Published online Sep 14, 2022. doi: 10.3748/wjg.v28.i34.5076
Peer-review started: March 7, 2022
First decision: April 5, 2022
Revised: April 19, 2022
Accepted: August 6, 2022
Article in press: August 6, 2022
Published online: September 14, 2022
Processing time: 183 Days and 22.5 Hours
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract. As most of them harbor a KIT mutation (75%), selective kinase inhibitors are the therapeutic option and show a sustained objective response among patients with metastatic or unresectable GISTs. A well-known higher risk of neoplasm has been described among renal transplant recipients (RTRs). Nevertheless, only few cases of GIST onset among transplant patients have been reported in the literature.
Here, we describe 2 cases of gastric GIST occurring during the follow-up of RTRs. We also review the existing literature concerning GIST occurrence in transplant patients. In total and in association with our 2 cases, 16 patients have been reported. The median age was 59.5 years and 69% were male. With a median tumor size of 45 mm, no patient displayed metastatic dissemination at diagnosis. Time from transplantation to diagnosis was highly variable between 5 mo and 21 years. Histopathological data mostly revealed high risk of progression (43%). Death increased to 29% during follow-up. Surgical treatment was systematically performed when the tumor was operable (94%). The use of adjuvant therapy was uncommon (19%).
GISTs represent rare but potentially severe malignant complication among transplant patients.
Core Tip: Although a well-known higher risk of neoplasm has been described among renal transplant recipients (RTRs), few cases of gastrointestinal stromal tumors (GISTs) have been reported. We describe 2 cases of gastric GIST among RTRs and provide a review of the literature. We report 16 patients with a median age of 59.5 years, and 69% were male. No patient displayed metastasis at diagnosis. Time from transplantation to diagnosis varied between 5 mo and 21 years. Histopathology revealed high risk of progression (43%). Death increased to 29%. Surgical treatment was commonly performed (94%). The use of adjuvant therapy was uncommon (19%).
- Citation: Stammler R, Anglicheau D, Landi B, Meatchi T, Ragot E, Thervet E, Lazareth H. Gastrointestinal tumors in transplantation: Two case reports and review of literature . World J Gastroenterol 2022; 28(34): 5076-5085
- URL: https://www.wjgnet.com/1007-9327/full/v28/i34/5076.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i34.5076
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal (GI) tract[1]. GISTs arise from interstitial cells of Cajal (ICC), which are specialized mesenchymal cells located within the muscle of the GI tract. ICC play a critical role in regulating smooth muscle function and GI tract motility[2]. GISTs are mainly located in the stomach (55%) or the small bowel (30%). About 10% to 47% of patients have metastatic disease at diagnosis[3-5]. About 95% of GISTs display positive staining for the receptor tyrosine kinase KIT (or CD117), 75% of these tumors harbor a KIT gene mutation and 10% a platelet-derived growth factor receptor A (PDGFRA) gene mutation[6]. Among KIT-negative GISTs, immunohistochemical expression of discovered on GIST-1 (DOG-1) was found in 76% of the cases[7]. Consequently, selective tyrosine kinase inhibitors targeting KIT receptor have been used. The first one, imatinib mesylate (Gleevec®; Novartis, Basel, Switzerland), has shown a sustained objective response in a phase III trial among patients with metastatic or unresectable GISTs in immunocompetent patients[8].
In renal transplant recipients (RTRs), an increased risk of cancer has been reported especially for non-melanoma skin cancer, virus-associated cancer and lymphoproliferative disorders[9]. Currently, malignancy represents a major cause of mortality among RTRs[10]. Nonetheless, only few cases of GIST have been reported among transplant patients. Overall, 8 cases of GIST[11-17] and 2 cases of extra GIST (EGIST)[14,18] have previously been reported in RTRs and respectively 3 cases[19-21] and 1 case[22] in liver transplant recipients.
We report 2 cases of GIST occurring in RTRs and provide a review of the existing literature concerning GIST occurrence in transplant patients.
Case 1: A 60-year-old Caucasian man without any symptoms.
Case 2: A 56-year-old Caucasian man presented with upper GI hemorrhage.
Case 1: Hepatic magnetic resonance imaging (MRI) was performed to explore abnormal hepatic tests. MRI revealed a 32 mm spherical tumor of the lesser curvature of the stomach.
Case 2: The upper GI hemorrhage led to gastric endoscopy, which revealed a spherical gastric tumor in the fundus.
Case 1: He had end-stage renal disease with a kidney biopsy compatible with nephronophthisis despite negative screening for mutation in hepatocyte nuclear factor 1 beta (HNF1B) gene. Hemodialysis was initiated in 2016. In October 2019, he received a kidney transplant from a deceased donor. The initial immunosuppressive therapy combined basiliximab, steroids, tacrolimus, and everolimus. Renal function at hospital discharge was 94 µmol/L, (normal range 53 µmol/L to 97 µmol/L). Initial maintenance immunosuppressive therapy associated steroids, tacrolimus, and everolimus. Due to relapsing lymphocele, everolimus was switched to mycophenolate mofetil (MMF). Moreover, a pre-existing mild cytolysis and cholestasis worsened after transplantation leading to the discontinuation of cotrimoxazole and MMF, which were replaced by atovaquone and belatacept (NULOJIX®; Bristol-Myers Squibb, New York, NY, United States), respectively.
Case 2: The patient developed end-stage renal disease of unknown origin. He received a kidney transplantation from a deceased donor. Due to preformed donor specific antibodies (anti-Cw15, mean fluorescence intensity of 6130) on the day of transplantation, induction immunosuppressive therapy combined basiliximab, steroids, MMF, cyclosporine, and intravenous immunoglobulins. At 10 d after surgery, a kidney biopsy was performed due to delayed graft function. It revealed acute tubular necrosis associated with possible acute humoral rejection (g1 cpt0 v0 i0 t0 according to Banff’s classification[23,24], C4d immunostaining was negative). A treatment with high dose steroids, five plasma exchanges and rituximab[25] was initiated allowing improvement of renal function with a nadir in serum creatinine level of 170 µmol/L. Maintenance immunosuppressive therapy included steroids, cyclosporine, and MMF.
Case 1: His other past medical history consisted in nonalcoholic steatohepatitis, Hashimoto’s thyroiditis, and hypertension.
Case 2: The patient had no significant personal or family history.
Case 1: On admission, physical examination was unremarkable.
Case 2: Physical examination was unremarkable except for hematemesis.
Case 1: The patient had mild cytolysis and cholestasis without any other biological abnormality.
Case 2: No abnormal blood test was noticed on admission.
Case 1: Body computed tomography (CT) scan confirmed the absence of metastatic dissemination.
Case 2: Body CT scan was consistent with local tumor without metastatic localizations.
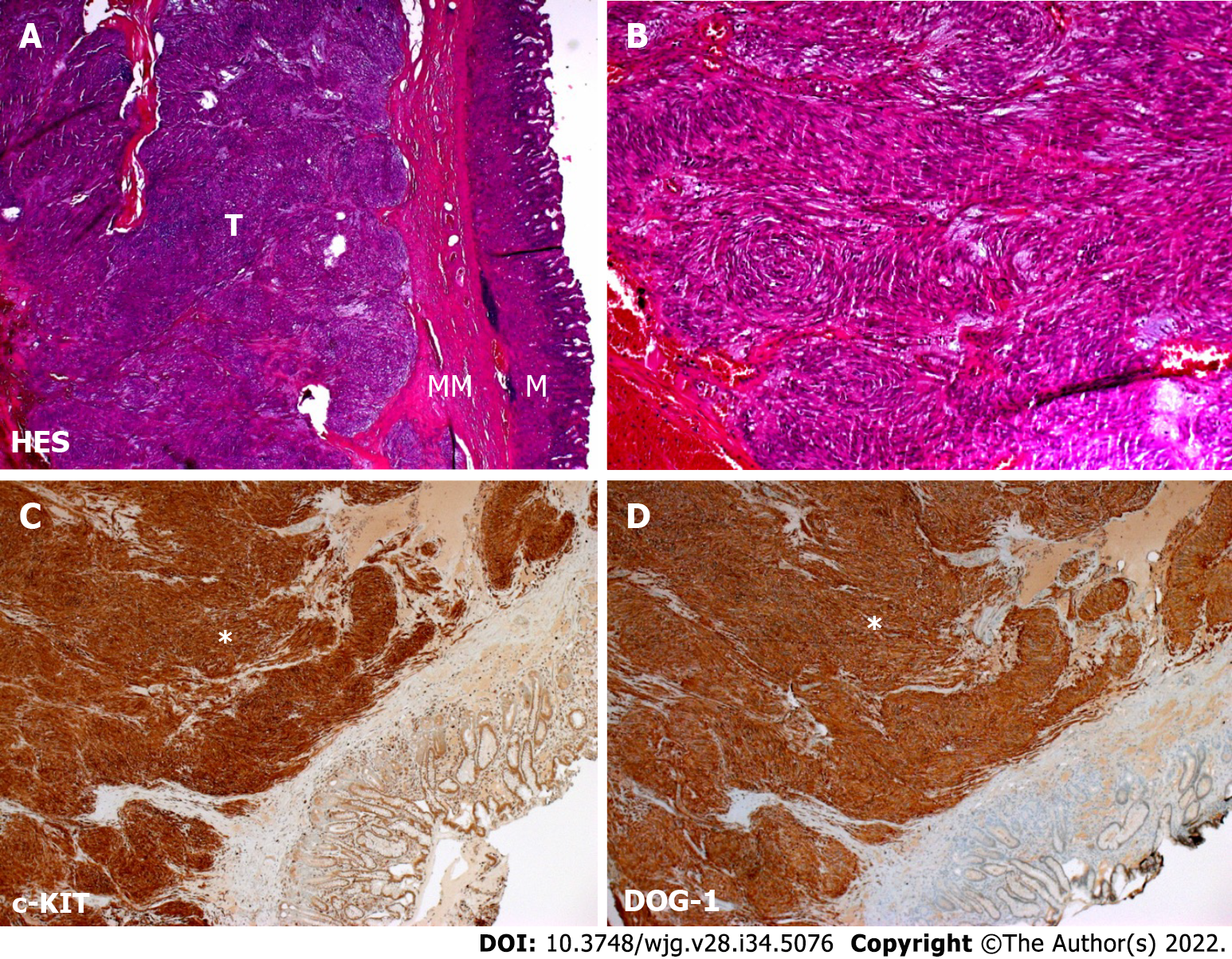
Case 1: Upper GI endoscopy found a 3 cm submucosal tumor of the lesser curvature of the stomach. Tumor biopsies were performed using endoscopic ultrasound guidance. Cytological examination revealed spindle-shaped cells that showed positive staining for c-KIT and DOG-1 in immunohistochemistry, confirming the diagnosis of GIST (Figure 1).
Case 2: The gastric endoscopy revealed a spherical gastric tumor in the fundus with a typical macroscopic aspect of GIST.
Case 1: Partial gastrectomy was performed without complication.
Case 2: Partial gastrectomy was performed.
Case 1: No complication associated with the GIST of its treatment was noticed.
Case 2: The patient was rapidly discharged after partial gastrectomy without complication.
Histopathology revealed a 27 mm stromal tumor strongly positive for KIT and moderately positive for DOG-1 with a mitotic count of 2 mitosis for 5 mm2. The tumor harbored an exon 11 (p. Val559Ala c.1676T>C) KIT mutation[23].
Histopathology report described a 51 mm GIST strongly positive for KIT harboring a mitotic count of 10 mitosis for 5 mm2. Of note, an exon 18 D842V PDGFRA mutation was identified.
Regarding the very low risk of progression, no adjuvant therapy was initiated.
No adjuvant treatment was initiated at the time of diagnosis.
The patient remains in remission at the 1-year follow-up.
Two years later, a follow-up MRI revealed hepatic vascular nodules compatible with metastatic lesions. Treatment with imatinib mesylate was initiated. In the absence of a tumor response, imatinib was discontinued 4 mo later and sunitinib (SUTENT©; Bayer, Germany), an anti-angiogenic multikinase inhibitor (anti vascular endothelial growth factor-1, -2, -3, PDGFR-α,-β, c-KIT, fms-like tyrosine kinase 3, and RET) was introduced. Five months later, the onset of thrombopenia, neutropenia, and hepatic cytolysis led to replacement of sunitinib with regorafenib (STIVARGA©; Bayer Pharma AG, Germany), another multikinase inhibitor. Due to side effects and tumor progression, regorafenib was discontinued and dasatinib (SPRYCEL©; Bristol-Myers Squibb, New York, NY, United States) was introduced. Disease progression finally led to stopping all therapies in April 2019. Selective transarterial embolization was performed complicated with artery dissection of the kidney transplant requiring stent implantation. The patient was finally admitted with a clinical presentation of hydrops concomitant with acute renal injury and peritoneal carcinosis. The patient eventually died due to disease progression.
GISTs represent an uncommon malignant complication of immunosuppression state in solid organ transplantation. We describe 2 cases of typical GIST occurring early in the course of kidney transplantation. The first patient developed an isolated gastric GIST 5 mo after transplantation and the second 4 years after. Both were nonmetastatic at diagnosis although the second patient developed multiple hepatic metastasis 2 years after complete tumor resection. Of note, the mutation of PDGFRA D842V in the second case was associated with resistance to imatinib mesylate.
We looked for previously reported cases of GIST in the literature during the course of transplantation. We searched PubMed and Web of Science databases using the following Medical Subject Headings words: “Gastrointestinal stromal tumors” AND “Kidney transplantation” or “Gastrointestinal stromal tumors” AND “Transplantation.” Using these terms, we found 8 and 31 articles, respectively. Only 12 articles were analyzed. From 2007 to 2020, 14 cases of GIST have been reported in transplant recipients[11-22]. We excluded reports of GIST occurring among nontransplant or bone marrow transplant patients. We also excluded article types different than case reports or case series.
Table 1 summarizes the main features of these patients including the 2 cases described in the present manuscript. Tables 2 and 3 give details on the 14 cases reported. In our literature review, the typical patient profile was a male patient with a median age of 59.5-years-old, who developed large nonmetastatic gastric tumors (median size, 45 mm). The delay between transplantation and diagnosis was highly variable, ranging between 5 mo and 21 years. Histopathological data mostly revealed high risk of progression (42.8%) and death occurred in 29% of the cases during follow-up. Surgical treatment was systematically performed if the tumor features were suitable (94%). The use of adjuvant therapy was uncommon (19%).
| Overall number | ||
| Male sex, n (%) | 16 | 11 (69) |
| Age (yr), median (min; max) | 16 | 59.5 (23; 74) |
| Type of organ transplantation, n (%) | 16 | |
| Kidney | 12 (75) | |
| Liver | 4 (25) | |
| Location of primitive tumor, n (%) | 16 | |
| Stomach | 9 (56) | |
| Small bowel | 3 (19) | |
| Colon | 1 (6) | |
| Other: pelvis, perineum, mesentery | 3 (19) | |
| Time from transplantation to diagnosis (mo), median (min; max) | 16 | 32 (5; 252) |
| Metastatic dissemination at diagnosis, n (%) | 16 | 0 (0) |
| Tumor size (mm), median (min; max) | 15 | 45 (10; 230) |
| Risk of progression according to Joensuu’s criteria, n (%) | 14 | |
| Very low | 2 (14) | |
| Low | 4 (29) | |
| Intermediate | 2 (14) | |
| High | 6 (43) | |
| Surgical treatment, n (%) | 16 | 15 (94) |
| Adjuvant treatment, n (%) | 16 | 3 (19) |
| Modification of immunosuppression, n (%) | 11 | 9 (82) |
| Death during follow-up, n (%) | 14 | 4 (29) |
| Ref. | Age (yr)/sex | Transplanted organ | Time from transplantation to diagnosis | Location of primitive GIST | Metastasis at diagnosis | Evolution/delay | Immunosuppression before diagnosis | Immunosuppression after diagnosis | |
| Agaimy and Wünsch[11] | 59/F | Kidney | 40 mo | Stomach | No | Relapse 68 mo | Not described | Not described | |
| Agaimy and Wünsch[11] | 58/F | Kidney | 96 mo | Small bowel | No | Not described | Not described | Not described | |
| Saidi et al[19] | 54/M | Liver | 11 mo | Colon | No | Not described | Tac, Aza | Not described | |
| Camargo et al[22] | 64/M | Liver | 7 mo | Perineum | No | Not described | Tac, mycophenolate sodium | Not described | |
| Tu et al[18] | 57/F | Kidney | 6 mo | Pelvis | No | Not described | Steroids, CsA, MMF | CsA and MMF at half dosage; rapamycin-containing regimensteroids withdrawn | |
| Mulder et al[12] | 72/M | Kidney | 21 yr | Stomach | No | Peritoneal metastasis/24 mo | Steroids, CsA | Steroids, CsA (60% reduction in dosage) | |
| Mrzljak et al[20] | 53/M | Liver | 12 mo | Jejunum | No | No | Tac, MMF | Same | |
| Cimen et al[13] | 46/F | Kidney | 18 yr | Stomach | No | Not described | Steroids, CsA, Aza | Same with reduced dosage of CsA | |
| Cheung et al[14] | 64/M | Kidney | 2 yr | Stomach | No | Yes/2 yr | Steroids, Tac, MMF | Steroids, Tac (reduced dosage), everolimus | |
| Cheung et al[14] | 48/M | Kidney | 1 yr | Mesentery | Multiple tumors | No | CsA, MMF | CsA withdrawal, sirolimus introduction | |
| Patiño et al[15] | 23/F | Kidney | 13 yr | Stomach | No | Local relapse/3 yr | Steroids, Tac, MMF | Not described | |
| Xie et al[21] | 60/M | Liver | 11 mo | Stomach | No | No | Tac, sirolimus, MMF | Same | |
| Elkabets et al[17] | 74/M | Kidney | 7 yr | Stomach | No | No | Steroids, CsA, MMF | Switch CsA to mTOR inhibitor | |
| Takahashi et al[16] | 64/M | Kidney | 72 mo | Small bowel | No | No | Steroids, CsA, MMF | Stop CsA | |
| Stammler et al | 60/M | Kidney | 5 mo | Stomach | No | No | Steroids, Tac, MMF | Switch MMF to belatacept | |
| Stammler et al | 64/M | Kidney | 51 mo | Stomach | No | Yes/23 mo | Steroids, CsA, MMF | Switch CsA to Tac | |
| Ref. | Size (mm) | Mitotic count | Fletcher’s criteria | Joensuu’s criteria | Mutation identified | Resection | Initial adjuvant treatment | Second line treatment | Outcome |
| Agaimy and Wünsch[11] | 35 | < 5/50 | Low | Low | Not described | Yes | No | Not described | Alive and relapse free at 68 mo |
| Agaimy and Wünsch[11] | 230 | 14/50 | High | High | Not described | Yes | No | Not described | Not described |
| Saidi et al[19] | 25 | 1/50 | Low | High | Not described | Yes | No | Not described | Alive and relapse free at 18 mo |
| Camargo et al[22] | 50 | 5/50 | Intermediate | High | Not described | Yes | No | Not described | Alive and relapse free at 20 mo |
| Tu et al[18] | 45 | 2-3/50 | Low | Low | PDGFRA exon 18 V824V | Yes | No | Not described | Alive and relapse free 24 mo |
| Mulder et al[12] | 50 | > 10/50 | High | High | Not described | Yes | No | Imatinib 400 mg/d then 200 mg/d | Died 44 mo |
| Mrzljak et al[20] | 10 | 1/50 | Very low | Very low | Not described | Yes | No | No | Died 3 yr after from acute leukemia |
| Cimen et al[13] | 150 | 14/50 | High | High | KIT T574del | Yes | Imatinib 400 mg/d | Not described | Alive and relapse free at 12 mo |
| Cheung et al[14] | 30 | 9/50 | Intermediate | Intermediate | Not described | Yes | No | No | Died from pneumonia at 2 yr |
| Cheung et al[14] | Not described | Not described | Not described | Not described | Not described | No | Imatinib 400 mg/d for 1 yr | Switch CsA to sirolimus | Alive and relapse free at 10 yr |
| Patiño et al[15] | 58 | Not described | Intermediate or high | Intermediate of high | Not described | Yes | No | Imatinib 400 mg/d | Alive and relapse free/5 yr after imatinib initiation |
| Xie et al[21] | 10 | < 5/50 | Very low | Very low | KIT exon 11 | Yes | No | No | Not described |
| Elkabets et al[17] | 31 | Not described | Not described | Not described | Not described | Yes | No | No | Alive and relapse free at 40 mo |
| Takahashi et al[16] | 110 | 20/50 | High | High | KIT exon 11 | Yes | Imatinib 400 mg/d reduced to 3000 mg/d | No | Alive and relapse free at 18 mo |
| Stammler et al | 27 | 2/5 | Low | Low | KIT exon 11 | Yes | No | No | Alive and relapse free at 2 mo |
| Stammler et al | 51 | 10/50 | High | High | PDGFRA exon 18 | Yes | No | Sunitinib then regorafenib then dasatinib | Died 56 mo later |
Several prognostic classifications have been used to evaluate the risk of recurrence of GIST after surgery. In 2002, Fletcher et al[26] claimed size of the tumor and mitotic count, Miettinen and Lasota[27] in 2006 added tumor location and Joensuu et al[28] in 2012 adjoined rupture of the tumoral capsule and male gender. Heinrich et al[29] demonstrated that PDGFRA and c-KIT were mutually exclusive proto-oncogenic mutations with similar biologicals consequences, even if associated with different prognostics. Molecular predictors of response to imatinib have been widely studied. Underlying KIT or PDGFRA mutations are the strongest predictors of imatinib sensitivity[30]. Mutations directly located in the PDGFRA binding site of imatinib or inducing variations in tridimensional conformation of the tyrosine kinase receptor and subsequently hiding the binding site, may explain inefficacy of therapy. For instance, KIT exon 9 mutation is less sensitive to imatinib and PDFGRA exon 18 D842V mutations is associated with imatinib resistance. Nevertheless, these mutations have been correlated with opposite courses of the disease, indolent for PDFGRA exon 18 D842V mutation but aggressive for KIT exon 9 mutation[31]. These data should highlight the importance of molecular biomarkers to evaluate prognosis of GIST or EGIST at diagnosis.
Guidelines for the diagnosis, treatment, and follow-up of GIST have recently been published[32]. Management of local or locoregional disease should always aim for complete resection whenever possible. Otherwise, neoadjuvant treatment with imatinib for 6 to 12 mo should be used in case of sensitive mutation with an overall response rate of 50%[30]. Moreover, high-risk patients, as previously described, should receive adjuvant imatinib for a duration of 3 years[33]. Imatinib remains the first-line therapy for metastatic GIST. Several other targeted therapies such as sunitinib or regorafenib have emerged as second- or third-line treatment, and more recently avapritinib and ripretinib. Several biomarkers, such as KIT or PDGFRA mutations, are used as predictive factors for tumoral response to refine therapeutic strategies[32]. Data are missing concerning the level of tyrosine kinase inhibitors’ efficacy in transplanted patients.
Data about the management of immunosuppressive therapy after the diagnosis of GIST are scarce. As both imatinib mesylate and cyclosporin are extensively metabolized by cytochrome P450 3A4, interaction occurrence has been documented[12]. Reduction in the dosage of cyclosporin should be performed if this treatment is maintained. Mammalian target of rapamycin inhibitors (mTORis) have shown antiproliferative properties among transplant patients. Schöffski et al[34] highlighted the potential efficacy of association of everolimus and imatinib in imatinib-resistant GIST in a phase II trial. Cheung et al[14] reported a case of complete tumoral response with sirolimus in a transplant patient with imatinib-resistant GIST. Among the patients described in Tables 1 and 2, mTORis have been initiated or switched in 4 of them. Three of them were alive and relapse-free at last follow-up and the last patient died from pneumonia 2 years after GIST diagnosis.
This study had several limitations. First, the retrospective analysis of GIST cases impairs the reliability of the data. Very few cases of GIST occurring after solid organ transplantation have been described in the last 15 years reducing the significance of this literature review. Moreover, it was unclear if GIST was a de novo feature in our first patient because of the short delay (5 mo) between transplantation and tumor discovery. Unfortunately, the latest available CT scan was performed 7 years before the transplantation. However, some previous cases reported GIST onset within the 1st year following transplantation[14,18-22].
To conclude, GISTs represent rare but potentially severe malignant complication among transplant patients. Further analysis of prognosis value of new biomarkers should improve therapeutic strategies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Harouachi A, Morocco; Karki S, Nepal; Shuang WB, China S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP
| 1. | Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 441] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 2. | Sanders KM, Kito Y, Hwang SJ, Ward SM. Regulation of Gastrointestinal Smooth Muscle Function by Interstitial Cells. Physiology (Bethesda). 2016;31:316-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 520] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 4. | DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1797] [Cited by in RCA: 1682] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 5. | Emile JF, Brahimi S, Coindre JM, Bringuier PP, Monges G, Samb P, Doucet L, Hostein I, Landi B, Buisine MP, Neuville A, Bouché O, Cervera P, Pretet JL, Tisserand J, Gauthier A, Le Cesne A, Sabourin JC, Scoazec JY, Bonvalot S, Corless CL, Heinrich MC, Blay JY, Aegerter P. Frequencies of KIT and PDGFRA mutations in the MolecGIST prospective population-based study differ from those of advanced GISTs. Med Oncol. 2012;29:1765-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:865-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 623] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 7. | Lopes LF, West RB, Bacchi LM, van de Rijn M, Bacchi CE. DOG1 for the diagnosis of gastrointestinal stromal tumor (GIST): Comparison between 2 different antibodies. Appl Immunohistochem Mol Morphol. 2010;18:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CD, Joensuu H. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3203] [Cited by in RCA: 3110] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 9. | Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823-2831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 840] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 10. | Awan AA, Niu J, Pan JS, Erickson KF, Mandayam S, Winkelmayer WC, Navaneethan SD, Ramanathan V. Trends in the Causes of Death among Kidney Transplant Recipients in the United States (1996-2014). Am J Nephrol. 2018;48:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 11. | Agaimy A, Wünsch PH. Gastrointestinal stromal tumours (GIST) in kidney transplant recipients--a report of two cases. Nephrol Dial Transplant. 2007;22:1489-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Mulder KE, Egorin MJ, Sawyer MB. Renal dysfunction in a renal transplant patient treated concurrently with cyclosporine and imatinib. Invest New Drugs. 2012;30:2400-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Cimen S, Guler S, Panek R, Alwayn I. Gastrointestinal stromal tumour in a recipient with kidney transplantation. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Cheung CY, Lo SH, Chan CK, Li FK, Cheng IK, Chau KF. Gastrointestinal stromal tumors in kidney transplant recipients: Report of two cases and literature review. Asia Pac J Clin Oncol. 2017;13:104-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Seculini Patiño CE, Tabares AH, Laborie MV, Diller A. [Gastrointestinal stromal tumor and renal transplant]. Medicina (B Aires). 2017;77:334-336. [PubMed] [DOI] [Full Text] |
| 16. | Takahashi R, Shinoda K, Ishida T, Hamamoto Y, Morita S, Akita H, Kitaoka S, Tamaki S, Asanuma H, Yoshida T, Jinzaki M, Kameyama K, Oya M. Small intestinal perforation due to a huge gastrointestinal stromal tumor in a kidney transplant recipient: a case report and literature review. BMC Nephrol. 2019;20:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Erratum for the Research Article: "mTORC1 Inhibition Is Required for Sensitivity to PI3K p110α Inhibitors in PIK3CA-Mutant Breast Cancer" by M. Elkabets, S. Vora, D. Juric, N. Morse, M. Mino-Kenudson, T. Muranen, J. Tao, A. B. Campos, J. Rodon, Y. H. Ibrahim, V. Serra, V. Rodrik-Outmezguine, S. Hazra, S. Singh, P. Kim, C. Quadt, M. Liu, A. Huang, N. Rosen, J. A. Engelman, M. Scaltriti, J. Baselga. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Tu H, Li Q, Cai J, Chen Z, Yang H, Jiang H, Mao Y, Shou Z, Chen J. Extragastrointestinal stromal tumor in a kidney transplant recipient. Clin Exp Nephrol. 2012;16:350-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Saidi RF, Sepehr A, Cosimi AB, Hertl M. Gastrointestinal stromal tumor in a liver transplant recipient. Transplantation. 2008;85:1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Mrzljak A, Košuta I, Škrtić A, Kardum-Skelin I, Vrhovac R. Metachronous gastrointestinal stromal tumor and acute leukemia after liver transplantation for cholangiocellular carcinoma: is there a link? Case Rep Oncol. 2013;6:163-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Xie M, Rao W, Zhang P, Zhao Q, Tian Z. Endoscopic full-thickness resection for a gastrointestinal stromal tumor in a liver transplant recipient: A case report. Medicine (Baltimore). 2019;98:e16669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Camargo MA, Boin I, Mainnardi JP, de Lourdes M, Ayrizono S, Coy CS, Leonardi MI, Meirelles L, Leonardi LS, Escanhoela CA. Extragastrointestinal stromal tumor and liver transplantation: case report and review. Transplant Proc. 2008;40:3781-3783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Pai T, Bal M, Shetty O, Gurav M, Ostwal V, Ramaswamy A, Ramadwar M, Desai S. Unraveling the spectrum of KIT mutations in gastrointestinal stromal tumors: An Indian Tertiary Cancer Center Experience. South Asian J Cancer. 2017;6:113-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Roufosse C, Simmonds N, Clahsen-van Groningen M, Haas M, Henriksen KJ, Horsfield C, Loupy A, Mengel M, Perkowska-Ptasińska A, Rabant M, Racusen LC, Solez K, Becker JU. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation. 2018;102:1795-1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 506] [Cited by in RCA: 544] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 25. | Roberts DM, Jiang SH, Chadban SJ. The treatment of acute antibody-mediated rejection in kidney transplant recipients-a systematic review. Transplantation. 2012;94:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 26. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 27. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1304] [Article Influence: 72.4] [Reference Citation Analysis (33)] |
| 28. | Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, Bordoni A, Magnusson MK, Linke Z, Sufliarsky J, Federico M, Jonasson JG, Dei Tos AP, Rutkowski P. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 671] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 29. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1722] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 30. | Kee D, Zalcberg JR. Current and emerging strategies for the management of imatinib-refractory advanced gastrointestinal stromal tumors. Ther Adv Med Oncol. 2012;4:255-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Palomba G, Paliogiannis P, Sini MC, Colombino M, Casula M, Manca A, Pisano M, Sotgiu G, Doneddu V, Palmieri G, Cossu A. KIT and PDGFRa mutational patterns in Sardinian patients with gastrointestinal stromal tumors. Eur J Cancer Prev. 2021;30:53-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A, Haas RL, Hassan B, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kasper B, Kopeckova K, Krákorová DA, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Pantaleo MA, Piana R, Picci P, Piperno-Neumann S, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Sundby Hall K, Unk M, Van Coevorden F, van der Graaf WTA, Whelan J, Wardelmann E, Zaikova O, Blay JY; ESMO Guidelines Committee and EURACAN. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv68-iv78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 289] [Article Influence: 41.3] [Reference Citation Analysis (1)] |
| 33. | Joensuu H, Eriksson M, Sundby Hall K, Reichardt A, Hartmann JT, Pink D, Ramadori G, Hohenberger P, Al-Batran SE, Schlemmer M, Bauer S, Wardelmann E, Nilsson B, Sihto H, Bono P, Kallio R, Junnila J, Alvegård T, Reichardt P. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. J Clin Oncol. 2016;34:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 34. | Schöffski P, Reichardt P, Blay JY, Dumez H, Morgan JA, Ray-Coquard I, Hollaender N, Jappe A, Demetri GD. A phase I-II study of everolimus (RAD001) in combination with imatinib in patients with imatinib-resistant gastrointestinal stromal tumors. Ann Oncol. 2010;21:1990-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |