Published online Jan 14, 2022. doi: 10.3748/wjg.v28.i2.176
Peer-review started: March 18, 2021
First decision: July 3, 2021
Revised: July 15, 2021
Accepted: December 31, 2021
Article in press: December 31, 2021
Published online: January 14, 2022
Processing time: 299 Days and 1.7 Hours
Hepatocellular carcinoma (HCC) is an epidemic burden and remains highly prevalent worldwide. The significant mortality rates of HCC are largely due to the tendency of late diagnosis and the multifaceted, complex nature of treatment. Meanwhile, current therapeutic modalities such as liver resection and trans
Core Tip: Hepatocellular carcinoma (HCC) is a global epidemic burden. The high mortality rate is mostly due to late diagnosis and complexity of treatment. Nanotheranostics is a potential approach for HCC management. We herein discuss the challenges of HCC management, the advancement of nanotheranostics in cancer, and the potential role of nanotheranostics to address the current challenges in HCC management.
- Citation: Ladju RB, Ulhaq ZS, Soraya GV. Nanotheranostics: A powerful next-generation solution to tackle hepatocellular carcinoma. World J Gastroenterol 2022; 28(2): 176-187
- URL: https://www.wjgnet.com/1007-9327/full/v28/i2/176.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i2.176
Hepatocellular carcinoma (HCC) is the most common primary cancer of the liver and remains the second leading cause of cancer-related deaths worldwide[1,2]. It accounts for around 90% of all primary liver cancer cases worldwide[3], and is an epidemic burden in both developing and developed countries[4]. Whilst certain endemic areas such as East Asia has shown a decreasing trend, regions such as Europe, Africa, and the United states display increasing trends in HCC incidence rate with substantial morbidity and mortality[5,6]. Concerningly, cases have doubled in Europe and America as a result of lifestyle factors such as alcohol abuse, smoking, obesity, and metabolic diseases[7-10]. Variations among age and gender are also interesting epidemiologic features of HCC. Men have a higher prevalence of HCC than women with a ratio of 462.4:185.8 new cases per year in developing countries[11]. Res
The risk factors and etiologies of HCC vary depending on geographic region and lifestyle. Hepatitis B and C infections are major etiological factors that significantly contribute to HCC globally[13-15], accounting for 44% and 21% of HCC cases resp
Due to the multifactorial nature of HCC, several cellular phenomena can be observed, including hypoxia, inflammation, oxidative stress, and tumor microenvironment. Indeed, the molecular mechanism of liver carcinogenesis involves multiple end
Early diagnosis is a major challenge in HCC management, and in most cases, the lack of early diagnostic modalities lead to less than optimal treatment outcomes. In developed countries, 30%-60% of HCC cases can be diagnosed early, enabling higher success rates of curative treatment. Contrastingly, HCC cases in developing countries are mostly diagnosed in late stages, leading to substantially lower likelihood of curative treatment[27]. Diagnosis of HCC is in fact, an important and critical phase that relates directly to the survival and prognosis of the patients.
Treatment of HCC itself is also complex and multifaceted, and outcome depends on the time of diagnosis and the presence of additional comorbidities. Prompt diagnosis of HCC is correlated with better outcomes of curative therapies. This is demonstrated by studies that show higher efficacy of local radiofrequency ablation and surgical intervention (liver transplantation and liver resection) in the very early and early-stage HCC as compared to later stages[8,28]. However, most HCC patients are excluded from definitive surgical resection due to late diagnosis. In such cases, liver tr
Issues such as tumor recurrence and drug resistance are also major obstacles that frequently complicate HCC management[32,33]. The 5-year recurrence probability of HCC is around 62% after liver ablation[34] and 80% after liver resection[35]. Palliative treatment often have unexpected and poor outcomes related with high refractoriness to systemic therapy that lead to development of multidrug resistance[36-38].
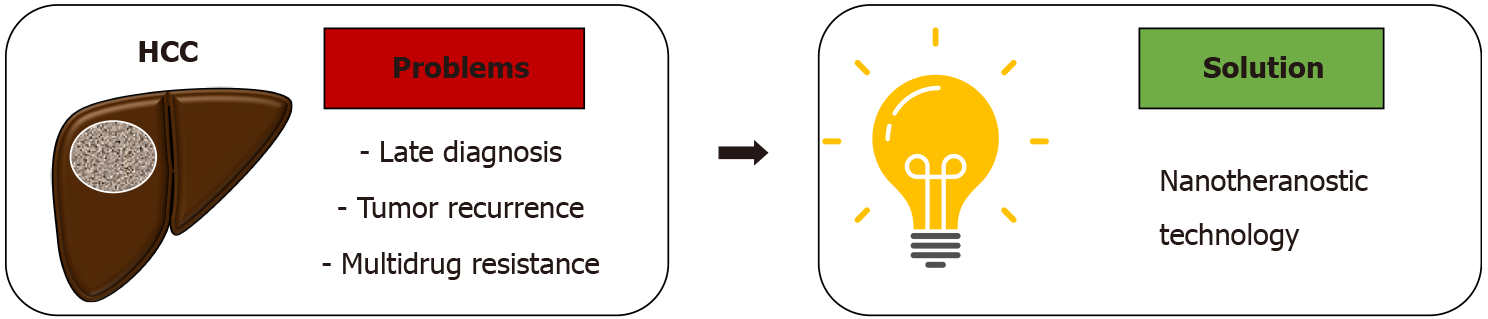
The challenges associated with both diagnosis and treatment of HCC has resulted in the high mortality rates across the globe, and calls upon innovative approaches that can improve the prognosis of HCC patients. In the following sections, we describe the rapid advances and implementation of theranostic-based nanomedicine and nanoparticles (nanotheranostic) as a promising option (Figure 1) for the improvement of HCC patient outcomes and quality of life.
Nanotheranostic modalities present a promising solution to the diagnostic and therapeutic challenges encountered in HCC management, through the use of biocompatible nanoparticles that simultaneously performs both diagnostic and therapeutic functions. This approach potentially provides a more personalized and targeted approach to cancer therapy, wherein the nanoparticles can be designed to detect specific biomarkers of the target malignant region, allow real-time monitoring or visualisation of the target, and finally deliver therapeutic modalities in a more precise manner. In recent years, the nanosensor and nanomedicine technologies have experienced major development, and have paved the way for promising means of nanotheranostics implementation in cancer management.
Nanotheranostic is a real-time combination of novel therapeutic and modern diagnostic tool or imaging into a single agent linked and integrated by nanoparticles[39,40]. Nanoparticles are the key components of the nanotheranostic agent[41] which include aptamer[42], DNA nanostructure[43], lactosome-based nanoparticles[44], metallic nanoparticles[45], gold nanoparticle[46], silver nanoparticle[47], dendrimer and copolymer-based nanoparticles[48], lipid-based nanomaterials[49], magnetic nanoparticles[50] iron oxide nanoparticle[51], mesoporous silica nanoparticle[52] and quantum dots nanoparticle[53].
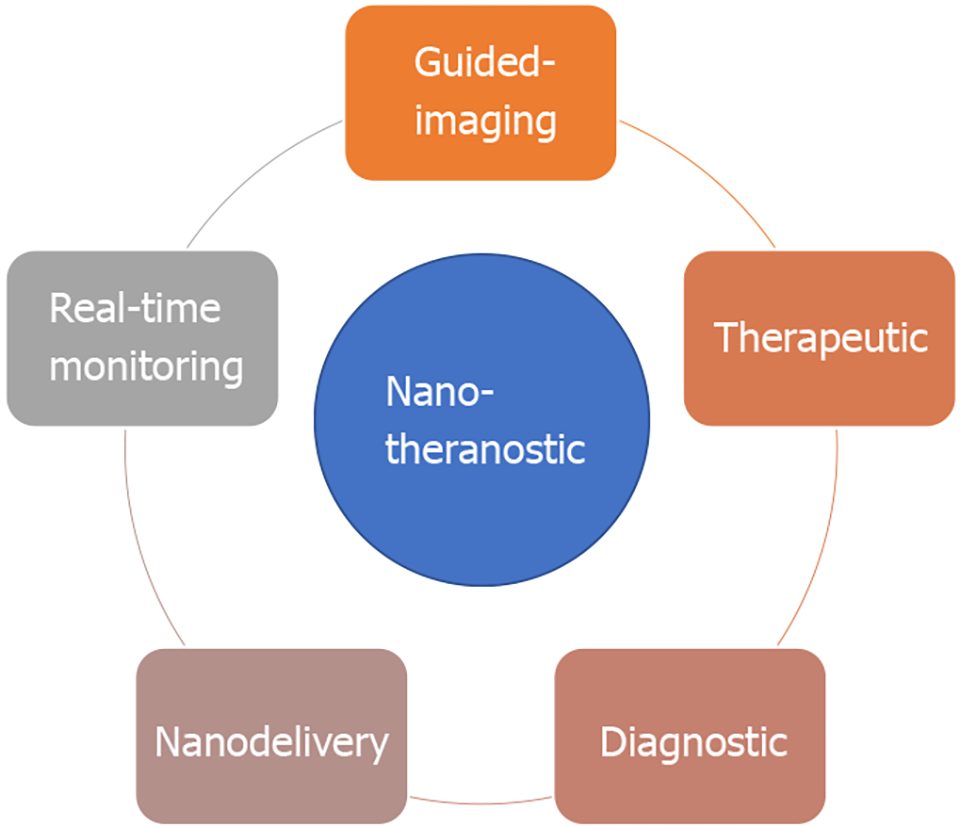
Nanotheranostic is an ideal choice for cancer treatment in the era of personalized medicine due to its potential to overcome the diagnostic and therapeutic challenges described prior[54]. Nanotheranostic not only provides the means for early diagnostic tools[55], nanoimaging-therapeutic integrated medicine[56], targeted-therapy[57] and tumor-specific nano-delivery agent[58], it also holds potential for real-time monitoring of drug response, and reduce side effects and drug toxicity in patients[59,60] as shown in Figure 2.
Successful demonstration of nanotheranostics for diagnostics and targeted therapy has been shown by Roy et al[61] in which highly sensitive, polymer-modified gadolinium-doped iron oxide-based T1 contrast agents were used for successful methotrexate drug delivery. In the second application, nanotheranostics have been utilized for simultaneous imaging and cancer monitoring[62]. An auto-fluorescent platform, constructed from a positively charged amphiphilic polymer polyethyleneimine-polylactide, was utilized to simultaneously load the antiangiogenesis agent cobretrastatin together with near-infrared (NIR) dye IR825 and heat-shock protein inhibitors. Altogether, the mechanism represents self-monitoring nanotheranostics, which in a mouse model demonstrated inhibitive properties in the tumour site through anti-angiogenesis and gene silencing enhanced photothermal therapy, while allowing real-time fluorescence monitoring.
The final and most widely developed application of nanotheranostics is for simultaneous imaging and targeted therapy, which has been shown to substantially increase the overall efficacy of therapies[63-65]. Theranostic platform choice has expanded rapidly in the past decade, and typically combines imaging modalities such as magnetic resonance imaging (MRI), NIR fluorescence, photoacoustic (PA) or ultrasound imaging, with therapeutic modalities such as chemotherapeutic agents, x-rays, hyperthermia, or free radicals.
Depending on the desired diagnostic and therapeutic modality, the nanoparticle of choice may be composed of metals, polymers, carbons and lipids. Each choice provides its own unique characteristics and physicochemical interactions, and also require different fabrication and functionalization procedures. As an example, successful magnetic-based imaging in a nanotheranostic platform is achievable using iron oxide, which is also desired due to its low toxicity and chemical stability. But many platforms prefer the use of multi-functional semiconducting polymers with hydrophobic properties, which simultaneously allow imaging through easy interactions with aromatic chemotherapeutic agents[66]. Nanoparticles can also be engineered to provide multimodal imaging[67] which utilizes modified ultrasmall Ag2Se nanodots to allow upconversion luminescence, downshifting luminescence, computed tomography and PA imaging techniques.
An increasingly common approach in cancer theranostics is the use of multimodal therapy. To illustrate, the study by Zhang et al[68] utilized Janus-type γ-Fe2O3/SiO2 nanoparticles to combine the glucose oxidase-mediated cancer starvation strategy with hydroxyl radicals as chemodynamic therapy. Interestingly, nanoparticles can also be designed to become responsive towards environmental stimuli in drug-resistant tumours, meaning that it can be developed specifically towards the pathological profile of the tumor microenvironment as well as the organ-specific tissues and compartments, which contribute to the overall specificity of the drug delivery. For highly complex pathologies such as HCC with drug resistance, this provides a myriad of options for exploration, and becomes an interesting approach for future implementation of HCC-specific nanotheranostics.
Finally, it is also worth noting that metastasis remains a major issue in cancers such as HCC where diagnosis tends to be late. An interesting strategy[69] showed the use of immunotherapy-based theranostics to specifically target metastatic tissue. In said study, magnetic-responsive immunostimulatory nanoagents were added with superparamagnetic iron oxide nanoparticles and cytosine-phosphate-guanine oligodeoxynucleotides. These engineered components allow for PA and MRI in addition to acting as a therapeutic agent for photothermally triggered immunotherapy.
To illustrate these advancements, we present the current modalities of cancer nanotheranostics in Table 1. In general, cancer nanotheranostics has been used for simultaneous diagnostics and therapeutic[70], real-time monitoring of malignancies[71], guided-imaging[72,73], drug-delivery[74] and multimodal-targeted therapy[75-79].
| Applications | Principle | Ref. |
| Diagnostic and therapeutic | Stimuli responsive nanoparticle and targeted drug delivery | [61] |
| Activatable nanotheranostic systems diagnosis and therapy of peritoneal metastasis | [70] | |
| Real-time monitoring and therapeutic | Self-monitoring and triple-collaborative therapy via auto-fluorescence nanoparticles | [62] |
| Real-time monitoring and tumor targeting via dual-fluorescent hydroxyapatite–doxorubicin | [71] | |
| Guided-imaging and nanodelivery | Nanoparticle conjugated with antibody for tumor targeting and guided drug delivery | [63] |
| A protein-stabilized multifunctional nanoplatform for multimodal imaging and drug-delivery | [64] | |
| Guided-imaging and therapeutic | Dual-targeting nanotheranostic with chemosensitizing agent for MDR chemotherapy | [65] |
| Multifunctional nanocarrier for fluorescence imaging guided chemo-photothermal | [66] | |
| Dual-modal imaging and synergistic cancer starvation/chemodynamic therapy | [68] | |
| Tetra-modal imaging guided photothermal therapy | [67] | |
| Bimodal imaging guided photothermal-triggered immunotherapy | [69] | |
| Hierarchical tumor acidity-responsive magnetic nanobomb photodynamic therapy | [73] | |
| Lipid based nanoparticles nanodelivery-anticancer drug and nanoimaging | [74] | |
| The self-assembly nanoparticles with guided imaging and chemotherapeutic drugs | [76] | |
| Biocompatible nanoparticles as targeted-nanodelivery of chemotherapeutic agent | [77] | |
| Dual-modality mapping guided photothermal ablation for metastatic cancer | [78] | |
| Magnetic nanoparticle-doxorubicin for enhancing nanoimaging and targeted therapy | [79] |
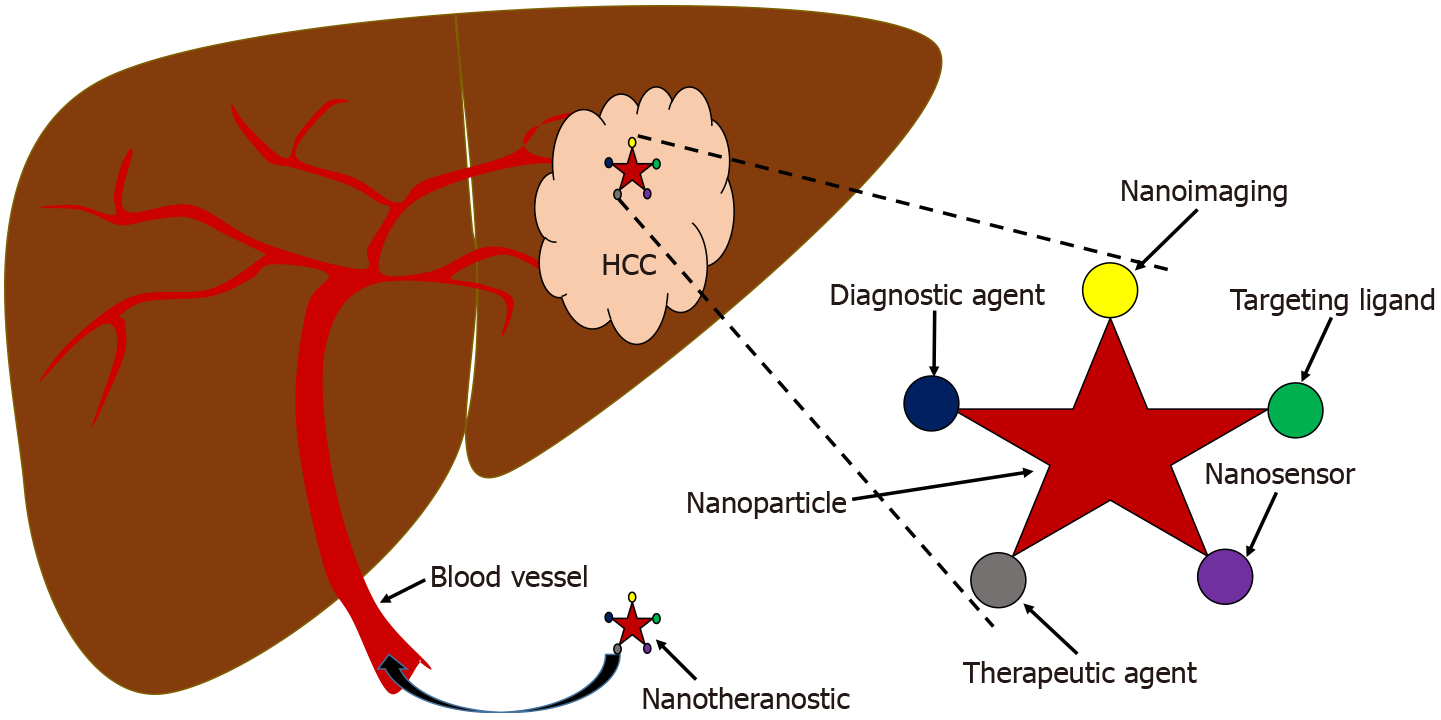
The nanotheranostic platform is a promising approach that is urgently needed to overcome the limitations of conventional therapy and diagnosis for more efficient HCC management. Figure 3 illustrates the multimodality of the nanotheranostic platform, which utilizes multipurpose nanoparticles for targeted nano-delivery, continuously controlled release of anticancer agents, guided imaging and early detection, for superior effectivity of transport[80]. Management of HCC requires powerful theranostic-based nanoparticles for early diagnostics and therapeutics with higher sensitivity and specificity, and to surpass the limitation of tissue penetration[81]. Previous studies have demonstrated the promising potential of silica-based nanomaterial as a potent nanotheranostic platform of HCC targeted-therapy nano-delivery[82-86]. In addition, many advances in HCC-specific nanotheranostics platforms are illustrated in Table 2, which demonstrates the multifunctional role of nanotheranostics as a detector to identify the HCC cell and tumor inhibitor by suppressing proliferation, migration and invasion of HCC[87,88].
| Applications | Principle | Ref. |
| Diagnostic and therapeutic | Conventional SELEX | [87] |
| CE-SELEX | [88] | |
| Magnetic nanoparticle-aptamer | [92] | |
| Enhancing therapeutic | Inducing tumor regression using siRNA-nanoparticle construction | [100] |
| Enhancing the anticancer efficacy using siRNA-nanoparticle construction | [101] | |
| Enhancing chemotherapy using microRNA 375-nanoparticle construction | [102] | |
| Synergistic antitumor effect of microRNA 375-nanoparticle construction | [103] | |
| Diagnostic and guided-imaging | ‘Activatable’ aptamer-based fluorescence probe | [104] |
| Streptavidin-fluorescent silica nanoparticles combination | [105] | |
| Aptamer- based electrochemical biosensors | [106] | |
| Gene editing | Next-generation CRISPR/Cas technology | [107] |
One of the most remarkable advancements of the nanotheranostic platform is imaging and nano-delivery integration as an innovative resolution for early HCC diagnosis and in situ drug release. In vivo and ex vivo investigations have observed specific nanoplatform activation by the tumor, with minimized toxicity towards non-target cells[89]. Integration of multifunctional nanoparticles with MRI may provide novel perspectives in tumor imaging technology to enhance HCC management and treatment strategy. High precision quantification and sensitivity of nanoimaging technology is needed for tissue penetration issue in early diagnosis of HCC[90].
Aptamer-based nanotheranostic is also a potential tool for HCC management due to its unique characteristics. This oligonucleotide nanomedicine has high specificity and affinity towards various types of target molecules[91]. In HCC clinical application, aptamer-based nanotheranostic development targeting the epithelial cell adhesion molecule demonstrated an improvement of MRI application and drug-delivery with high efficiency of doxorubicin released specifically towards cancer cells[92].
Improvement of therapeutic success is urgently needed for patients with unresectable and advanced HCC. The combination between nanomedicine as a nano-delivery system with cancer immunotherapy holds great potential for enhancing the nanotherapeutic outcome for this population. A promising targeted-nano-delivery immunotherapy for advanced HCC that is currently undergoing clinical trial is the 4th generation chimeric antigen receptor (CAR) T cells targeting glypican-3 (GPC3) (ClinicalTrials.gov Identifier: NCT03884751)[93]. This study showed promising phase I results in regard to antitumor activity and safety profile of CAR-GPC3 T-cell immunotherapy. The antitumor activity is positively associated with tumor response with no grade 3/4 neurotoxicity effect in any patients[94]. Several studies have also been done to achieve said goal by conjugating anticancer drugs with nanoparticles, rendering the treatment safer with more effective systemic administration due to the platform’s capability of controlling and postponing drug release. In the in vivo mouse model, tumor specific uptake of the controlled drug release for several weeks was observed, with minimal toxicity[95].
Molecular-targeted nano-therapies have also been constructed for nano-delivery using a modular design of polymeric nanoparticles for selective accumulation of drug pay load within tumor lesions. In in vivo mouse models, the intravenous drug injection was more effective for tumor inhibition than oral administration. This has revolutionized anticancer therapy by enhancing the efficacy and potency of therapeutics through inhibition of the angiogenesis pathway, tumor growth, tube formation and metastasis[96]. Targeted drug delivery using mesoporous silica nanoparticle is also promising. Nanoconstruction of silica nanorattle encapsulated docetaxel exhibited low toxicity with high antitumor activity, making it a prospective candidate for nano-delivery system[97]. Moreover, modified silica nanoparticles targeting low density lipoprotein and loaded with two anticancer drugs for liver cancer chemotherapy showed increased delivery efficiency based on in vitro and in vivo analysis[98].
In addition to anticancer drug nano-delivery for HCC treatment, the nanotheranostic platform is also suitable for targeted nano-delivery of small interfering RNA based therapeutics. This can be used as gene therapy to knock down a specific gene[99-101], and micro RNA for enhancing chemotherapy efficiency[102] to overcome multi-drug resistance in HCC[103].
HCC is an extremely complex and heterogeneous disease with diverse molecular profiles, aetiology and subtypes. Since conventional approaches still fail to overcome limitations in HCC management, nanotheranostic is a promising alternative to overcome the problems. Rapid development in nanotechnology has added a tremendous value on cancer therapy. The future of cancer nanomedicine lies on multimodal nanoplatforms that combine targeting ligands, imaging agents, diagnostic agents and therapeutic components into one unit of functionalized nanoparticles. Thus, multifunctionality is a powerful and unique advantage of nanotheranostic over traditional methods, and evidence has shown its capacity to work efficiently and noninvasively in vivo without systemic toxicity. Development of nanotheranostic in the right direction requires improvement of platforms so that it can be optimized simultaneously for proficient performance as the best clinical outcome in HCC.
In summary, nanotheranostic is an emerging and promising approach for HCC diagnosis/imaging and therapy in the future. Nanotheranostic is a powerful, unique, and multifunctional tool that yields positive impact both in the basic research and clinical application of HCC. We predict that in a near future nanotheranostic platform will continue to exponentially grow and progressively implemented in the development of novel and efficacious diagnostic and therapeutic agents towards cancers, including HCC. Further expansion would be needed to assist clinical translation of the promising preclinical studies in HCC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Indonesia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de la Pinta C, Liu ZW, Zhang L S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55824] [Article Influence: 7974.9] [Reference Citation Analysis (132)] |
| 2. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1216] [Article Influence: 202.7] [Reference Citation Analysis (1)] |
| 3. | Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 1873] [Article Influence: 208.1] [Reference Citation Analysis (4)] |
| 4. | Dimitroulis D, Damaskos C, Valsami S, Davakis S, Garmpis N, Spartalis E, Athanasiou A, Moris D, Sakellariou S, Kykalos S, Tsourouflis G, Garmpi A, Delladetsima I, Kontzoglou K, Kouraklis G. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J Gastroenterol. 2017;23:5282-5294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 228] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (4)] |
| 5. | Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol. 2016;34:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 358] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 6. | Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68:526-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 523] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 7. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 8. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3596] [Article Influence: 276.6] [Reference Citation Analysis (4)] |
| 9. | Tang A, Hallouch O, Chernyak V, Kamaya A, Sirlin CB. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol (NY). 2018;43:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 302] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 10. | Rawla P, Sunkara T, Muralidharan P, Raj JP. Update in global trends and aetiology of hepatocellular carcinoma. Contemp Oncol (Pozn). 2018;22:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 11. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21368] [Article Influence: 2136.8] [Reference Citation Analysis (3)] |
| 12. | Mittal S, Kramer JR, Omino R, Chayanupatkul M, Richardson PA, El-Serag HB, Kanwal F. Role of Age and Race in the Risk of Hepatocellular Carcinoma in Veterans With Hepatitis B Virus Infection. Clin Gastroenterol Hepatol. 2018;16:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Hemming AW, Berumen J, Mekeel K. Hepatitis B and Hepatocellular Carcinoma. Clin Liver Dis. 2016;20:703-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Goto K, Roca Suarez AA, Wrensch F, Baumert TF, Lupberger J. Hepatitis C Virus and Hepatocellular Carcinoma: When the Host Loses Its Grip. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 15. | de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 382] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 16. | Baecker A, Liu X, La Vecchia C, Zhang ZF. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev. 2018;27:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 17. | Seyda Seydel G, Kucukoglu O, Altinbasv A, Demir OO, Yilmaz S, Akkiz H, Otan E, Sowa JP, Canbay A. Economic growth leads to increase of obesity and associated hepatocellular carcinoma in developing countries. Ann Hepatol. 2016;15:662-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (1)] |
| 18. | Zheng H, Li P, Kwok JG, Korrapati A, Li WT, Qu Y, Wang XQ, Kisseleva T, Wang-Rodriguez J, Ongkeko WM. Alcohol and hepatitis virus-dysregulated lncRNAs as potential biomarkers for hepatocellular carcinoma. Oncotarget. 2018;9:224-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 2902] [Article Influence: 483.7] [Reference Citation Analysis (17)] |
| 20. | Kew MC. Aflatoxins as a cause of hepatocellular carcinoma. J Gastrointestin Liver Dis. 2013;22:305-310. [PubMed] |
| 21. | André F. Hepatitis B epidemiology in Asia, the Middle East and Africa. Vaccine. 2000;18 Suppl 1:S20-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 174] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 532] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 23. | Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48:2047-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 512] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 24. | Hui AM, Makuuchi M. Molecular basis of multistep hepatocarcinogenesis: genetic and epigenetic events. Scand J Gastroenterol. 1999;34:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1103] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 26. | Feitelson MA, Sun B, Satiroglu Tufan NL, Liu J, Pan J, Lian Z. Genetic mechanisms of hepatocarcinogenesis. Oncogene. 2002;21:2593-2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 243] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 27. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4520] [Article Influence: 347.7] [Reference Citation Analysis (2)] |
| 28. | de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56 Suppl 1:S75-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 484] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 29. | Vitale A, Peck-Radosavljevic M, Giannini EG, Vibert E, Sieghart W, Van Poucke S, Pawlik TM. Personalized treatment of patients with very early hepatocellular carcinoma. J Hepatol. 2017;66:412-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 30. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6059] [Article Influence: 865.6] [Reference Citation Analysis (3)] |
| 31. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 866] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 32. | Daher S, Massarwa M, Benson AA, Khoury T. Current and Future Treatment of Hepatocellular Carcinoma: An Updated Comprehensive Review. J Clin Transl Hepatol. 2018;6:69-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 33. | Guo J, Li L, Guo B, Liu D, Shi J, Wu C, Chen J, Zhang X, Wu J. Mechanisms of resistance to chemotherapy and radiotherapy in hepatocellular carcinoma. Transl Cancer Res. 2018;7:765-781. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Facciorusso A, Del Prete V, Antonino M, Crucinio N, Neve V, Di Leo A, Carr BI, Barone M. Post-recurrence survival in hepatocellular carcinoma after percutaneous radiofrequency ablation. Dig Liver Dis. 2014;46:1014-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, Makuuchi M. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1234] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 36. | Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, Wu F, Wang Q, Wang S, Rong D, Reiter FP, De Toni EN, Wang X. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther. 2020;5:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 700] [Article Influence: 140.0] [Reference Citation Analysis (0)] |
| 37. | Marin JJG, Macias RIR, Monte MJ, Romero MR, Asensio M, Sanchez-Martin A, Cives-Losada C, Temprano AG, Espinosa-Escudero R, Reviejo M, Bohorquez LH, Briz O. Molecular Bases of Drug Resistance in Hepatocellular Carcinoma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 38. | Duan B, Huang C, Bai J, Zhang YL, Wang X, Yang J, Li J. Multidrug Resistance in Hepatocellular Carcinoma. In: Tirnitz-Parker JEE. Hepatocellular Carcinoma. Brisbane (AU): Codon Publications, 2019. |
| 39. | Muthu MS, Leong DT, Mei L, Feng SS. Nanotheranostics - application and further development of nanomedicine strategies for advanced theranostics. Theranostics. 2014;4:660-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 371] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 40. | Jeelani S, Reddy RC, Maheswaran T, Asokan GS, Dany A, Anand B. Theranostics: A treasured tailor for tomorrow. J Pharm Bioallied Sci. 2014;6:S6-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 41. | Madanayake NH, Rienzie R, Adassooriya NM. Nanoparticles in Nanotheranostics Applications. In: Rai M, Jamil B. Nanotheranostics: Applications and Limitations. Cham: Springer International Publishing, 2019: 19–40. |
| 42. | Ravichandran G, Rengan AK. Aptamer-Mediated Nanotheranostics for Cancer Treatment: A Review. ACS Appl Nano Mater. 2020;3:9542-9559. [DOI] [Full Text] |
| 43. | Nicolson F, Ali A, Kircher MF, Pal S. DNA Nanostructures and DNA-Functionalized Nanoparticles for Cancer Theranostics. Adv Sci (Weinh). 2020;7:2001669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 44. | Lim MSH, Ohtsuki T, Takenaka F, Kobayashi K, Akehi M, Uji H, Kobuchi H, Sasaki T, Ozeki E, Matsuura E. A Novel 89Zr-labeled DDS Device Utilizing Human IgG Variant (scFv): "Lactosome" Nanoparticle-Based Theranostics for PET Imaging and Targeted Therapy. Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Silva CO, Pinho JO, Lopes JM, Almeida AJ, Gaspar MM, Reis C. Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems. Pharmaceutics. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 46. | Gao Q, Zhang J, Gao J, Zhang Z, Zhu H, Wang D. Gold Nanoparticles in Cancer Theranostics. Front Bioeng Biotechnol. 2021;9:647905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 47. | Zhang P, Hu C, Ran W, Meng J, Yin Q, Li Y. Recent Progress in Light-Triggered Nanotheranostics for Cancer Treatment. Theranostics. 2016;6:948-968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 48. | Ray S, Li Z, Hsu CH, Hwang LP, Lin YC, Chou PT, Lin YY. Dendrimer- and copolymer-based nanoparticles for magnetic resonance cancer theranostics. Theranostics. 2018;8:6322-6349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Tang WL, Tang WH, Li SD. Cancer theranostic applications of lipid-based nanoparticles. Drug Discov Today. 2018;23:1159-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 50. | Carvalho de Jesus P da C, Pellosi DS, Tedesco AC. Chapter 12 - Magnetic nanoparticles: applications in biomedical processes as synergic drug-delivery systems. In: Holban A-M, Grumezescu AM. Materials for Biomedical Engineering. Elsevier, 2019: 371–396. |
| 51. | Zhao S, Yu X, Qian Y, Chen W, Shen J. Multifunctional magnetic iron oxide nanoparticles: an advanced platform for cancer theranostics. Theranostics. 2020;10:6278-6309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 52. | Tao Y, Wang J, Xu X. Emerging and Innovative Theranostic Approaches for Mesoporous Silica Nanoparticles in Hepatocellular Carcinoma: Current Status and Advances. Front Bioeng Biotechnol. 2020;8:184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Chen H, Liu Z, Wei B, Huang J, You X, Zhang J, Yuan Z, Tang Z, Guo Z, Wu J. Redox responsive nanoparticle encapsulating black phosphorus quantum dots for cancer theranostics. Bioact Mater. 2021;6:655-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 54. | Mura S, Couvreur P. Nanotheranostics for personalized medicine. Adv Drug Deliv Rev. 2012;64:1394-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 323] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 55. | Sonali, Viswanadh MK, Singh RP, Agrawal P, Mehata AK, Pawde DM, Narendra, Sonkar R, Muthu MS. Nanotheranostics: Emerging Strategies for Early Diagnosis and Therapy of Brain Cancer. Nanotheranostics. 2018;2:70-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 56. | Sivasubramanian M, Chuang YC, Chen NT, Lo LW. Seeing Better and Going Deeper in Cancer Nanotheranostics. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Zhang P, Chen H, Liu J, Liu G. Genetically Engineered Plasma Membrane Nanovesicles for Cancer-Targeted Nanotheranostics. Methods Mol Biol. 2019;2054:283-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Mendes LP, Lima EM, Torchilin VP. Chapter 9 - Targeted Nanotheranostics for Selective Drug Delivery in Cancer. In: Conde J. Handbook of Nanomaterials for Cancer Theranostics. Elsevier, 2018: 245–277. |
| 59. | Fan Z, Fu PP, Yu H, Ray PC. Theranostic nanomedicine for cancer detection and treatment. J Food Drug Anal. 2014;22:3-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 60. | Kundu P, Singh D, Singh A, Sahoo SK. Cancer Nanotheranostics: A Nanomedicinal Approach for Cancer Therapy and Diagnosis. Anticancer Agents Med Chem. 2020;20:1288-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Roy E, Patra S, Madhuri R, Sharma PK. Stimuli-responsive poly(N-isopropyl acrylamide)-co-tyrosine@gadolinium: Iron oxide nanoparticle-based nanotheranostic for cancer diagnosis and treatment. Colloids Surf B Biointerfaces. 2016;142:248-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Shao L, Li Q, Zhao C, Lu J, Li X, Chen L, Deng X, Ge G, Wu Y. Auto-fluorescent polymer nanotheranostics for self-monitoring of cancer therapy via triple-collaborative strategy. Biomaterials. 2019;194:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 63. | Chen F, Hong H, Zhang Y, Valdovinos HF, Shi S, Kwon GS, Theuer CP, Barnhart TE, Cai W. In vivo tumor targeting and image-guided drug delivery with antibody-conjugated, radiolabeled mesoporous silica nanoparticles. ACS Nano. 2013;7:9027-9039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 64. | Han L, Xia JM, Hai X, Shu Y, Chen XW, Wang JH. Protein-Stabilized Gadolinium Oxide-Gold Nanoclusters Hybrid for Multimodal Imaging and Drug Delivery. ACS Appl Mater Interfaces. 2017;9:6941-6949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 65. | Yang C, Pang X, Chen W, Wang X, Lin G, Chu C, Zhang X, Deng X, Chen X, Liu G. Environmentally responsive dual-targeting nanotheranostics for overcoming cancer multidrug resistance. Science Bulletin. 2019;64:705-714. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 66. | Jiang Y, Cui D, Fang Y, Zhen X, Upputuri PK, Pramanik M, Ding D, Pu K. Amphiphilic semiconducting polymer as multifunctional nanocarrier for fluorescence/photoacoustic imaging guided chemo-photothermal therapy. Biomaterials. 2017;145:168-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 67. | Du K, Lei P, Dong L, Zhang M, Gao X, Yao S, Feng J, Zhang H. In situ decorating of ultrasmall Ag2Se on upconversion nanoparticles as novel nanotheranostic agent for multimodal imaging-guided cancer photothermal therapy. Appl Mater Today. 2020;18:100497. [DOI] [Full Text] |
| 68. | Zhang Y, Wan Y, Liao Y, Hu Y, Jiang T, He T, Bi W, Lin J, Gong P, Tang L, Huang P. Janus γ-Fe2O3/SiO2-based nanotheranostics for dual-modal imaging and enhanced synergistic cancer starvation/chemodynamic therapy. Science Bulletin. 2020;65:564-572. [RCA] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 69. | Guo Y, Ran Y, Wang Z, Cheng J, Cao Y, Yang C, Liu F, Ran H. Magnetic-responsive and targeted cancer nanotheranostics by PA/MR bimodal imaging-guided photothermally triggered immunotherapy. Biomaterials 2019; 219: 119370.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 70. | Ling S, Yang X, Li C, Zhang Y, Yang H, Chen G, Wang Q. Tumor Microenvironment-Activated NIR-II Nanotheranostic System for Precise Diagnosis and Treatment of Peritoneal Metastasis. Angew Chem Int Ed Engl. 2020;59:7219-7223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 71. | Kang Y, Sun W, Fan J, Wei Z, Wang S, Li M, Zhang Z, Xie Y, Du J, Peng X. Ratiometric real-time monitoring of hydroxyapatite–doxorubicin nanotheranostic agents for on-demand tumor targeted chemotherapy. Mater Chem Front. 2018;2:1791-1798. [DOI] [Full Text] |
| 72. | Dehghani S, Hosseini M, Haghgoo S, Changizi V, Akbari Javar H, Khoobi M, Riahi Alam N. Multifunctional MIL-Cur@FC as a theranostic agent for magnetic resonance imaging and targeting drug delivery: in vitro and in vivo study. J Drug Target. 2020;28:668-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 73. | Yang HY, Jang MS, Li Y, Fu Y, Wu TP, Lee JH, Lee DS. Hierarchical tumor acidity-responsive self-assembled magnetic nanotheranostics for bimodal bioimaging and photodynamic therapy. J Control Release. 2019;301:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 74. | Parhi P, Sahoo SK. Trastuzumab guided nanotheranostics: A lipid based multifunctional nanoformulation for targeted drug delivery and imaging in breast cancer therapy. J Colloid Interface Sci. 2015;451:198-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Dai Y, Wang B, Sun Z, Cheng J, Zhao H, Wu K, Sun P, Shen Q, Li M, Fan Q. Multifunctional Theranostic Liposomes Loaded with a Hypoxia-Activated Prodrug for Cascade-Activated Tumor Selective Combination Therapy. ACS Appl Mater Interfaces. 2019;11:39410-39423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 76. | Tang J, Zheng F, Zhao J. Self-assembled multifunctional nanotheranostics loading GEM for targeted lung cancer therapy. Mater Sci Eng C Mater Biol Appl. 2020;112:110786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Unnikrishnan BS, Sen A, Preethi GU, Joseph MM, Maya S, Shiji R, Anusree KS, Sreelekha TT. Folic acid-appended galactoxyloglucan-capped iron oxide nanoparticles as a biocompatible nanotheranostic agent for tumor-targeted delivery of doxorubicin. Int J Biol Macromol. 2021;168:130-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | Wang S, Zhang Q, Luo XF, Li J, He H, Yang F, Di Y, Jin C, Jiang XG, Shen S, Fu de L. Magnetic graphene-based nanotheranostic agent for dual-modality mapping guided photothermal therapy in regional lymph nodal metastasis of pancreatic cancer. Biomaterials. 2014;35:9473-9483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 79. | Mosafer J, Abnous K, Tafaghodi M, Mokhtarzadeh A, Ramezani M. In vitro and in vivo evaluation of anti-nucleolin-targeted magnetic PLGA nanoparticles loaded with doxorubicin as a theranostic agent for enhanced targeted cancer imaging and therapy. Eur J Pharm Biopharm. 2017;113:60-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 80. | Siafaka PI, Okur NÜ, Karantas ID, Okur ME, Gündoğdu EA. Current update on nanoplatforms as therapeutic and diagnostic tools: A review for the materials used as nanotheranostics and imaging modalities. Asian J Pharm Sci. 2021;16:24-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 81. | Xiang D, Zheng C, Zhou SF, Qiao S, Tran PH, Pu C, Li Y, Kong L, Kouzani AZ, Lin J, Liu K, Li L, Shigdar S, Duan W. Superior Performance of Aptamer in Tumor Penetration over Antibody: Implication of Aptamer-Based Theranostics in Solid Tumors. Theranostics. 2015;5:1083-1097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 82. | Wang JK, Zhou YY, Guo SJ, Wang YY, Nie CJ, Wang HL, Wang JL, Zhao Y, Li XY, Chen XJ. Cetuximab conjugated and doxorubicin loaded silica nanoparticles for tumor-targeting and tumor microenvironment responsive binary drug delivery of liver cancer therapy. Mater Sci Eng C Mater Biol Appl. 2017;76:944-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 83. | Zhang B, Luo Z, Liu J, Ding X, Li J, Cai K. Cytochrome c end-capped mesoporous silica nanoparticles as redox-responsive drug delivery vehicles for liver tumor-targeted triplex therapy in vitro and in vivo. J Control Release. 2014;192:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 84. | Lv Y, Li J, Chen H, Bai Y, Zhang L. Glycyrrhetinic acid-functionalized mesoporous silica nanoparticles as hepatocellular carcinoma-targeted drug carrier. Int J Nanomedicine. 2017;12:4361-4370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 85. | Xu X, Wu C, Bai A, Liu X, Lv H, Liu Y. Folate-Functionalized Mesoporous Silica Nanoparticles as a Liver Tumor-Targeted Drug Delivery System to Improve the Antitumor Effect of Paclitaxel. J Nanomater. 2017;2017:e2069685. [DOI] [Full Text] |
| 86. | Zhao R, Li T, Zheng G, Jiang K, Fan L, Shao J. Simultaneous inhibition of growth and metastasis of hepatocellular carcinoma by co-delivery of ursolic acid and sorafenib using lactobionic acid modified and pH-sensitive chitosan-conjugated mesoporous silica nanocomplex. Biomaterials. 2017;143:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 87. | Lee YJ, Lee SW. Regression of hepatocarcinoma cells using RNA aptamer specific to alpha-fetoprotein. Biochem Biophys Res Commun. 2012;417:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Dong L, Tan Q, Ye W, Liu D, Chen H, Hu H, Wen D, Liu Y, Cao Y, Kang J, Fan J, Guo W, Wu W. Screening and Identifying a Novel ssDNA Aptamer against Alpha-fetoprotein Using CE-SELEX. Sci Rep. 2015;5:15552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 89. | Lei Y, Tang J, Shi H, Ye X, He X, Xu F, Yan L, Qiao Z, Wang K. Nature-Inspired Smart DNA Nanodoctor for Activatable In Vivo Cancer Imaging and In Situ Drug Release Based on Recognition-Triggered Assembly of Split Aptamer. Anal Chem. 2016;88:11699-11706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 90. | Malla RR, Kumari S, Kgk D, Momin S, Nagaraju GP. Nanotheranostics: Their role in hepatocellular carcinoma. Crit Rev Oncol Hematol. 2020;151:102968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 91. | Ladju RB, Pascut D, Massi MN, Tiribelli C, Sukowati CHC. Aptamer: A potential oligonucleotide nanomedicine in the diagnosis and treatment of hepatocellular carcinoma. Oncotarget. 2018;9:2951-2961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 92. | Pilapong C, Sitthichai S, Thongtem S, Thongtem T. Smart magnetic nanoparticle-aptamer probe for targeted imaging and treatment of hepatocellular carcinoma. Int J Pharm. 2014;473:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 93. | Carsgen Therapeutics, Ltd. A Phase I Clinical Study of Chimeric Antigen Receptor T Cells Targeting Glypican-3 (CAR-GPC3 T Cells) in Patients With Advanced Hepatocellular Carcinoma. [accessed 2021 Mar 18]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03884751. |
| 94. | Shi D, Shi Y, Kaseb AO, Qi X, Zhang Y, Chi J, Lu Q, Gao H, Jiang H, Wang H, Yuan D, Ma H, Li Z, Zhai B. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials. Clin Cancer Res. 2020;26:3979-3989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 95. | Xu L, Xu S, Wang H, Zhang J, Chen Z, Pan L, Wang J, Wei X, Xie H, Zhou L, Zheng S, Xu X. Enhancing the Efficacy and Safety of Doxorubicin against Hepatocellular Carcinoma through a Modular Assembly Approach: The Combination of Polymeric Prodrug Design, Nanoparticle Encapsulation, and Cancer Cell-Specific Drug Targeting. ACS Appl Mater Interfaces. 2018;10:3229-3240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 96. | Wang J, Wang H, Li J, Liu Z, Xie H, Wei X, Lu D, Zhuang R, Xu X, Zheng S. iRGD-Decorated Polymeric Nanoparticles for the Efficient Delivery of Vandetanib to Hepatocellular Carcinoma: Preparation and in Vitro and in Vivo Evaluation. ACS Appl Mater Interfaces. 2016;8:19228-19237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 97. | Li L, Tang F, Liu H, Liu T, Hao N, Chen D, Teng X, He J. In vivo delivery of silica nanorattle encapsulated docetaxel for liver cancer therapy with low toxicity and high efficacy. ACS Nano. 2010;4:6874-6882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 98. | Ao M, Xiao X, Ao Y. Low density lipoprotein modified silica nanoparticles loaded with docetaxel and thalidomide for effective chemotherapy of liver cancer. Braz J Med Biol Res. 2018;51:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 99. | Varshosaz J, Farzan M. Nanoparticles for targeted delivery of therapeutics and small interfering RNAs in hepatocellular carcinoma. World J Gastroenterol. 2015;21:12022-12041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 100. | Huang KW, Lai YT, Chern GJ, Huang SF, Tsai CL, Sung YC, Chiang CC, Hwang PB, Ho TL, Huang RL, Shiue TY, Chen Y, Wang SK. Galactose Derivative-Modified Nanoparticles for Efficient siRNA Delivery to Hepatocellular Carcinoma. Biomacromolecules. 2018;19:2330-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 101. | Wu JY, Wang ZX, Zhang G, Lu X, Qiang GH, Hu W, Ji AL, Wu JH, Jiang CP. Targeted co-delivery of Beclin 1 siRNA and FTY720 to hepatocellular carcinoma by calcium phosphate nanoparticles for enhanced anticancer efficacy. Int J Nanomedicine. 2018;13:1265-1280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Zhao P, Li M, Wang Y, Chen Y, He C, Zhang X, Yang T, Lu Y, You J, Lee RJ, Xiang G. Enhancing anti-tumor efficiency in hepatocellular carcinoma through the autophagy inhibition by miR-375/sorafenib in lipid-coated calcium carbonate nanoparticles. Acta Biomater. 2018;72:248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 103. | Xue H, Yu Z, Liu Y, Yuan W, Yang T, You J, He X, Lee RJ, Li L, Xu C. Delivery of miR-375 and doxorubicin hydrochloride by lipid-coated hollow mesoporous silica nanoparticles to overcome multiple drug resistance in hepatocellular carcinoma. Int J Nanomedicine. 2017;12:5271-5287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 104. | Lai Z, Tan J, Wan R, Zhang Z, Hu Z, Li J, Yang W, Wang Y, Jiang Y, He J, Yang N, Lu X, Zhao Y. An 'activatable' aptamer-based fluorescence probe for the detection of HepG2 cells. Oncol Rep. 2017;37:2688-2694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 105. | Hu Z, Tan J, Lai Z, Zheng R, Zhong J, Wang Y, Li X, Yang N, Li J, Yang W, Huang Y, Zhao Y, Lu X. Aptamer Combined with Fluorescent Silica Nanoparticles for Detection of Hepatoma Cells. Nanoscale Res Lett. 2017;12:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 106. | Kashefi-Kheyrabadi L, Mehrgardi MA, Wiechec E, Turner AP, Tiwari A. Ultrasensitive detection of human liver hepatocellular carcinoma cells using a label-free aptasensor. Anal Chem. 2014;86:4956-4960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 107. | Kong H, Ju E, Yi K, Xu W, Lao YH, Cheng D, Zhang Q, Tao Y, Li M, Ding J. Advanced Nanotheranostics of CRISPR/Cas for Viral Hepatitis and Hepatocellular Carcinoma. Adv Sci (Weinh). 2021;e2102051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |