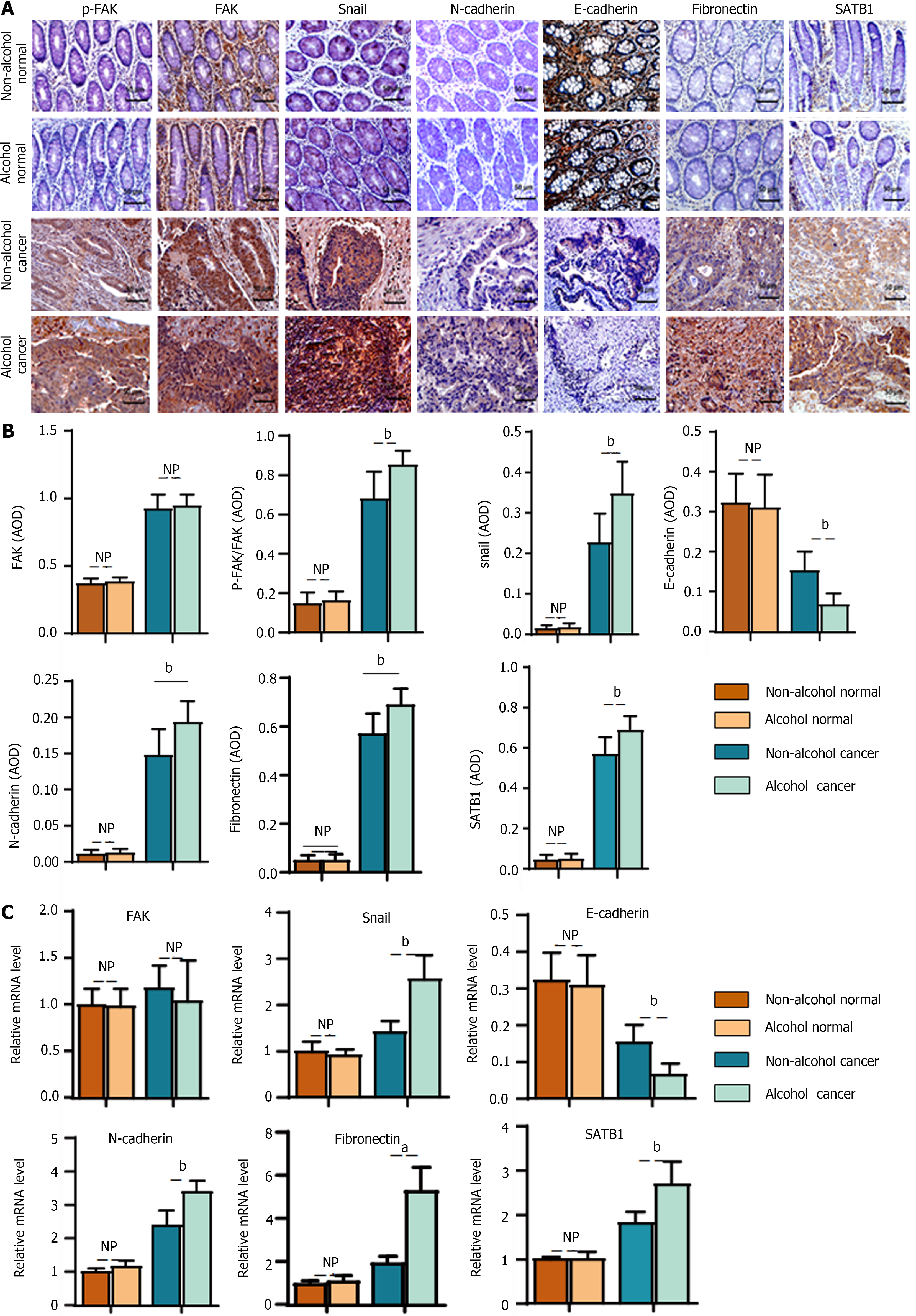
In the animal experiment, we found that alcohol may abnormally change the expression levels of EMT-associated biomarkers in CRC rats in the early stage. To verify the clinical implications of this finding, the levels of EMT biomarkers were detected in clinical samples. The results of IHC (Figure 8A and B) showed that there were significant differences in EMT-associated biomarkers (P < 0.01) in cancer tissues between the alcohol and non-alcohol group. In cancer tissues, the protein levels of p-FAK, N-cadherin, fibronectin and snail were significantly higher in alcohol group was significantly higher than that in non-alcohol group; however, the expression of E-cadherin in alcohol group was significantly lower in alcohol group than that in non-alcohol group. The qRT-PCR results (Figure 8C) also showed that the mRNA levels of N-cadherin, fibronectin and snail in the cancer tissues of alcohol group was higher than that in non-alcohol group. The abovementioned findings were consistent with the results of animal experiments. The abnormal expression of EMT biomarkers in CRC patients was different from that in alcohol group, and the abnormal expression of LAMC2 and ITGB1 was more severe in alcohol group.
In animal experiments, alcohol consumption was found to increase the expression of SATB1 in CRC rats. IHC and qRT-PCR were used to evaluate the protein and mRNA levels of SATB1 in clinical samples (Figure 8A-C). The obtained results showed that protein and mRNA levels of SATB1 in cancer tissues were significantly higher in alcohol group than that in non-Alcohol group. Our results indicate that the expression of SATB1 is higher in alcohol group than those in non-Alcohol group, which is consistent with the higher metastasis rate of CRC patients who drink alcohol as described by TNM staging. The above results suggest that alcohol consumption may up-regulate the expression of SATB1, thereby induce the early EMT event and leads to malignant metastasis of the tumor[45].
Metastasis is the prominent cause of death in CRC[7]. Chronic alcohol intake has been extensively reported to enhance CRC metastasis; nevertheless, the specific mechanism remains unknown. In the present study, the results of TNM stage, pathological grade and other clinicopathological parameters suggested that the tumor deterioration rate of patients in alcohol group was higher than that in non-alcohol group, and the results were consistent with previous reports[6,7]. The present study demon
A key step in the formation of metastasis is the extravasation of circulating tumor cells to distant organs and their adaptation to the new environment[46,47]. Thus, the interaction between disseminated cancer cells and the microenvironment of the premetastatic niche is necessary for metastasis[10]. Primary tumor cells regulate the metastasis by secreting a variety of molecules that promote the mobilization and recruitment of various cells to the premetastatic niche and alter ECM protein expression and ECM properties in secondary organs[25,48]. The ECM constitutes a scaffold that supports the attachment and thus reactivation of survival signaling in cancer cells[16]. And the remodeling of ECM is essential during formation of premetastatic niche, which may support cancer cell attachment, metabolism and migration of recruited cells[17,18]. Laminin, as an important component of the ECM and the main structural component of basement membrane, is involved in the formation of pre-metastasis niche[49]. Among them, LAMC2 is an important component of the survival-dependent environment of colorectal cancer epithelial cells and plays a key role in the regulation of tumor cell motility by premetastatic niche formation[50]. LAMC2 plays a role in premetastatic niche signaling by stimulating the formation of premetastatic local contacts and promoting the migration of cancer cells through its interaction with ITGB1[51]. It has been reported that the interaction of ITGB1 and LAMC2 may promote the tumor budding of CRC cells, which leads to malignant metastasis of CRC, and the focal adhesion formed by the interaction of LAMC2 and ITGB1 plays an important role in the prognosis of CRC. This adhesion is not only the basis of cancer cell survival but is also closely related to the migration and metastasis of cancer cells[52]. FAK is involved in LAMC2 and ITGB1 signal transduction, and the overexpression of FAK was found to play a key regulatory role in ITGB1-mediated signal transduction. As the phosphorylated FAK is activated, it transmits signals to the transcription factor snail, thereby inducing EMT[32,33]. The interaction of LAMC2 and ITGB1 and the overexpression of FAK were seen as markers of premetastatic niche formation, which may lead to malignant metastasis of CRC[52]. According to the results of our study, more interacting signals between LAMC2 and ITGB1, and higher level of FAK were found in the alcohol group than those in the non-alcohol group at early stage. In addition, the higher ratio of p-FAK/FAK in the alcohol group in the early stage indicates a higher degree of FAK activation, which may induce early EMT events and promote CRC metastasis.
CRC metastasis depends on EMT, during EMT, epithelial cells lose the connectivity and top-to-base polarity, resulting in a mesenchymal phenotype capability of migration and invasion[32]. There is evidence that some cancer cells may acquire the ability to spread and metastasize before they are fully malignant, which suggests that EMT may be an early event involving in the metastasis[53]. In addition, SATB1 has been found to be associated with early EMT events and can be used as a marker of CRC metastasis[54]. SATB1 was found to be a driver of the malignant phenotype in CRC, and its expression was shown to be positively associated with invasion depth, poor degree of differentiation and advanced TNM stage of CRC. And SATB1 organizes chromatin into spatial rings that provide the necessary "docking sites" for further binding of transcription factors and chromatin modifying enzymes[45]. SATB1 was found to regulate entire genes, even those located on distant chromosomes[55]. The results of the animal experiment suggested that the expression of SATB1 was significantly higher in the alcohol group than that in the non-alcohol group during precancerous lesions, which may predict the early EMT events.
In addition, there is an increasing evidence that proinflammatory cytokines play an important role in the formation of premetastatic niches. Previous studies have shown that the high expression of IL-6 and IL-1β may activate ITGB1[56,57] to stimulate the adhesion of bone marrow cells to endothelial cells, thereby promote the formation of premetastatic niche, and inducing EMT to promote metastasis[58]. Pal et al[55] found that TNF-α induces EMT in human HCT116 cells and promotes colorectal cancer invasion and metastasis. According to our study, the levels of the proinflammatory cytokines IL-1β, TNF-α and IL-6 in CRC rats in the alcohol group were significantly increased at the early stage of precancerous lesions, which may serve as paracrine signals to enhance the proliferation and invasion of early disseminated cancer cells. It may help cancer cells change their phenotypes through EMT to enhance metastasis.
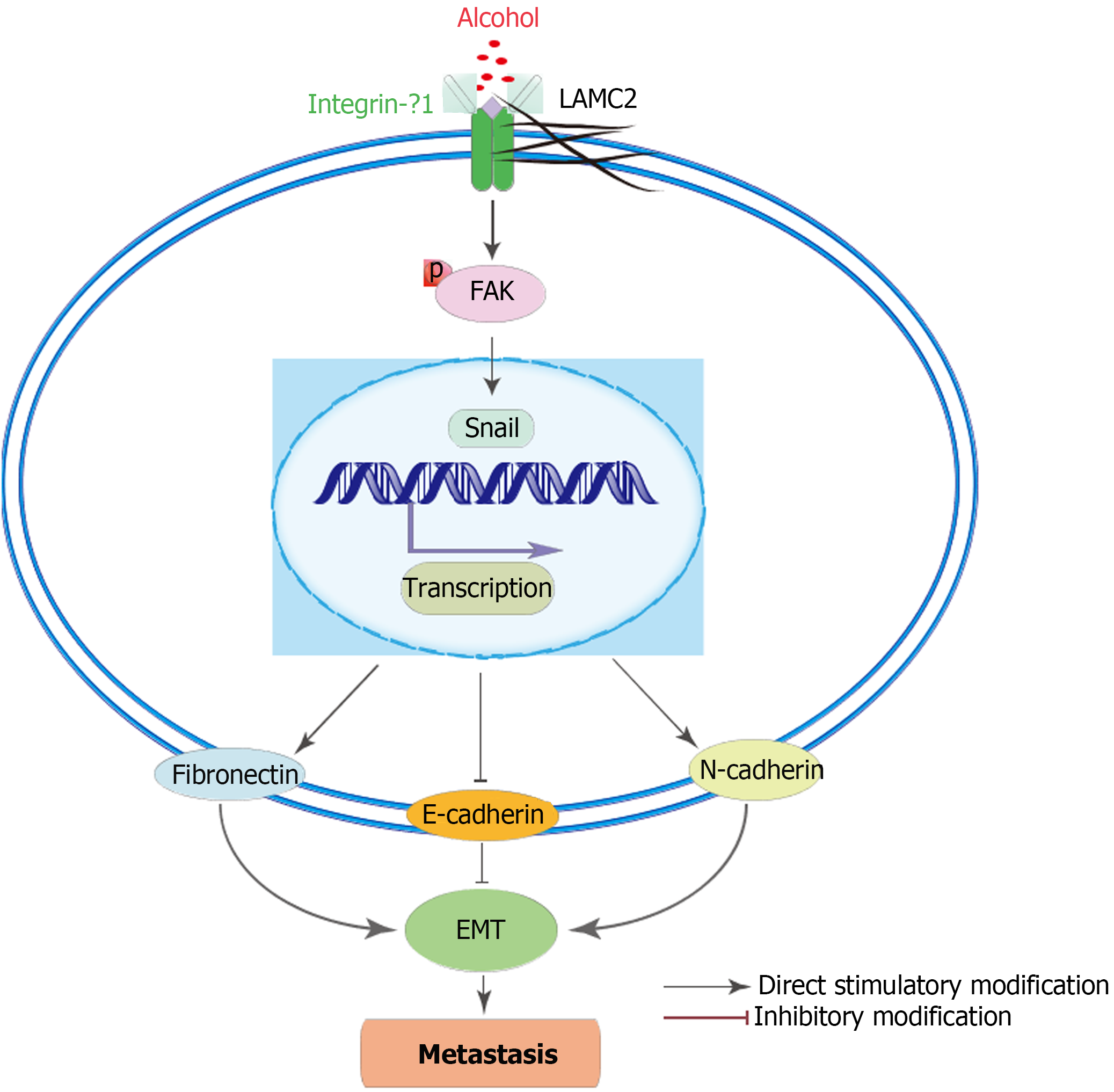
The EMT event is also a key event involving in the formation of the premetastatic niche[32,33], which is almost featuring the loss of E-cadherin expression and overexpression of snail, N-cadherin and fibronectin[56,59]. The results of animal experiments suggested that long-term alcohol consumption (shown in Figure 9), as a risk factor of CRC, may further activate p-FAK by enhancing the interaction between LAMC2 and ITGB1 and forming a premetastatic niche, thus upregulating the expression of snail in precancerous lesions. Thus, the expression levels of N-cadherin and fibronectin were increased and the expression level of E-cadherin was downregulated, leading to the mesenchymal phenotype of cancer cells and colorectal cancer metastasis. This may be one of the reasons for the higher metastasis rate of CRC rats in the alcohol group. We found the similar results in clinical samples. The main limitation of the clinical study is the relatively small sample size. Larger-scale functional studies are required to improve our understanding of the effects of alcohol on the premetastatic niche in CRC. However, the obtained results provide some interesting suggestions for future studies with an increased sample size. Based on the abovementioned results, the present study demonstrated that alcohol may promote the formation of premetastatic niche via enhancing the EMT event mediated by the LAMC2- ITGB1 interaction, leading to the high incidence of CRC metastasis in the alcohol group.
In summary, the present study attempted to examine the nonspecific effect of alcohol on the premetastatic niche of colorectal cancer. Our study suggests that alcohol has an effect on the colorectal carcinogenesis paradigm and may accelerate the malignant metastasis of CRC. The molecular mechanism may involve the effect of alcohol on the early EMT-mediated premetastatic niche of colorectal cancer induced by LAMC2 and ITGB1 interactions.
Colorectal cancer (CRC) is a common malignant tumor.
Alcohol consumption is positively correlated with CRC malignant metastasis; however, the mechanism is unclear.
To investigate the effects of alcohol on CRC metastasis
The interaction between laminin-γ2 (LAMC2) and integrin-β1 (ITGB1) was measured by Duolink assay, and the expression levels of LAMC2, ITGB1 and focal adhesion kinase, snail, fibronectin, N-cadherin and special AT-rich sequence binding protein 1 were measured by quantitative real-time polymerase chain reaction, immunohistochemistry and western blotting. Interleukin-1β (IL-1β), tumor necrosis factor-α and IL-6 levels were measured via enzyme-linked immunosorbent assay, histopathological assessment via hematoxylin eosin staining, and determination of aberrant crypt foci via methylene blue.
Alcohol may promote CRC metastasis by influencing the molecular mechanism of the premetastatic niche.
Our study suggests that alcohol promotes epithelial mesenchymal transformation-mediated premetastatic niche formation of CRC by activating the early interaction between LAMC2 and ITGB1 and lead to CRC metastasis.
To explore the effect of alcohol on colorectal cancer metastasis.
We are very grateful to all the staff of Guangzhou University of Chinese Medicine and The First Affiliated Hospital of Guangxi University of Chinese Medicine for their help in the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Legaz I, Spain; Spataru A, Canada S-Editor: Zhang H L-Editor: A P-Editor: Cai YX
| 1. | Ladabaum U, Dominitz JA, Kahi C, Schoen RE. Strategies for Colorectal Cancer Screening. Gastroenterology. 2020;158:418-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 412] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 2. | Akimoto N, Ugai T, Zhong R, Hamada T, Fujiyoshi K, Giannakis M, Wu K, Cao Y, Ng K, Ogino S. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol. 2021;18:230-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 404] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 3. | Murphy N, Moreno V, Hughes DJ, Vodicka L, Vodicka P, Aglago EK, Gunter MJ, Jenab M. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol Aspects Med. 2019;69:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 180] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 4. | Zheng K, Yu J, Chen Z, Zhou R, Lin C, Zhang Y, Huang Z, Yu L, Zhao L, Wang Q. Ethanol promotes alcohol-related colorectal cancer metastasis via the TGF-β/RUNX3/Snail axis by inducing TGF-β1 upregulation and RUNX3 cytoplasmic mislocalization. EBioMedicine. 2019;50:224-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Mohr AM, Gould JJ, Kubik JL, Talmon GA, Casey CA, Thomas P, Tuma DJ, McVicker BL. Enhanced colorectal cancer metastases in the alcohol-injured liver. Clin Exp Metastasis. 2017;34:171-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Diao XY, Peng T, Kong FG, Huang JG, Han S, Shang YS, Liu H. Alcohol consumption promotes colorectal cancer by altering intestinal permeability. Eur Rev Med Pharmacol Sci. 2020;24:9370-9377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Hosseini H, Obradović MMS, Hoffmann M, Harper KL, Sosa MS, Werner-Klein M, Nanduri LK, Werno C, Ehrl C, Maneck M, Patwary N, Haunschild G, Gužvić M, Reimelt C, Grauvogl M, Eichner N, Weber F, Hartkopf AD, Taran FA, Brucker SY, Fehm T, Rack B, Buchholz S, Spang R, Meister G, Aguirre-Ghiso JA, Klein CA. Early dissemination seeds metastasis in breast cancer. Nature. 2016;540:552-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 521] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 8. | Tsai HW, Yuan CC, Wang PH. Umbilicus as the only site of metastasis in recurrent ovarian cancer. J Chin Med Assoc. 2006;69:233-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Attaran S, Bissell MJ. The role of tumor microenvironment and exosomes in dormancy and relapse. Semin Cancer Biol. 2022;78:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Radin DP, Tsirka SE. Interactions between Tumor Cells, Neurons, and Microglia in the Glioma Microenvironment. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Osipov A, Saung MT, Zheng L, Murphy AG. Small molecule immunomodulation: the tumor microenvironment and overcoming immune escape. J Immunother Cancer. 2019;7:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 12. | Zhang Z, Qiao J, Zhang D, Zhu W, Zhu J, Leng X, Li S. Noncoding RNAs Act as Tumor-Derived Molecular Components in Inducing Premetastatic Niche Formation. Biomed Res Int. 2019;2019:9258075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Han P, Cao P, Hu S, Kong K, Deng Y, Zhao B, Li F. Esophageal Microenvironment: From Precursor Microenvironment to Premetastatic Niche. Cancer Manag Res. 2020;12:5857-5879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Jing B, Wang T, Sun B, Xu J, Xu D, Liao Y, Song H, Guo W, Li K, Hu M, Zhang S, Ling J, Kuang Y, Zhang T, Zhou BP, Yao F, Deng J. IL6/STAT3 Signaling Orchestrates Premetastatic Niche Formation and Immunosuppressive Traits in Lung. Cancer Res. 2020;80:784-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Doglioni G, Parik S, Fendt SM. Interactions in the (Pre)metastatic Niche Support Metastasis Formation. Front Oncol. 2019;9:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 16. | Choi J, Gyamfi J, Jang H, Koo JS. The role of tumor-associated macrophage in breast cancer biology. Histol Histopathol. 2018;33:133-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 113] [Reference Citation Analysis (0)] |
| 17. | Dong Q, Liu X, Cheng K, Sheng J, Kong J, Liu T. Pre-metastatic Niche Formation in Different Organs Induced by Tumor Extracellular Vesicles. Front Cell Dev Biol. 2021;9:733627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 18. | Kim H, Chung H, Kim J, Choi DH, Shin Y, Kang YG, Kim BM, Seo SU, Chung S, Seok SH. Macrophages-Triggered Sequential Remodeling of Endothelium-Interstitial Matrix to Form Pre-Metastatic Niche in Microfluidic Tumor Microenvironment. Adv Sci (Weinh). 2019;6:1900195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 19. | Najafi M, Farhood B, Mortezaee K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J Cell Biochem. 2019;120:2782-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 471] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 20. | Trapani V, Bonaldo P, Corallo D. Role of the ECM in notochord formation, function and disease. J Cell Sci. 2017;130:3203-3211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Mal'tseva DV, Makarova YA, Raigorodskaya MP, Rodin SA. Effects of Laminins 332 and 411 on the Epithelial-Mesenchymal Status of Colorectal Cancer Cells. Bull Exp Biol Med. 2019;166:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Urooj T, Wasim B, Mushtaq S, Shah SNN, Shah M. Cancer Cell-derived Secretory Factors in Breast Cancer-associated Lung Metastasis: Their Mechanism and Future Prospects. Curr Cancer Drug Targets. 2020;20:168-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Maltseva DV, Rodin SA. [Laminins in Metastatic Cancer]. Mol Biol (Mosk). 2018;B: 411-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Chang YC, Wang JD, Chang HY, Zhou P, Hahn RA, Gordon MK, Laskin JD, Gerecke DR. Expression of Laminin γ2 Proteolytic Fragments in Murine Skin Following Exposure to Sulfur Mustard. Anat Rec (Hoboken). 2020;303:1642-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Meireles Da Costa N, Mendes FA, Pontes B, Nasciutti LE, Ribeiro Pinto LF, Palumbo Júnior A. Potential Therapeutic Significance of Laminin in Head and Neck Squamous Carcinomas. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Tohmatsu Y, Imura J, Sakai T, Takagi K, Minamisaka T, Tanaka S, Noguchi A, Nakajima T, Nagata T, Makino T, Shimizu T, Fujii T. Expression of laminin-5 gamma 2 chain predicts invasion of extramammary Paget's disease cell. APMIS. 2021;129:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Huang C, Chen J. Laminin332 mediates proliferation, apoptosis, invasion, migration and epithelialtomesenchymal transition in pancreatic ductal adenocarcinoma. Mol Med Rep. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Wang SH, Liou GG, Liu SH, Chang JS, Hsiao JR, Yen YC, Chen YL, Wu WL, Chang JY, Chen YW. Laminin γ2-enriched extracellular vesicles of oral squamous cell carcinoma cells enhance in vitro lymphangiogenesis via integrin α3-dependent uptake by lymphatic endothelial cells. Int J Cancer. 2019;144:2795-2810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Liu CC, Lin JH, Hsu TW, Hsu JW, Chang JW, Su K, Hsu HS, Hung SC. Collagen XVII/laminin-5 activates epithelial-to-mesenchymal transition and is associated with poor prognosis in lung cancer. Oncotarget. 2018;9:1656-1672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Brenner H, Chen C. The colorectal cancer epidemic: challenges and opportunities for primary, secondary and tertiary prevention. Br J Cancer. 2018;119:785-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 31. | Erin N, Grahovac J, Brozovic A, Efferth T. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist Updat. 2020;53:100715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 347] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 32. | Wang Y, Shi J, Chai K, Ying X, Zhou BP. The Role of Snail in EMT and Tumorigenesis. Curr Cancer Drug Targets. 2013;13:963-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 688] [Article Influence: 62.5] [Reference Citation Analysis (1)] |
| 33. | Wu X, Cai J, Zuo Z, Li J. Collagen facilitates the colorectal cancer stemness and metastasis through an integrin/PI3K/AKT/Snail signaling pathway. Biomed Pharmacother. 2019;114:108708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 34. | Wang Y, Xu M, Ke ZJ, Luo J. Cellular and molecular mechanisms underlying alcohol-induced aggressiveness of breast cancer. Pharmacol Res. 2017;115:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Cho S, Shin A, Park SK, Shin HR, Chang SH, Yoo KY. Alcohol Drinking, Cigarette Smoking and Risk of Colorectal Cancer in the Korean Multi-center Cancer Cohort. J Cancer Prev. 2015;20:147-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Ferretti S, Patriarca S, Carbone A, Zanetti R. [TNM classification of malignant tumours, VII edition 2009. Changes and practical effects on cancer epidemiology]. Epidemiol Prev. 2010;34:125-128. [PubMed] |
| 37. | National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press (US); 2011. [PubMed] |
| 38. | Martin B, Schäfer E, Jakubowicz E, Mayr P, Ihringer R, Anthuber M, Schenkirsch G, Schaller T, Märkl B. Interobserver variability in the H&E-based assessment of tumor budding in pT3/4 colon cancer: does it affect the prognostic relevance? Virchows Arch. 2018;473:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Bird RP, Lafave LM. Varying effect of dietary lipids and azoxymethane on early stages of colon carcinogenesis: enumeration of aberrant crypt foci and proliferative indices. Cancer Detect Prev. 1995;19:308-315. [PubMed] |
| 40. | Sivaranjani A, Sivagami G, Nalini N. Chemopreventive effect of carvacrol on 1,2-dimethylhydrazine induced experimental colon carcinogenesis. J Cancer Res Ther. 2016;12:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Mansour DF, Abdallah HMI, Ibrahim BMM, Hegazy RR, Esmail RSE, Abdel-Salam LO. The Carcinogenic Agent Diethylnitrosamine Induces Early Oxidative Stress, Inflammation and Proliferation in Rat Liver, Stomach and Colon: Protective Effect of Ginger Extract. Asian Pac J Cancer Prev. 2019;20:2551-2561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 42. | Huang G, Bao J, Shao X, Zhou W, Wu B, Ni Z, Wang L. Inhibiting pannexin-1 alleviates sepsis-induced acute kidney injury via decreasing NLRP3 inflammasome activation and cell apoptosis. Life Sci. 2020;254:117791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 43. | Bai N, Zhang Q, Zhang W, Liu B, Yang F, Brann D, Wang R. G-protein-coupled estrogen receptor activation upregulates interleukin-1 receptor antagonist in the hippocampus after global cerebral ischemia: implications for neuronal self-defense. J Neuroinflammation. 2020;17:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 44. | Suarez-Carmona M, Lesage J, Cataldo D, Gilles C. EMT and inflammation: inseparable actors of cancer progression. Mol Oncol. 2017;11:805-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 420] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 45. | Coupland LA, Parish CR. Platelets, selectins, and the control of tumor metastasis. Semin Oncol. 2014;41:422-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Høye AM, Erler JT. Structural ECM components in the premetastatic and metastatic niche. Am J Physiol Cell Physiol. 2016;310:C955-C967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 47. | Zhuyan J, Chen M, Zhu T, Bao X, Zhen T, Xing K, Wang Q, Zhu S. Critical steps to tumor metastasis: alterations of tumor microenvironment and extracellular matrix in the formation of pre-metastatic and metastatic niche. Cell Biosci. 2020;10:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 48. | Guo Y, Ji X, Liu J, Fan D, Zhou Q, Chen C, Wang W, Wang G, Wang H, Yuan W, Ji Z, Sun Z. Effects of exosomes on pre-metastatic niche formation in tumors. Mol Cancer. 2019;18:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 315] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 49. | Wang Y, Shi M, Yang N, Zhou X, Xu L. GPR115 Contributes to Lung Adenocarcinoma Metastasis Associated With LAMC2 and Predicts a Poor Prognosis. Front Oncol. 2020;10:577530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Xu L, Hou Y, Tu G, Chen Y, Du YE, Zhang H, Wen S, Tang X, Yin J, Lang L, Sun K, Yang G, Liu M. Nuclear Drosha enhances cell invasion via an EGFR-ERK1/2-MMP7 signaling pathway induced by dysregulated miRNA-622/197 and their targets LAMC2 and CD82 in gastric cancer. Cell Death Dis. 2017;8:e2642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 51. | Moon YW, Rao G, Kim JJ, Shim HS, Park KS, An SS, Kim B, Steeg PS, Sarfaraz S, Changwoo Lee L, Voeller D, Choi EY, Luo J, Palmieri D, Chung HC, Kim JH, Wang Y, Giaccone G. LAMC2 enhances the metastatic potential of lung adenocarcinoma. Cell Death Differ. 2015;22:1341-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 52. | Cavaco ACM, Eble JA. A 3D Spheroid Model as a More Physiological System for Cancer-Associated Fibroblasts Differentiation and Invasion In Vitro Studies. J Vis Exp. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 53. | Wang G, Yang Q, Li M, Zhang Y, Cai Y, Liang X, Fu Y, Xiao Z, Zhou M, Xie Z, Huang H, Huang Y, Chen Y, He Q, Peng F, Chen Z. Quantitative proteomic profiling of tumor-associated vascular endothelial cells in colorectal cancer. Biol Open. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Pavan Kumar P, Purbey PK, Sinha CK, Notani D, Limaye A, Jayani RS, Galande S. Phosphorylation of SATB1, a global gene regulator, acts as a molecular switch regulating its transcriptional activity in vivo. Mol Cell. 2006;22:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 55. | Pal M, Bhattacharya S, Kalyan G, Hazra S. Cadherin profiling for therapeutic interventions in Epithelial Mesenchymal Transition (EMT) and tumorigenesis. Exp Cell Res. 2018;368:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 56. | Schmid MC, Avraamides CJ, Foubert P, Shaked Y, Kang SW, Kerbel RS, Varner JA. Combined blockade of integrin-α4β1 plus cytokines SDF-1α or IL-1β potently inhibits tumor inflammation and growth. Cancer Res. 2011;71:6965-6975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 57. | Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18:773-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 744] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 58. | Deng Y, Chakraborty P, Jolly MK, Levine H. A Theoretical Approach to Coupling the Epithelial-Mesenchymal Transition (EMT) to Extracellular Matrix (ECM) Stiffness via LOXL2. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 59. | Noronha C, Ribeiro AS, Taipa R, Castro DS, Reis J, Faria C, Paredes J. Cadherin Expression and EMT: A Focus on Gliomas. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |