Published online Apr 28, 2022. doi: 10.3748/wjg.v28.i16.1671
Peer-review started: November 16, 2021
First decision: January 11, 2022
Revised: January 21, 2022
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: April 28, 2022
Processing time: 158 Days and 16.7 Hours
Coronavirus disease 2019 (COVID-19) has a spectrum of clinical syndromes with serious involvement of the lung and frequent effection of the liver and hemostatic system. Blood biomarkers are affordable, rapid, objective, and useful in the evaluation and prognostication of COVID-19 patients.
To investigate the association between aspartate transferase-to-platelet ratio index (APRI) and in-hospital mortality to develop a COVID-19 mortality prediction model.
A multicenter cohort study with a retrospective design was conducted. Medical records of all consecutive adult patients admitted to Al-Azhar University Hospital (Assiut, Egypt) and Chest Hospital (Assiut, Egypt) with confirmed COVID-19 from July 1, 2020 to October 1, 2020, were retrieved and analyzed. The patient cohort was classified into the following two categories based on the APRI: (1) COVID-19 presenting with APRI ≤ 0.5; and (2) COVID-19 presenting with APRI (> 0.5 and ≤ 1.5). The association between APRI and all-cause in-hospital mortality was analyzed, and the new model was developed through logistic regression analyses.
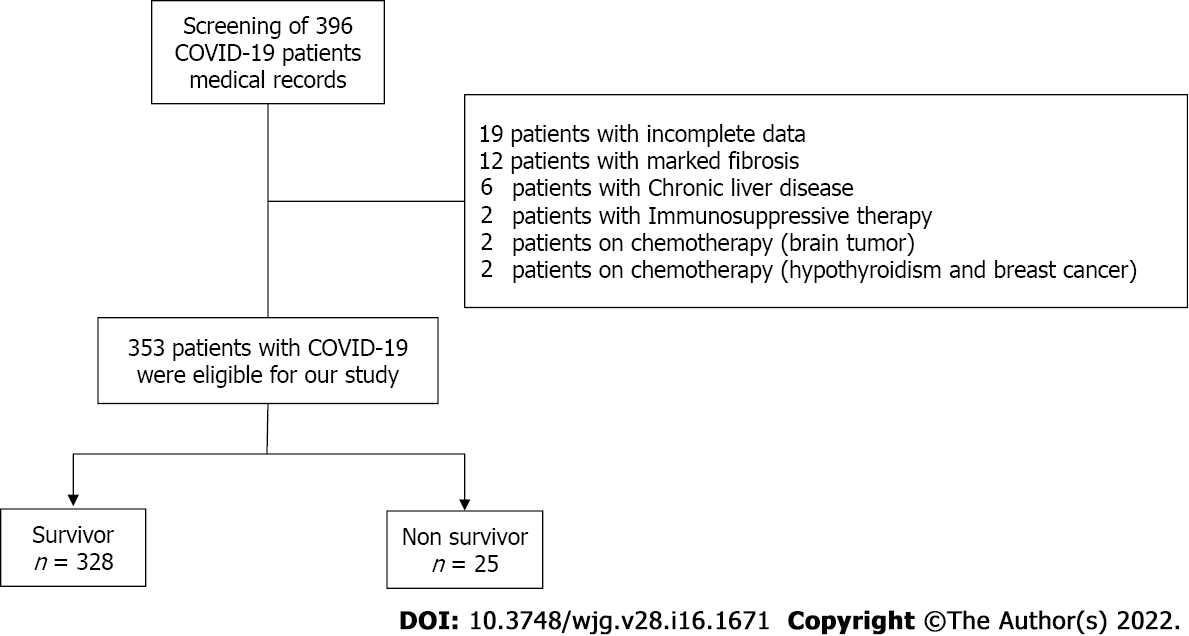
Of the 353 patients who satisfied the inclusion criteria, 10% were admitted to the intensive care unit (n = 36) and 7% died during the hospital stay (n = 25). The median age was 40 years and 50.7% were male. On admission, 49% had aspartate transferase-dominant liver injury. On admission, APRI (> 0.5 and ≤ 1.5) was independently associated with all-cause in-hospital mortality in unadjusted regression analysis and after adjustment for age and sex; after stepwise adjustment for several clinically relevant confounders, APRI was still significantly associated with all-cause in-hospital mortality. On admission, APRI (> 0.5 and ≤ 1.5) increased the odds of mortality by five-times (P < 0.006). From these results, we developed a new predictive model, the APRI-plus, which includes the four predictors of age, aspartate transferase, platelets, and serum ferritin. Performance for mortality was very good, with an area under the receiver operating curve of 0.90.
APRI-plus is an accurate and simplified prediction model for mortality among patients with COVID-19 and is associated with in-hospital mortality, independent of other relevant predictors.
Core Tip: Aspartate transferase-to-platelet ratio index-plus can be used to predict the severity of coronavirus disease 2019. The performance of the model for mortality was very good, with an area under the receiver operating curve of 0.90. This new prediction model could help in estimating the risk of mortality and may, therefore, assist in triaging patients. Moreover, our study confirmed that an aspartate transferase-dominant pattern, diabetes mellites, leukocytosis, and increased ferritin levels are associated with fatal outcomes.
- Citation: Madian A, Eliwa A, Abdalla H, A Azeem Aly H. Aspartate transferase-to-platelet ratio index-plus: A new simplified model for predicting the risk of mortality among patients with COVID-19. World J Gastroenterol 2022; 28(16): 1671-1680
- URL: https://www.wjgnet.com/1007-9327/full/v28/i16/1671.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i16.1671
The coronavirus disease 2019 (COVID-19) pandemic has prompted a global race to develop a variety of vaccine platforms. As a result, the first vaccine had been approved for emergency use by approximately 1 year from the beginning of the outbreak[1]. However, the ongoing limited availability of vaccines, particularly in low-income countries, means that the approach will not be sufficient to achieve global herd immunity. Therefore, effective and efficient allocation of health care resources is essential during the management of COVID-19 patients.
Blood biomarkers are affordable, rapid, readily available, and objective. It has been proven that blood biomarkers are useful in the evaluation and prognostication of COVID-19 patients. Many studies have demonstrated lymphopenia and thrombocytopenia among COVID-19 patients on hospital admission[2,3]. Furthermore, both lymphopenia[3,4] and thrombocytopenia[5] have been shown as predictive of COVID-19 severity and mortality.
Previously, many reports have cited hepatocellular injury with an aspartate aminotransferase (AST)-dominant pattern at hospital admission[6,7]. In addition, it has been demonstrated that AST elevation at admission is associated with severe COVID-19 disease status and poor outcomes, including intensive care admission, need for mechanical ventilation[7], and all-cause in-hospital mortality[6]. It has also been reported that elevated serum ferritin on admission is associated with fatal outcomes in patients with COVID-19[8,9].
Patients with COVID-19 associated with thrombocytopenia and hepatocellular injury progress to severe disease and poor outcomes. Previously, a simple scoring system, the AST-to-platelet ratio index (APRI), was developed to predict fibrosis in patients with chronic hepatitis C, being based upon routine blood biomarkers, including AST and platelets[10]. Using this as a premise, we hypothesized that it is possible to build a similar prediction model for patients with COVID-19 using objective, inexpensive, and readily available items.
The aim of the current study was to investigate whether APRI is associated with all-cause in-hospital mortality among patients with COVID-19 and develop a predictive model using objective and readily available factors.
We conducted a multicenter retrospective cohort study using medical records of all consecutive adult patients admitted to Al-Azhar University Hospital in Assiut, Egypt and Chest Hospital in Assiut, Egypt with confirmed COVID-19 from July 1, 2020 to October 1, 2020, as the Egyptian Ministry of Health had allocated both hospitals for the management of COVID-19 patients. The inclusion criteria for patients were complete medical record data, hospitalized adults of ages ≥ 18 years, and COVID-19 diagnosis established by examination of nasopharyngeal swab specimens using reverse-transcriptase PCR [CerTest Viasure® SARS-CoV-2 Real Time PCR Detection Kit (CerTest; Biotec, Spain)] for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). All laboratory tests were operated in the accredited laboratories at the Clinical Pathology Departments (Microbiology and Immunology units) in Al-Azhar University Hospital and Chest Hospital. The exclusion criteria were pediatric patients of ages < 18 years, patients with known chronic liver disease or newly diagnosed with chronic liver disease at hospital admission, pregnant females, patients with incomplete data, patients with splenomegaly, splenectomy, chemotherapy, or radiotherapy within 1 mo of hospital admission, and patients on immunosuppressive therapy. In addition, we excluded any patient with marked fibrosis, as determined by both APRI and fibrosis-4 (FIB-4) scores (FIB-4 > 2.67 and APRI > 1.5). The institutional review boards approved the study protocol.
AST and platelets were measured within 24 h of hospital admission. The normal upper limit for AST was 40 U/L, and the normal upper limit and lower limit for platelets were 350 ´ 109/L and 150 ´ 109/L, respectively. APRI was calculated according to the following equation: “AST Level/ULN ÷ Platelet count × 100”[10]. We categorized our patient cohort into the following two categories based on APRI: (1) COVID-19 presenting with APRI ≤ 0.5; and (2) COVID-19 presenting with APRI (> 0.5 and ≤ 1.5).
For every participant of our cohort, demographic criteria, including age, sex, and smoking status, comorbidities, including as diabetes mellites (DM), hypertension (HTN), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), and Deyo-Charlson index, and laboratory biomarkers, including complete blood count, liver enzymes, serum albumin, serum total bilirubin, blood urea, and serum creatinine and creatine kinase, were retrieved and analyzed. The FIB-4 was calculated by the following equation:
Age (year) × AST(U/L) ÷ Platelets (1000/μL) × √ALT(U/L)[11].
The primary outcome of interest was all-cause in-hospital mortality. The vital status of each patient in the study cohort was confirmed from hospital records. Follow-up of outcome was ended on October 15, 2020.
We summarized continuous variables as medians and interquartile ranges, and categorical variables were summarized as absolute numbers and percentages. We utilized unadjusted binary logistic regression for analyses of group differences. We performed a stepwise analysis adjusted for age and sex, as well as for relevant confounding factors, to investigate clinical confounders. Spearman’s correlation coefficients were utilized to analyze the relationships between variables. All P values calculated were two-tailed; values less than 0.05 were considered as indicative of statistical significance. Prediction performance was evaluated by the area under the curve of the receiver operating curve (AUROC). Overall fit of the models was evaluated by the Hosmer-Lemeshow test and the Bayesian information criterion. We used Stata Software (Stata Statistical Software: Release 16. College Station, TX, United States: Stata Corp LP) for data visualization and analysis.
Out of the 396 patients considered, 353 satisfied the inclusion criteria. Among those, 10% of the study cohort was admitted to the intensive care unit (n = 36) and 7% of the study participants succumbed during hospitalization (n = 25) (Figure 1). The median age at hospital admission was 40 years [interquartile range (IQR): 28-55 years], and 50.7% were males (n = 179) (Table 1). The frequency of thrombocytopenia was 9.6% (n = 34). Thrombocytosis was reported among 15% (n = 54) of the study cohort. However, there was no statistically significant difference in platelet count between the survivor and death groups (Table 1). On admission, 49% of study participants had AST-dominant liver injury (n = 174). Study participants were grouped based on all-cause in-hospital death into survivor and nonsurvivor groups, and their features are provided in Table 1.
| Characteristics | Survivors, n = 328 | Non-survivors, n = 25 | Unadjusted OR | P value |
| Age in yr, median (IQR) | ||||
| < 40 | 178 (98.89) | 2 (1.11) | Ref. | |
| 40-60 | 112 (94.92) | 6 (5.08) | 4.76 (0.94-24.03) | 0.05 |
| > 60 | 38 (69.09) | 17 (30.91) | 39.81 (8.82-179.59) | 0.0001 |
| Male sex, n (%) | 167 (93.30) | 12 (6.70) | 0.88 (0.39-2.00) | 0.77 |
| Comorbidities | ||||
| Chronic kidney disease, n (%) | 4 (44.44) | 5 (55.56) | 20.25 (5.04-81.31) | 0.0001 |
| Diabetes mellites, n (%) | 55 (77.46) | 16 (22.54) | 8.82 (3.70-20.98) | 0.0001 |
| Chronic obstructive pulmonary disease, n (%) | 55 (88.71) | 7 (11.29) | 1.93 (0.76-4.84) | 0.16 |
| Hypertension, n (%) | 84 (84.00) | 16 (16.00) | 5.16 (2.19-12.12) | 0.0001 |
| Deyo-Charlson index, n (%) | ||||
| 0-1 | 267 (97.45) | 7 (2.55) | Ref. | |
| 2-3 | 60 (80.00) | 15 (20.00) | 9.53 (3.72-24.40) | 0.0001 |
| > 3 | 1 (25.00) | 3 (75.00) | 114.42 (10.54-1241.77) | 0.0001 |
| Laboratory biomarkers | ||||
| Hemoglobin < 12 mg/dL, n (%) | 125 (92.59) | 10 (7.41) | 1.08 (0.47-2.48) | 0.85 |
| Total leukocytic count > 11 × 109/L, n (%) | 50 (80.65) | 12 (19.35) | 5.13 (2.21-11.89) | 0.0001 |
| Platelet | ||||
| 150-350 | 248 (93.58) | 17 (6.42) | Ref. | |
| < 150 × 109/L, n (%) | 32 (94.12) | 2 (5.88) | 0.91 (0.20-4.13) | 0.90 |
| > 350 × 109/L, n (%) | 48 (88.89) | 6 (11.11) | 1.82 (0.68-4.86) | 0.23 |
| Serum AST, n (%) | ||||
| < 40 U/L | 239 (97.15) | 7 (2.85) | Ref. | |
| 40-80 U/L | 81 (84.38) | 15 (15.63) | 6.32 (2.49-16.05) | 0.0001 |
| > 80 U/L | 8 (72.73) | 3 (27.27) | 12.80 (2.78-58.83) | 0.001 |
| Serum ALT, n (%) | ||||
| < 40 U/L | 217 (93.94) | 14 (6.06) | Ref. | |
| 40-80 U/L | 90 (90.00) | 10 (10.00) | 1.72 (0.73-4.02) | 0.20 |
| > 80 U/L | 21 (95.45) | 1 (4.55) | 0.73 (0.09-5.89) | 0.77 |
| Serum albumin < 3.5 g/dL, n (%) | 112 (86.15) | 18 (13.85) | 4.95 (2.01-12.22) | 0.001 |
| Serum total bilirubin > 1.5 mg/dL, n (%) | 2 (40.00) | 3 (60.00) | 22.22 (3.52-140.03) | 0.001 |
| Serum creatinine > 1.1 mg/dL for males; > 0.95 mg/dL for females, n (%) | 64 (80.00) | 16 (20.00) | 7.33 (3.09-17.34) | 0.0001 |
| Serum ferritin > 400 μg/L for males; > 150 μg/L for females, n (%) | 169 (87.56) | 24 (12.44) | 22.57 (3.01-168.86) | 0.002 |
| D-dimer > 0.5 μg/mL | 319 (92.73) | 25 (7.27) | 1 | - |
| C-reactive protein ≥ 1 mg/L, n (%) | 330 (92.70) | 26 (7.30) | 1 | - |
| Creatine kinase > 117 IU/L, n (%) | 0.00 (100.00) | 0.00 (0.00) | - | - |
| APRI, n (%) | ||||
| ≤ 0.5 | 254 (95.85) | 11 (4.15) | Ref. | |
| > 0.5 | 74 (84.09) | 14 (15.19) | 4.36 (1.90-10.02) | 0.001 |
| FIB-4, n (%) | ||||
| ≤ 2 | 300 (96.15) | 12 (3.85) | Ref. | |
| > 2 | 17 (73.91) | 6 (26.09) | 8.82 (2.95-26.37) | 0.0001 |
| > 2.67 | 11 (61.11) | 7 (38.89) | 15.90 (5.24-48.24) | 0.0001 |
Variables associated with in-hospital mortality were first assessed by univariate analysis. Significant variables from univariate analysis (P < 0.05) or clinically relevant variables were then utilized for multivariate analysis by forward logistic regression to identify independent predictors associated with in-hospital death among patients with COVID-19.
Regression analysis: (1) Binary logistic regression analysis. Age, Deyo-Charlson index, CKD, DM, and HTN were the clinical predictors significantly associated with hospital mortality. Additionally, the unadjusted regression demonstrated that APRI, serum ferritin, total leukocytic count, serum total bilirubin, serum creatinine, serum AST, and serum albumin levels were significant biochemical markers associated with hospital death. There was no association between hospital mortality and sex, COPD, hemoglobin, platelets, serum alanine transferase (ALT), C-reactive protein (CRP), creatine kinase, or D-dimer levels (Table 1). (2) Multivariable logistic regression analysis. The APRI (> 0.5 - ≤ 1.5) at admission was significantly associated with hospital mortality in the unadjusted binary logistic regression analysis and remained a significant predictor of the odds of hospital mortality in model 1 adjusted for age and sex (Table 2). In addition, in model 2, after adjustment for several covariates, APRI was still a significant predictor of hospital mortality and associated with increased odds of hospital mortality by five times (P < 0.005) (Table 2).
| Unadjusted | Model I (Adjusted for age and sex) | Model II | |||||||
| APRI | OR | CI | P value | OR | CI | P value | OR | CI | P value |
| ≤ 0.5 | Ref. | Ref. | Ref. | ||||||
| > 0.5 - ≤ 1.5 | 4.36 | 1.90-10.02 | 0.001 | 3.23 | 1.29-8.12 | 0.01 | 5.03 | 1.63-15.52 | 0.005 |
| Covariates | |||||||||
| Age in yr | |||||||||
| < 40 | Ref. | ||||||||
| 40-60 | 1.75 | 0.25-11.89 | 0.56 | ||||||
| > 60 | 10.45 | 1.51-72.09 | 0.01 | ||||||
| Sex | 1.19 | 0.39-3.65 | 0.75 | ||||||
| CKD | 8.24 | 1.37-49.53 | 0.02 | ||||||
| DM | 7.77 | 1.82-33.04 | 0.006 | ||||||
| HTN | 0.25 | 0.05-1.11 | 0.07 | ||||||
| WBC | 4.20 | 1.32-13.32 | 0.01 | ||||||
| Albumin | 1.17 | 0.33-4.09 | 0.81 | ||||||
| Bilirubin | 10.10 | 0.97-104.45 | 0.05 | ||||||
| Ferritin | 12.94 | 1.38-121.08 | 0.02 | ||||||
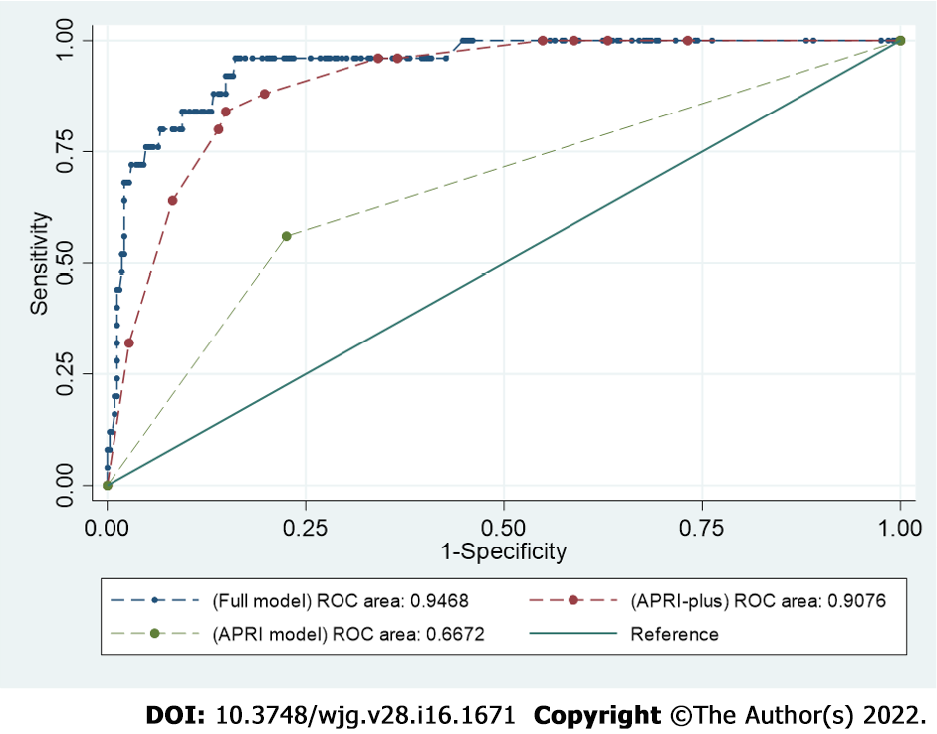
Variables in the best model (full model) for the prediction of death among patients with COVID-19 included APRI, age, CKD, DM, total leukocytic count, and serum ferritin. The AUROC for the prediction of death among patients with COVID-19 was 0.94 [95% confidence interval (CI): 0.90-0.98]. When the prediction model comprised APRI alone (APRI model), the AUROC for the prediction of mortality was 0.66 (95%CI: 0.56-0.76). The model with APRI, age, and serum ferritin (APRI-plus model) had better accuracy than the APRI model, and the AUROC became 0.90 (95%CI: 0.86-0.95) (P < 0.0001) (Figure 2). In addition, the APRI-plus model showed good calibration (Hosmer-Lemeshow χ2 = 1.7, P = 0.97) and was a better-fitting model than the full model (Bayesian information criterion 148.4) (Table 3).
| AUROC | HL-χ2 | BIC | |
| APRI-plus model | 0.90 (95%CI: 0.86–0.95) | 0.97 | 148.4 |
| Full model | 0.94 (95%CI: 0.90–0.98) | 0.95 | 164.9 |
The adjusted equation for the APRI-plus in patients with COVID-19 was P (in-hospital mortality) = -0.18 + 0.06 × (APRI > 0.5 and ≤ 1.5) (yes = 1, no = 0) + 0.004 × age + 0.00013 × ferritin.
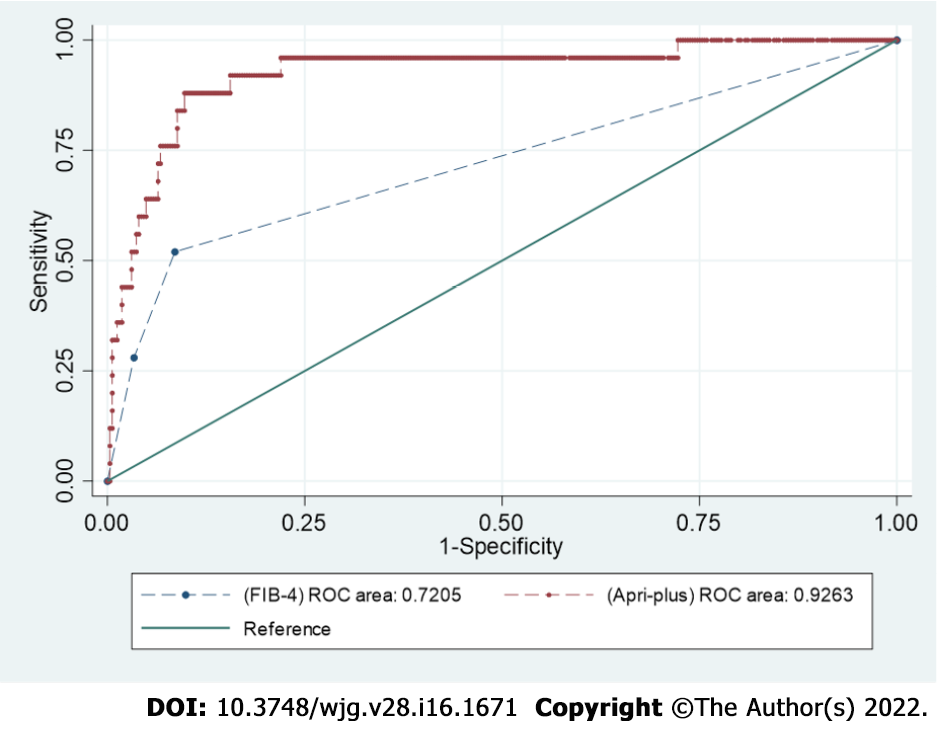
The AUROC for FIB-4 score was 0.72 (95%CI: 0.61-0.82). Upon comparison of the APRI-plus with FIB-4, the AUROC was significantly higher for APRI-plus, at 0.92 (95%CI: 0.86-0.98) (P < 0.0001) (Figure 3).
D-dimer was examined as a marker of thrombosis. On admission, platelets, serum ferritin, CRP, total leukocytic count, and AST were correlated with D-dimer (r = 0.1; P < 0.03, r = 0.4; P < 0.0001, r = 0.4; P < 0.0001, r = 0.1; P < 0.01, and r = 0.1; P < 0.03, respectively).
Serum ferritin, CRP, and total leukocytic count were investigated as inflammatory markers. Serum levels of AST were correlated with serum ferritin (r = 0.2; P < 0.0001), CRP (r = 0.2; P < 0.0001), and total leucocytic count (r = 0.1; P < 0.007). Platelets were correlated with serum ferritin (r = 0.1; P = 0.04), CRP (r = 0.07; P = 0.1), and total leukocytic count (r = 0.1; P = 0.05).
In the current study, we developed APRI-plus, a new, simple, inexpensive, and objective clinical score that is significantly associated with mortality among patients with COVID-19. APRI (> 0.5 and ≤ 1.5) increased the odds of mortality by five-fold. Since patients with chronic liver disease were excluded from participation in our analysis, this association is likely due to inflammation related to COVID-19 and the direct impact of SARS-Cov-2 on the liver and hemostatic system.
We observed a significant difference in AST between survivors and nonsurvivors at hospital admission. The AST-dominant pattern of hepatocellular injury among patients with COVID-19 has been reported many times in previous studies[6,7]. Likewise, the levels of serum ferritin were significantly higher among nonsurvivors. Platelets have been reported to be one of the strongest indicators of adverse outcomes and mortality among patients with COVID-19[2,3]. However, in our cohort, we did not find any statistically significant difference in platelets between survivors and nonsurvivors. Thus, platelets alone are less ideal in predicting outcomes among patients with COVID-19.
APRI includes AST in the numerator and platelets in the denominator. These criteria make the APRI score a better predictor of COVID-19 outcomes than AST or platelets alone. Regarding its accuracy in predicting mortality among patients with COVID-19, the APRI has an AUROC of 0.66. However, when age and serum ferritin were incorporated in the regression model (APRI-plus model), the performance of the model had an AUROC of 0.90. In contrast, compared with another noninvasive score, FIB-4 (composed of AST, ALT, age, and platelets), we found that the AUROC was significantly lower for FIB-4 (0.72). In line with our findings, the reported AUROC in an earlier study for FIB-4 was 0.79[11]. Using APRI-plus, it is possible to predict mortality among patients with COVID-19 with good performance.
Liver stiffness among patients with COVID-19 is likely to be multifactorial. Hepatic congestion, hepatocellular injury, and systemic inflammation may all play a role in the development of liver stiffness among patients with COVID-19. It has been reported that COVID-19 is associated with increased pressure within the right ventricle[12]; thus, this may result in hepatic venous congestion and liver stiffness[11].
Severe liver injury caused by SARS-CoV-2 Likely results in mitochondrial damage and defective clearance of AST, leading to an AST-dominant pattern. At the onset of severe SARS-CoV-2 infection, severe liver injury is likely associated with extensive inflammation, hepatocyte swelling, and tissue edema, especially among patients with an AST-dominant pattern. Similar to acute viral hepatitis, extensive necroinflammatory activity among patients with COVID-19 increases liver stiffness[13].
Our cohort revealed that platelet count did not have a significant association with mortality. Interestingly, not all previous studies have found platelet counts to be a predictor of COVID-19 mortality[14]. Inflammatory mediators associated with SARS-CoV-2 infection may result in the activation of platelets. Activated platelets have two pathways. First, enhanced platelet clearance/ sequestration from circulation by the spleen results in thrombocytopenia. Second, consumptive coagulopathy, such as inflammation-mediated endothelial damage/activation in addition to platelet activation, leads to the formation of platelet aggregates through systemic circulation and/or pulmonary circulation[15]. Therefore, we can speculate that the early phase of SARS-CoV-2 infection results in the activation of platelets followed by thrombocytopenia. Our results support this speculation, as inflammatory markers (CRP, serum ferritin, and leukocytosis) were correlated with D-dimer. This may suggest that the mechanism and progression of platelet activation rather than platelet number were associated with worse outcomes.
The rising question is why we did not find a significant difference in D-dimer between the survivor and nonsurvivor groups. This could be explained by COVID-19-associated coagulopathy. Coagulation changes associated with COVID-19 have the following three proposed stages: stage 1 presents with raised D-dimer; stage 2 presents with raised D-dimer together with modestly increased prothrombin time/international normalization ratio, activated partial thromboplastin time, and mild thrombocytopenia; and stage 3 presents with critical illness and laboratory markers, directing towards classic disseminated intravascular coagulopathy[14]. Therefore, our data may be retrieved early during platelet activation and before firing of stage 1 COVID-19-associated coagulopathy.
The retrospective design of the study reflected the association between APRI-plus and risk of mortality among patients with COVID-19 but did not reflect causality. We used APRI at the time of admission for group categorization without knowing follow-up changes. Because of the lack of follow-up, we do not know whether these necroinflammatory changes were self-limiting or progressive. Moreover, we do not know the extent and duration of resolution. We did not evaluate platelet activity, fibrin degradation products, or coagulation profiles. Elastography should be evaluated in different age groups to dissect the impact of age from COVID-19 on liver stiffness. Validation of the APRI-plus is required.
The current study showed that, on admission, APRI-plus among patients with COVID-19 has good performance in predicting mortality. A prediction model could help stratify the risk of mortality. This association may be explained by the impact of SARS-CoV-2 infection on the liver and hemostatic system. Further studies are required to investigate this association.
Coronavirus disease 2019 (COVID-19) has a spectrum of clinical syndromes with serious involvement of the lung and frequent effection of the liver and hemostatic system.
Development of a prediction model using objective, inexpensive, and readily available items is needed for patients with COVID-19.
To investigate whether the aspartate transferase-to-platelet ratio index (APRI) is associated with all-cause in-hospital mortality among patients with COVID-19 and develop a predictive model using objective and readily available factors.
A retrospective cohort study was carried out with 353 consecutive adult patients admitted to Al-Azhar University Hospital (Assiut, Egypt) and Chest Hospital (Assiut, Egypt) with confirmed COVID-19 from July 1, 2020 to October 1, 2020.
The prediction model comprised APRI alone (APRI model), and the area under the receiver operating curve (AUROC) for the prediction of mortality was 0.66 [95% confidence interval (CI): 0.56-0.76]. A modified model of APRI that included age and serum ferritin (APRI-plus model) had better accuracy than the APRI model, as the AUROC became 0.90 (95%CI: 0.86-0.95) (P < 0.0001).
APRI-plus among patients with COVID-19 showed good performance in predicting mortality. A prediction model could help stratify the risk of mortality.
Further studies are required to investigate this association. Validation of the APRI-plus is required.
We appreciate the effort of all medical staff and technicians who agreed to participate in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Ani RM, Iraq; Omar BJ, India S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Lamb YN. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs. 2021;81:495-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 244] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 2. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18877] [Article Influence: 3775.4] [Reference Citation Analysis (7)] |
| 3. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30120] [Article Influence: 6024.0] [Reference Citation Analysis (3)] |
| 4. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6660] [Article Influence: 1332.0] [Reference Citation Analysis (0)] |
| 5. | Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y, Shang Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469-1472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 359] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 6. | Madian A, Eliwa A, Abdalla H, Aly HAA. Hepatocellular injury and the mortality risk among patients with COVID-19: A retrospective cohort study. World J Hepatol. 2021;13:939-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (4)] |
| 7. | Weber S, Hellmuth JC, Scherer C, Muenchhoff M, Mayerle J, Gerbes AL. Liver function test abnormalities at hospital admission are associated with severe course of SARS-CoV-2 infection: a prospective cohort study. Gut. 2021;70:1925-1932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 8. | Deng F, Zhang L, Lyu L, Lu Z, Gao D, Ma X, Guo Y, Wang R, Gong S, Jiang W. [Increased levels of ferritin on admission predicts intensive care unit mortality in patients with COVID-19]. Med Clin (Barc). 2021;156:324-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Vargas-Vargas M, Cortés-Rojo C. Ferritin levels and COVID-19. Rev Panam Salud Publica. 2020;44:e72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 10. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3246] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 11. | Li Y, Regan J, Fajnzylber J, Coxen K, Corry H, Wong C, Rosenthal A, Atyeo C, Fischinger S, Gillespie E, Chishti R, Baden L, Yu XG, Alter G, Kim A, Li JZ. Liver Fibrosis Index FIB-4 Is Associated With Mortality in COVID-19. Hepatol Commun. 2021;5:434-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Li Y, Li H, Zhu S, Xie Y, Wang B, He L, Zhang D, Zhang Y, Yuan H, Wu C, Sun W, Li M, Cui L, Cai Y, Wang J, Yang Y, Lv Q, Zhang L, Xie M. Prognostic Value of Right Ventricular Longitudinal Strain in Patients With COVID-19. JACC Cardiovasc Imaging. 2020;13:2287-2299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 326] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 13. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G, Marra F, Pinzani M. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 574] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 14. | Wool GD, Miller JL. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology. 2021;88:15-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 15. | Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1779] [Cited by in RCA: 1710] [Article Influence: 342.0] [Reference Citation Analysis (0)] |