Published online Apr 21, 2022. doi: 10.3748/wjg.v28.i15.1574
Peer-review started: November 17, 2021
First decision: January 9, 2022
Revised: January 18, 2022
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 21, 2022
Processing time: 148 Days and 21.1 Hours
Intrahepatic cholangiocarcinoma (ICC) is a highly malignant tumour. Hepatectomy is an effective treatment for early ICC, but postoperative recurrence greatly affects patient survival. Studies on recurrent ICC after hepatectomy are lacking.
To investigate the clinical characteristics of patients with recurrent ICC after hepatectomy, analyse prognostic factors and explore diagnosis and treatment strategies.
A retrospective analysis was performed on all ICC patients undergoing hepatectomy from January 2013 to August 2021. Patients with postoperative recurrence were selected according to the inclusion and exclusion criteria. Cumulative overall survival was plotted by the Kaplan-Meier method, and differences were assessed by univariate survival analysis using the log-rank test. Multivariate analysis of cumulative survival was performed using the Cox proportional risk model.
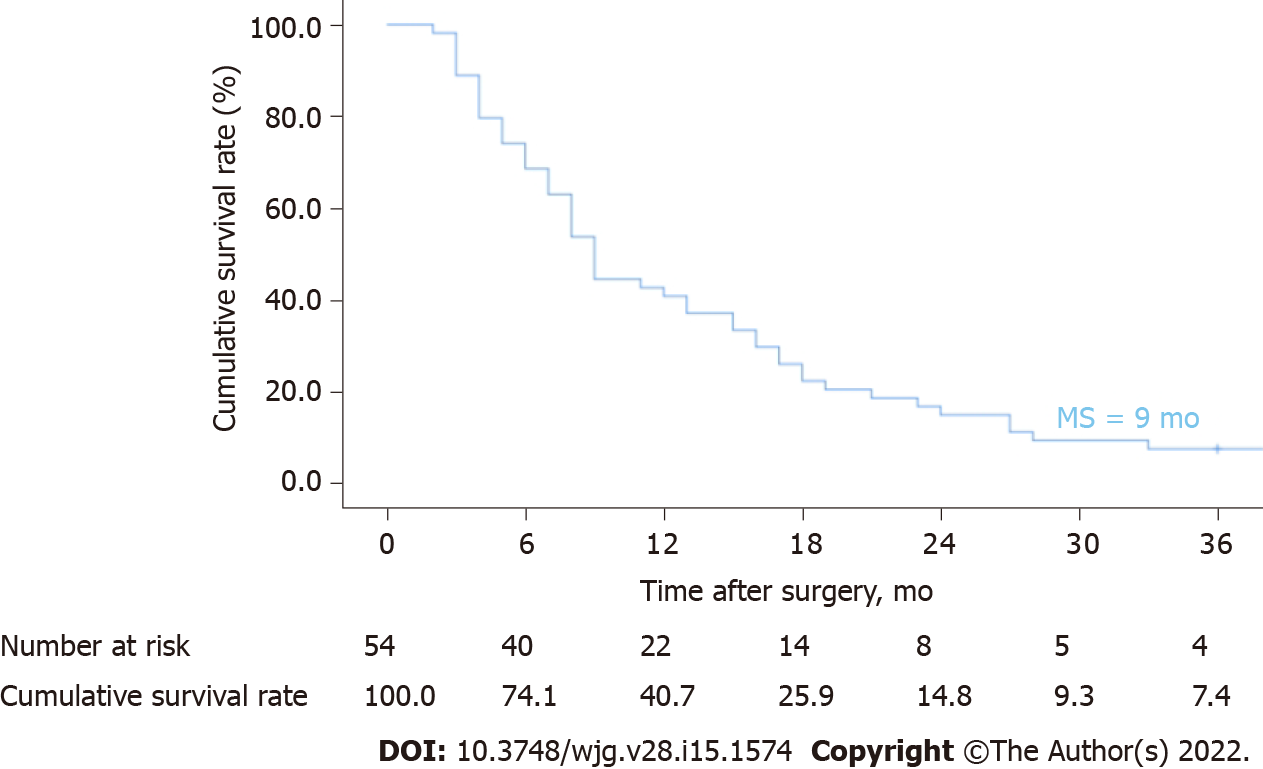
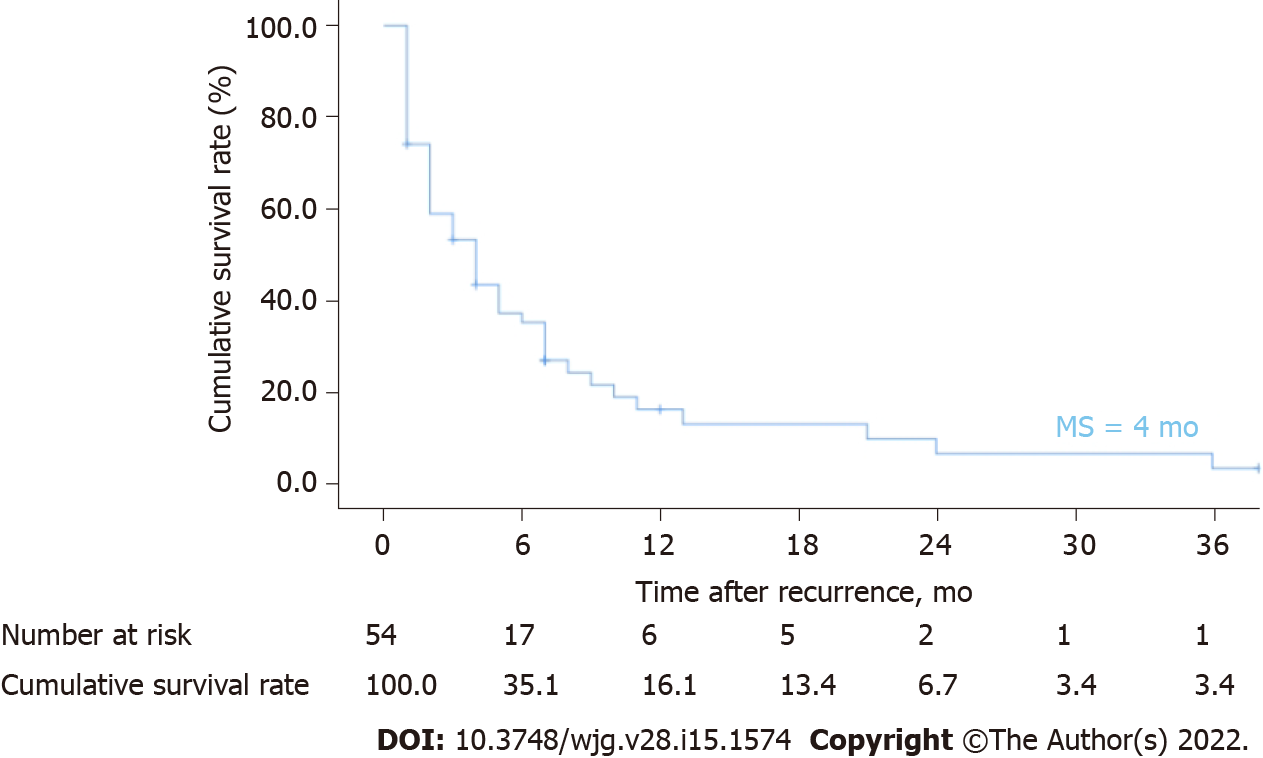
During the 8-year study period, 103 patients underwent ICC-related hepatectomy, and 54 exhibited postoperative recurrence. The median disease-free survival (DFS) was 6 mo, the median overall survival (OS) was 9 mo, and the cumulative OS rates at 1, 2 and 3 years after the operation were 40.7%, 14.8% and 7.4%, respectively. The median OS after recurrence was 4 mo, and the cumulative OS rates at 1, 2 and 3 years after recurrence were 16.1%, 6.7% and 3.4%, respectively. Multivariate analysis showed that alcohol consumption [hazard ratio (HR) = 4.64, 95% confidence interval (CI): 1.53-14.04, P = 0.007] and DFS < 6 mo (HR = 3.47, 95%CI: 1.59-7.60, P = 0.002) were independent risk factors for the cumulative survival of patients with recurrence, while treatment after recurrence (HR = 0.21, 95%CI: 0.08-0.55, P = 0.001) was an independent protective factor. The median OS time of patients receiving multimodality therapy after recurrence of ICC was 7 mo, which was significantly higher than that of patients receiving only local therapy (3 mo), patients receiving systematic therapy (4 mo) and patients receiving the best supportive therapy (1 mo). Patients with recurrent ICC who received multimodality therapy had a significantly better long-term survival after recurrence than those who did not (P = 0.026).
The prognosis of patients with recurrence after ICC-related hepatectomy is poor. Alcohol consumption and DFS < 6 mo are independent risk factors in terms of the cumulative survival of patients with recurrence, while treatment after recurrence is an independent protective factor. Multimodality therapy can effectively improve the prognosis of patients.
Core Tip: With this 8-year retrospective study, we aimed to investigate the clinical characteristics, analyse the prognostic factors, and discuss therapeutic strategies for patients with recurrent intrahepatic cholangiocarcinoma (ICC) after hepatectomy. Multivariate analysis showed that alcohol consumption [hazard ratio (HR) = 4.64, 95% confidence interval (CI): 1.53-14.04, P = 0.007] and disease-free survival < 6 mo (HR=3.47, 95%CI: 1.59-7.60, P = 0.002) were independent risk factors for cumulative survival for patients with recurrence, while treatment after recurrence (HR=0.21, 95%CI: 0.08-0.55, P = 0.001) was an independent protective factor. We propose that multimodality therapy should be developed to improve long-term outcomes through the combined approach of local therapy, chemotherapy, targeted therapy, and immunotherapy.
- Citation: Yuan ZB, Fang HB, Feng QK, Li T, Li J. Prognostic factors of recurrent intrahepatic cholangiocarcinoma after hepatectomy: A retrospective study. World J Gastroenterol 2022; 28(15): 1574-1587
- URL: https://www.wjgnet.com/1007-9327/full/v28/i15/1574.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i15.1574
Intrahepatic cholangiocarcinoma (ICC) is a highly malignant tumour originating from intrahepatic bile duct epithelial cells[1]. Liver cancer ranks sixth in the world in terms of incidence rate and third in terms of mortality rate[2]. ICC accounts for 10% to 15% of primary liver cancers[1]. In the last 30 years, the incidence and mortality rates of ICC have significantly increased worldwide[3]. Hepatectomy is an effective method for the treatment of early ICC[4]. However, ICC has highly malignant biological behaviour, and early recurrence and metastasis are extremely common, so the prognosis is poor[5]. The postoperative 5-year survival rate is only 20%-35%, and the recurrence rate is as high as 50%-70%, and these rates are much worse than those for hepatocellular carcinoma[6,7].
Prevention of ICC recurrence and treatment strategies after recurrence are extremely important to improve the overall survival (OS) time. The early recurrence of ICC is related to the characteristics of the tumour, while late recurrence is related to underlying liver diseases[8]. Studies[9] have shown that the presence of multiple tumours, microvascular invasion, and lymph node metastasis are risk factors for recurrence after hepatectomy. Age, liver disease, lymph node involvement, vascular invasion, multiple tumours, and tumour size are related to prognosis[10]. However, the risk factors affecting the long-term prognosis of patients with recurrent ICC after hepatectomy are not clear. The European Association for Liver Research[11] and the Italian Clinical Practice Guide[12] have pointed out that the treatment strategy for recurrent ICC is based on the clinical characteristics of the site of tumour recurrence. Recently, some studies[6,13] have reported various treatments for different types of recurrence. However, the best treatment strategy for the postoperative recurrence of ICC is still unclear.
In this study, the clinical characteristics and treatment statistics of patients with recurrent ICC after hepatectomy in our hospital were assessed to identify survival-related factors and explore strategies for diagnosis and treatment.
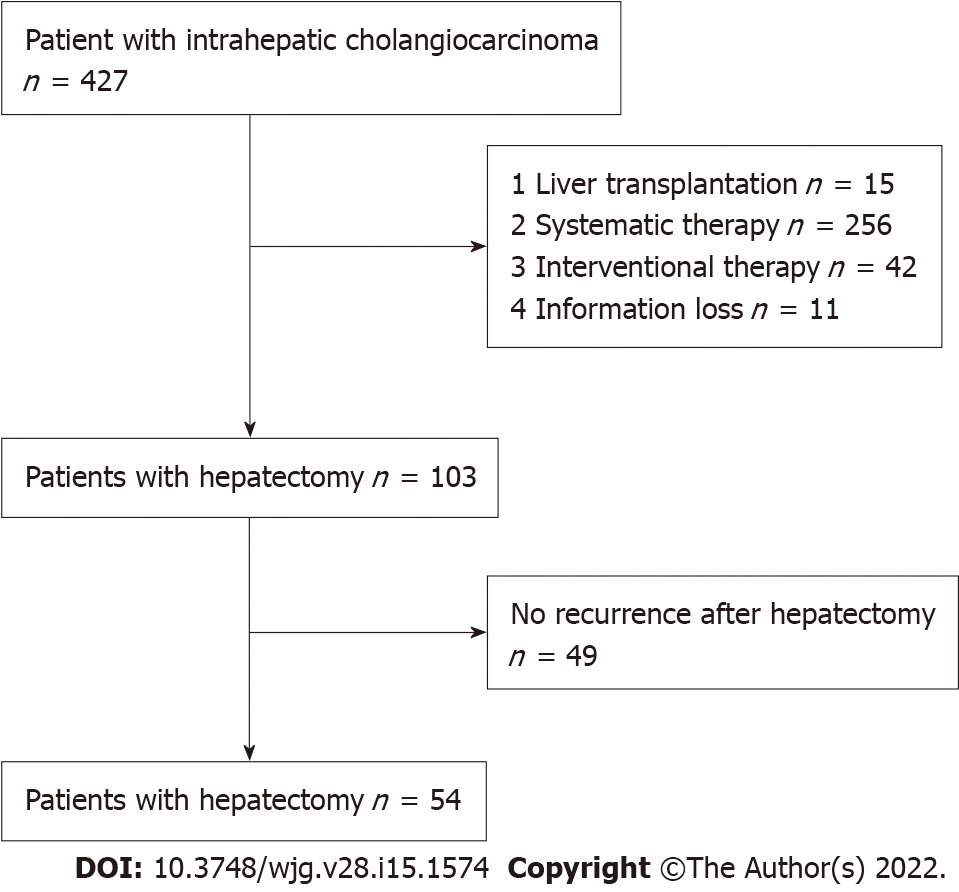
The clinical data of 103 ICC patients who underwent hepatectomy in the First Affiliated Hospital of Zhengzhou University were analyzed retrospectively from January 2013 to August 2021. The diagnosis of ICC was based on liver pathological examination, and histological grading was based on the WHO grading system[14]. The tumour stage was determined according to the American Joint Council on Cancer (AJCC) 8th edition tumour-node-metastasis classification system[15]. The inclusion criteria were as follows: (1) Primary intrahepatic cholangiocarcinoma was confirmed by postoperative histopathology; (2) Liver function was considered Child-Pugh grade A or B; (3) Preoperative evaluation indicated that the patient could tolerate surgery without serious heart, lung, brain, and kidney vital organ lesions; and (4) Relapse was observed after hepatectomy. The exclusion criteria were as follows: (1) The patient had a preoperative history of malignant tumour; (2) Postoperative histopathology confirmed hepatocellular carcinoma or mixed liver cancer; or (3) Clinical records and follow-up information were incomplete. Finally, a total of 54 patients with recurrent ICC after hepatectomy were included (Figure 1). The study was approved by the Scientific Research and Clinical Trial Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Ethical number 2021-KY-0464-001).
The mode of operation was determined according to the location and size of the tumour and the patient’s liver function. The scope of resection was classified according to the international consensus standard[16]: Extended hepatectomy was performed in 16 cases (resection of liver tissue more than 3 segments), and local hepatectomy was performed in 38 cases (marginal partial hepatectomy or resection of liver tissue no more than 3 segments). Abnormal enlargement of lymph nodes was found during the operation or imaging examination before the operation, and the hepatic hilum, hepatoduodenal ligament, and posterior pancreatic lymph nodes were dissected. Lymph node dissection was performed in 19 of the 54 patients. According to National Comprehensive Cancer Network (NCCN) practice guidelines[17], 20 ICC patients were treated with adjuvant therapy after hepatectomy.
After hepatectomy, all patients were followed up via outpatient visits or telephone calls. Follow-up was initiated 1 mo after intrahepatic cholangiocarcinoma resection, followed by follow-up visits every 3 mo for 2 years and every 6 mo after 2 years. Patients with postoperative recurrence of ICC were followed up once a month, and the last follow-up was in August 2021. During the follow-up period, the patient examinations included (1) Haematology examination, including assessment of liver and kidney function, serum tumour markers, and hepatitis viral load; and (2) Imaging examination, including chest plain film or nonenhanced CT, abdominal enhanced CT or MRI. To evaluate the progression of the disease, patients with recurrent ICC were examined by whole-body bone scan or PET-CT. Follow-up began at the time of hepatectomy and ended at the time of death or the last follow-up. Disease-free survival (DFS) was defined as the time from the date of surgery to the first recurrence of ICC. OS was defined as the time from the first recurrence after hepatectomy to death or the last follow-up.
For those who are diagnosed with tumour recurrence or metastasis, the treatment plan is determined according to the evaluation of the reserve function of the liver, the condition of the whole body, and the site of recurrence. The inclusion criteria of secondary hepatectomy were the same as those of primary hepatectomy. Patients with unresectable ICC are treated with local therapy, chemotherapy, targeted therapy, immunotherapy, and multimodality therapy.
For descriptive statistics, continuous variables are expressed as medians, and categorical variables are expressed as numbers (%). Cumulative survival was plotted by the Kaplan-Meier method. The log-rank test was used to assess differences in the univariate survival analysis. Multivariate analysis of cumulative survival was performed using the Cox proportional risk model. Statistical analysis was performed using SPSS 26.0 software (IBM, Armonk, NY, United States). Differences were considered statistically significant at P < 0.05. An online tool (http://www.bioinformatics.com.cn) was applied to draw Venn digrams related to recurrent patterns.
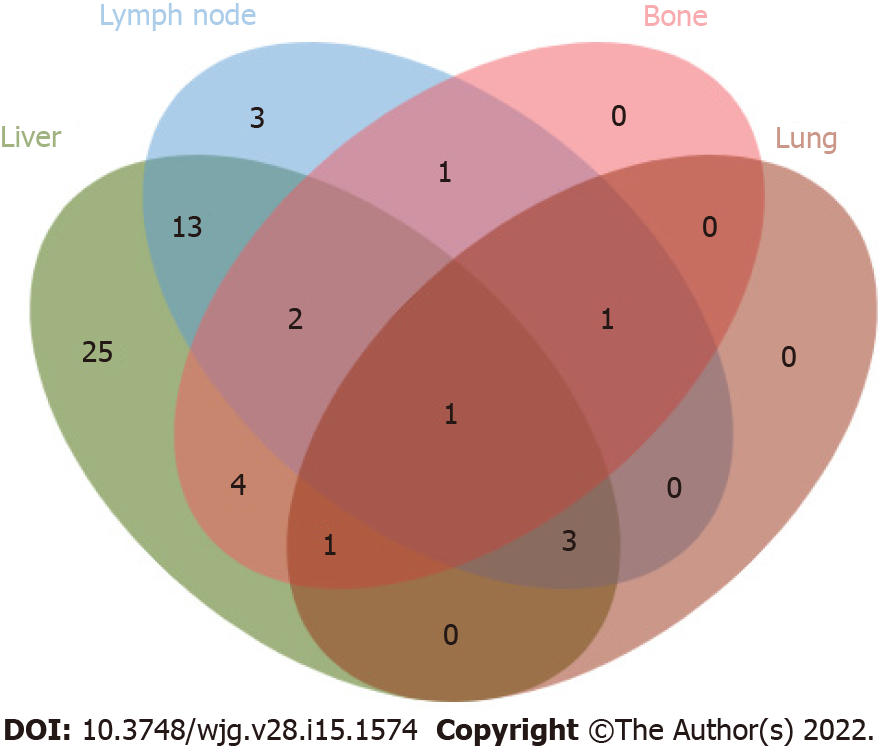
By the end of follow-up, 54 ICC patients (54/103, 52.4%) had recurrence after hepatectomy. Patients were followed up for 2-94 mo, with a median DFS of 6 mo and a median OS of 9 mo. The 1-year, 2-year, and 3-year cumulative OS rates were 40.7%, 14.8%, and 7.4%, respectively (Figure 2). The median OS after recurrence was 4 mo, and the 1-year, 2-year and 3-year cumulative survival rates after recurrence were 16.1%, 6.7%, and 3.4%, respectively (Figure 3). The majority of patients (45/54, 83.3%) relapsed within 1 year, and the recurrence rate was 50% (27/54) within 6 mo after surgery. Venn diagrams showed that intrahepatic lesions (25/54, 46.3%) were the most common recurrence sites, followed by concurrent liver and lymph lesions (13/54, 24.1%) (Figure 4). The clinical and pathological features of patients with recurrence are shown in Table 1. Most of the patients were male (34/54, 63.0%), smokers (34/54, 63.0%), and alcohol consumers (44/54, 81.5%). Sixteen patients had hypertension, and 9 patients had diabetes. Twenty-four patients were associated with hepatitis B virus (HBV), and 9 patients associated with hepatitis C virus (HCV). Sixteen patients were treated with extensive hepatectomy, and lymph node dissection was performed in 19 patients. Postoperative pathological reports showed that 46 patients had single tumours, 22 patients had poorly differentiated tumours, and 12 patients had vascular tumour thrombi.
| Factors | Cases, n (%) | Median survival after recurrence, mo (95%CI) | P value1 |
| Sex | 0.123 | ||
| Male | 34 (63.0) | 4.0 (1.2-6.8) | |
| Female | 20 (37.0) | 3.0 (1.1-4.9) | |
| Age (yr) | 0.031 | ||
| < 65 | 42 (77.8) | 4.0 (2.3-5.8) | |
| ≥ 65 | 12 (22.2) | 3.0 (1.3-5.1) | |
| Smoking | 0.059 | ||
| No | 20 (37.0) | 5.0 (2.0-8.0) | |
| Yes | 34 (63.0) | 3.0 (1.0-4.1) | |
| Alcohol consumption | 0.004 | ||
| No | 10 (18.5) | 10.0 (3.0-17.0) | |
| Yes | 44 (81.5) | 3.0 (1.9-4.2) | |
| Hypertension | 0.309 | ||
| No | 38 (70.4) | 4.0 (2.4-5.6) | |
| Yes | 16 (29.6) | 4.0 (0.0-11.3) | |
| Diabetes | 0.193 | ||
| No | 45 (83.3) | 4.0 (2.5-5.5) | |
| Yes | 9 (16.7) | 8.0 (1.0-15.0) | |
| Hepatitis B | 0.904 | ||
| Negative | 30 (55.6) | 3.0 (1.3-4.7) | |
| Positive | 24 (44.4) | 5.0 (2.0-8.0) | |
| Hepatitis C | 0.175 | ||
| Negative | 45 (83.3) | 4 .0 (2.2-5.8) | |
| Positive | 9 (16.7) | 5.0 (2.1-7.9) | |
| Anti-hepatitis-virus | 0.969 | ||
| No | 29 (53.7) | 4.0 (2.4-5.6) | |
| Yes | 25 (46.3) | 5.0 (1.9-8.1) | |
| Cholelithiasis | 0.181 | ||
| No | 44 (81.5) | 4.0 (2.1-5.9) | |
| Yes | 10 (18.5) | 2.0 (0.0-4.8) | |
| CA19-9 (U/mL) (initial) | 0.002 | ||
| < 200 | 33 (61.1) | 5.0 (1.8-8.2) | |
| ≥ 200 | 21 (38.9) | 2.0 (1.3-2.7) | |
| CEA (ng/mL) (initial) | 0.356 | ||
| < 5 | 34 (63.0) | 4.0 (2.1-5.9) | |
| ≥ 5 | 20 (37.0) | 3.0 (0.8-5.2) | |
| Hepatectomy | 0.855 | ||
| Limited | 38 (70.4) | 4.0 (2.1-5.9) | |
| Extended | 16 (29.6) | 3 .0 (0.0-6.9) | |
| Lymph node dissection | 0.909 | ||
| No | 35 (64.8) | 4.0 (2.2-5.8) | |
| Yes | 19 (35.2) | 5.0 (2.1-7.9) | |
| Adjuvant treatment | 0.619 | ||
| No | 34 (63.0) | 3 .0 (0.1-5.9) | |
| Yes | 20 (37.0) | 4 .0 (2.7-5.3) | |
| Tumor size (cm) | 0.884 | ||
| < 5 | 14 (25.9) | 4.0 (0.4-7.6) | |
| ≥ 5 | 40 (74.1) | 4 .0 (2.0-6.0) | |
| Multiplicity | 0.803 | ||
| Solitary | 46 (85.2) | 4.0 (1.4-6.6) | |
| Multiple | 8 (14.8) | 3.0 (1.3-4.7) | |
| Satellite nodules | 0.953 | ||
| No | 34 (63.0) | 4.0 (2.1-5.9) | |
| Yes | 20 (37.0) | 4.0 (2.4-5.6) | |
| Histological grade | 0.021 | ||
| PD | 22 (40.7) | 2.0 (1.2-2.8) | |
| WD or MD | 32 (59.3) | 5.0 (3.5-6.5) | |
| Vascular invasion | 0.705 | ||
| No | 49 (90.7) | 4.0 (2.1-5.9) | |
| Yes | 5 (9.3) | 5.0 (1.7-8.3) | |
| Lymph node metastasis | 0.531 | ||
| No | 29 (53.7) | 3.0 (0.9-5.1) | |
| Yes | 25 (46.3) | 5.0 (2.6-7.4) | |
| Perineural invasion | 0.428 | ||
| No | 45 (83.3) | 4.0 (2.5-5.5) | |
| Yes | 9 (16.7) | 2.0 (1.3-2.7) | |
| Biliary invasion | 0.003 | ||
| No | 35 (64.8) | 5.0 (2.6-7.4) | |
| Yes | 19 (35.2) | 2.0 (1.2-2.8) | |
| Vascular tumour thrombi | 0.002 | ||
| No | 42 (77.8) | 5.0 (3.2-6.8) | |
| Yes | 12 (22.2) | 2.0 (1.2-2.6) | |
| AJCC T category | 0.196 | ||
| T1–2 | 14 (25.9) | 4.0 (0.0-11.3) | |
| T3–4 | 40 (74.1) | 4.0 (2.1-5.9) | |
| DFS | 0.003 | ||
| < 6 mo | 27 (50.0) | 2.0 (0.7-3.3) | |
| ≥ 6 mo | 27 (50.0) | 6.0 (2.3-9.7) | |
| CA19-9 (U/mL) (recurrence) | 0.002 | ||
| < 200 | 35 (64.8) | 4.0 (1.3-6.7) | |
| ≥ 200 | 19 (35.2) | 2.0 (1.2-2.8) | |
| CEA (ng/mL) (recurrence) | 0.378 | ||
| < 5 | 41 (75.9) | 4.0 (2.3-5.7) | |
| ≥ 5 | 13 (24.1) | 7.0 (1.7-12.3) | |
| NLR (recurrence) | 0.804 | ||
| < 2 | 24 (44.4) | 5.0 (2.8-7.2) | |
| ≥ 2 | 30 (55.6) | 2.0 (0.9-3.1) | |
| Recurrent site | 0.334 | ||
| Intrahepatic | 24 (44.4) | 4.0 (2.3-5.7) | |
| Extrahepatic | 6 (11.2) | 3.0 (0.0-11.6) | |
| Intrahepatic + extrahepatic | 24 (44.4) | 2.0 (0.4-3.6) | |
| Treatment after recurrence | < 0.001 | ||
| No | 12 (22.2) | 2.0 (1.0-2.8) | |
| Yes | 42 (77.8) | 5.0 (2.9-7.1) |
According to the univariate analysis of patients with recurrent ICC, nine factors significantly affected the survival of patients (Table 1). Age, alcohol consumption, histological grade, biliary invasion, vascular tumour thrombi, DFS, preoperative and post-recurrence CA19-9 level, and treatment after recurrence were significant favorable prognostic indicators in patients with recurrent ICC. Multivariate Cox regression analysis showed that alcohol consumption, DFS < 6 mo and treatment after recurrence were independent factors affecting cumulative survival in patients with recurrence (Table 2). Early recurrence in ICC patients was associated with biliary invasion, vascular tumour thrombi, and high post-recurrence CA19-9 levels. Multivariate analysis proved that the risk of death from alcohol consumption was 4.64 times that of non-alcohol consumption, and this was independent of other prognostic factors. The mortality risk of patients with DFS < 6 mo was 3.47 times that of patients with DFS > 6 mo. Treatment after recurrence could significantly reduce the mortality risk.
| Factors | Multivariate analysis | ||
| HR | 95%CI | P value1 | |
| Age (≥ 65 yr) | 2.12 | 0.88-5.12 | 0.096 |
| Alcohol consumption (Yes) | 4.64 | 1.53-14.04 | 0.007 |
| CA19-9 (≥ 200 U/mL) (initial) | 2.63 | 0.94-7.35 | 0.065 |
| Histological grade (PD) | 1.18 | 0.59-2.35 | 0.646 |
| Biliary invasion (Yes) | 1.04 | 0.41-2.63 | 0.940 |
| Vascular tumor thrombus (Yes) | 1.80 | 0.70-4.65 | 0.222 |
| DFS (< 6 mo) | 3.47 | 1.59-7.60 | 0.002 |
| CA19-9 (≥ 200 U/mL) (recurrence) | 1.13 | 0.47-2.71 | 0.785 |
| Treatment after recurrence (Yes) | 0.21 | 0.08-0.55 | 0.001 |
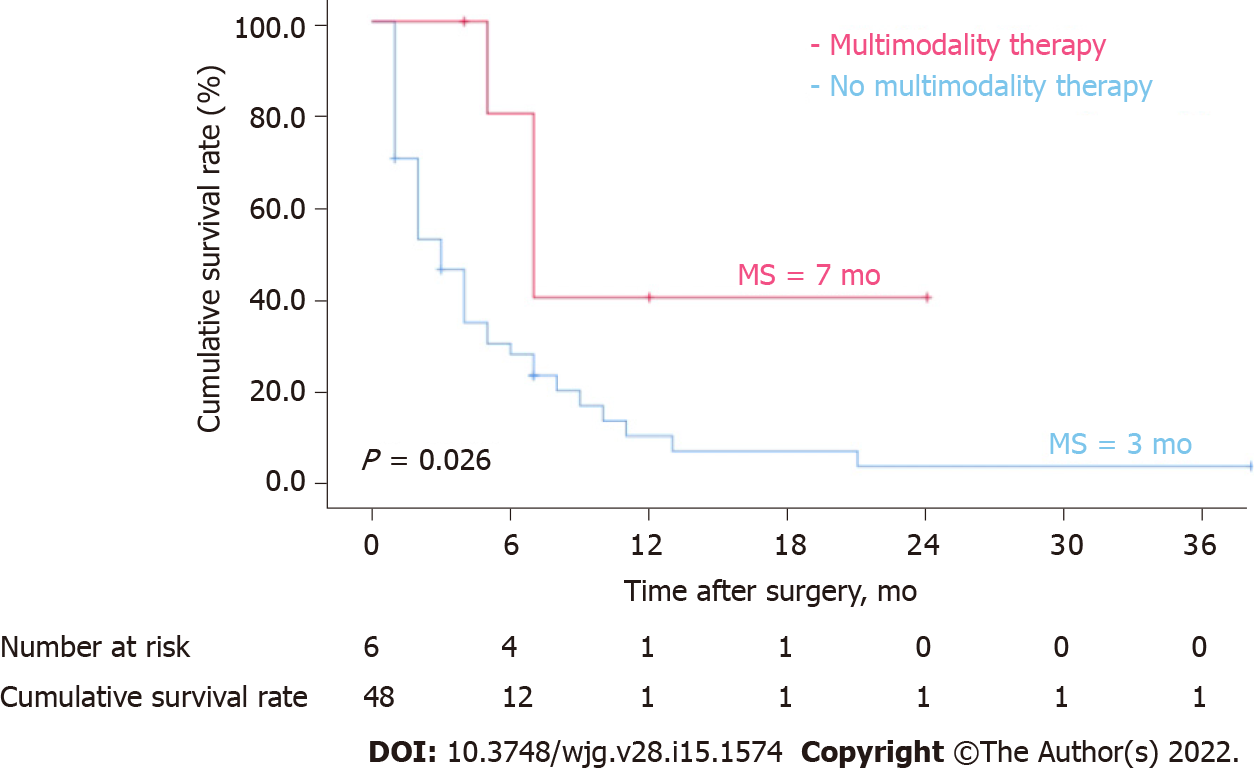
Treatment patterns for patients with recurrent ICC are shown in Table 3. Fourteen patients received local therapy, 22 patients received systematic therapy, 6 patients received multimodality therapy, and 12 patients received supportive care therapy based on their condition. Figure 5 shows patients with recurrent ICC who received multimodality therapy had a significantly better long-term survival after recurrence than those who did not (P = 0.026, log-rank test). Among the patients who received local treatment, 2 patients had hepatectomy after recurrence; 1 patient had received local liver resection, and hepatectomy was performed again after 7 mo. the patient died due to multiple metastases. Another patient underwent laparoscopic hepatectomy for the primary lesion, but the tumour recurred 13 mo after surgery. Local hepatectomy and lymph node dissection were performed for the recurrent lesion. Table 4 shows the clinicopathological features of the 6 patients who received multimodality therapy for recurrence, of which 2 patients (No. 4, 6) survived, and 4 patients (No. 1, 2, 3, 5) died due to tumour-related complications. Three patients with recurrence (No. 1, 2, 3) received the GEMOX regimen (1 g/m² gemcitabine on d 1 and 8 + 100 mg/m² oxaliplatin on d 1 with 21 d/cycle) after transarterial chemoembolization (TACE). One patient (No. 4) received local therapy after intrahepatic recurrence. Due to extrahepatic metastasis, the patient was switched to the SOX regimen (60 mg/d tegafur on d 1-14 + 130 mg/m² oxaliplatin on d 1 with 21 d/cycle) maintenance therapy. One patient with recurrence (No. 5) received the FOLFOX-4 regimen (400 mg/m² fluorouracil on d 1 and 2 + 200 mg/m² calcium folate on d 1 and 2 + 85 mg/m² oxaliplatin on d 1 with 14 d/cycle) after RFA. One patient (No. 6) received a tyrosine kinase inhibitor regimen + PD-1 inhibitor (250 mg/d apatinib mesylate + 200 mg camrelizumab on d 1 with 21 d/cycle) after RFA, and no disease progression was observed up to the submission date.
| Treatment | n | Median DFS, mo (range) | MS after recurrence, mo (range) |
| Local therapy | 14 | 6 (1-86) | 3 (1-36) |
| Hepatectomy | 2 | 10 (7-13) | 25 (13-36) |
| TACE | 8 | 3 (1-23) | 2 (1-7) |
| RFA | 4 | 7 (2-86) | 3 (1-7) |
| Systemic therapy | 22 | 5 (1-29) | 4 (1-38) |
| Chemotherapy | 3 | 11 (10-29) | 4 (1-38) |
| Targeted therapy | 12 | 3 (1-23) | 4 (1-10) |
| Targeted + immunization therapy | 7 | 4 (1-13) | 7 (2-24) |
| Multimodality therapy | 6 | 4 (2-11) | 7 (4-24) |
| Best supportive care | 12 | 6 (3-20) | 1 (1-6) |
| Clinical features | Pathological characteristics | Recurrent | Survival | ||||||||||
| Case | Age (yr) | Sex | Hepatitis | Surgery | Lymph node metastasis | Tumour size (cm) | Tumour number | Histological grade | Recurrent site | Treatment | DFS (mo) | SAR (mo) | Outcome |
| 1 | 62 | M | HBV | ERH | Yes | 12 | 1 | MD | Liver + lymph node | TACE + GEMOX | 4 | 3 | Dead |
| 2 | 48 | M | HBV | ERH | None | 8 | 1 | MD | Liver | TACE + GEMOX | 11 | 13 | Dead |
| 3 | 40 | M | HBV | ERH | None | 5 | 2 | MD | Liver + lymph node + bone | TACE + GEMOX | 2 | 5 | Dead |
| 4 | 53 | M | None | LH | None | 11 | 1 | MD | Liver + lymph node | RFA + TACE + SOX | 6 | 6 | Alive |
| 5 | 44 | M | HBV | RH | None | 14 | 1 | MD | Liver + bone | RFA + FOLFOX-4 | 3 | 2 | Dead |
| 6 | 64 | M | None | ERH | Yes | 22 | 1 | MD | Liver | RFA + PD-1 + TKI | 4 | 1 | Alive |
ICC is a rare invasive biliary tract tumour and primary liver malignancy with an increasing incidence worldwide[3]. Most patients are initially diagnosed with advanced ICC, and only 30% of ICCs can be surgically resected[18]. Surgical principles include negative margins and tumour-related lymph node resection[19-21]. Due to the biological characteristics of ICC and the vascular system of the liver, local recurrence and lymphatic metastasis are highly likely to occur after surgery. Even after R0 resection, the recurrence rate 5 years after hepatectomy is as high as 70%[6]. At present, the choice of treatment for recurrent ICC remains controversial. Since ICC after recurrence seriously affects the postoperative survival of patients, it is necessary to determine the risk factors for survival after recurrence and explore diagnosis and treatment strategies.
Previous studies[22,23] have explored the clinical characteristics and prognostic factors of the postoperative recurrence of ICC, but few studies have shown the prognosis of ICC patients after recurrence. Chan et al[22] reported that tumour diameter > 5 cm, tumour type, lymph node invasion, and vascular invasion are independent risk factors for recurrence in patients after hepatectomy. In other studies, Addeo et al[23] found that the risk factors influencing patient recurrence were related to the degree of tumour differentiation and the number of tumours. Regarding the prognosis of patients with recurrent ICC, Ohira et al[10] reported that tumour type and nonsurgical treatment were related to a poor prognosis. Our study found that alcohol consumption and DFS < 6 mo were independent risk factors affecting the cumulative survival rate of patients with recurrence, and treatment after recurrence was an independent protective factor.
Previous studies[24] have shown that alcohol consumption is a risk factor for ICC. Alcohol may interfere with DNA synthesis and repair through the mechanism of acetaldehyde, a product of ethanol oxidation, to promote the occurrence of liver cancer[25]. Although alcohol drinking is associated with the aetiology of ICC, it is not clear whether alcohol drinking affects the prognosis and survival of patients with recurrent ICC. In this study, multivariate analysis showed that alcohol consumption may be an independent risk factor for recurrent ICC. For patients with recurrent ICC, we recommend reducing alcohol consumption as much as possible to improve the prognosis and survival time of patients.
In this study, the first recurrence of most patients after hepatectomy occurred within 1 year after surgery. Multivariate analysis showed that DFS < 6 was an independent risk factor for survival after ICC recurrence. Park et al[26] and Si et al[27] also reported that DFS was associated with prognosis. Compared with the clinical characteristics of patients with advanced recurrence, early recurrence is often accompanied by bile duct invasion and lymph node metastasis, and the median survival time after recurrence is 2 mo, which is much lower than the time of late recurrence. Currently, immunohistochemical markers commonly used to predict early recurrence after hepatectomy include B-lymphocyte chemokine 13 (CXCL13)[28], pancreatic secreted trypsin inhibitor (PSTI)[29], and insulin-like growth factor-II mRNA binding protein 3 (IMP3)[30]. Even if the prognosis of patients with early recurrence of ICC after primary hepatectomy is poor, surgical treatment should be considered to improve the prognosis.
The survival time of patients with recurrent ICC after surgical resection is higher than that of patients without surgical resection. Furthermore, compared with other treatments, secondary hepatectomy significantly improved the OS time of patients with recurrent ICC. Studies[31] have shown that the prognosis of recurrent intrahepatic resection of ICC is comparable to that of primary resection. In a multicentre study of 356 patients with ICC who underwent hepatectomy, approximately 60% exhibited postoperative recurrence, and 37 of them underwent reresection, with a 5-year survival rate of 44%[32]. Recent studies[33] have reported that repeat resection after recurrence significantly prolongs OS compared with palliative treatment. Therefore, we suggest that patients with resectable intrahepatic recurrent ICC can undergo reoperation to improve patient outcomes.
Most recurrent ICCs are highly invasive and have limitations, such as insufficient remaining liver, making patients ineligible for secondary hepatectomy. Multimodality therapies include strategies that combine regional therapy, systemic chemotherapy, targeted therapy, and immunotherapy. In this study, 6 patients with recurrent ICC who were mainly treated with multimodality therapies achieved a higher postoperative median OS (7 mo) than those with local treatment (3 mo), systemic treatment (4 mo), and supportive treatment (1 mo).
Systemic chemotherapy combined with local therapy can significantly improve patient prognosis. Intra-arterial therapies combined with chemotherapy can shrink the lesion to achieve R0 resection[34]. In this study, the median survival of 3 patients with recurrent ICC treated with TACE combined with the GEMOX regimen was 5 mo, which was longer than that of patients with recurrent ICC using chemotherapy alone (median OS, 4 mo). RFA is suitable for local tumours with diameters < 5 cm, and tumour numbers < 3. RFA was superior to systematic chemotherapy in this study[35]. Among the 6 patients with recurrent ICC, 2 patients underwent RFA combined with chemotherapy. One patient had recurrence 3 mo after hepatectomy, and local intrahepatic lesions were treated with RFA combined with the FOLFOX-4 regimen. The other patient relapsed 6 mo after surgery, and 2 mo after RFA, multiple intrahepatic metastases occurred. TACE combined with the SOX regimen was performed again. Currently, the patient is still alive. A new approach of radiotherapy combined with chemotherapy in the open treatment of advanced ICC. Studies[36] have shown that radiotherapy with chemotherapy can not only relieve pain and other complications in patients with advanced ICC but can also improve the disease control rate and patient survival time. Japanese researchers[37] found that 60% of patients with advanced ICC underwent radical hepatocellular carcinoma after radiotherapy combined with systemic chemotherapy, and the 5-year survival rate was 24%. Radiotherapy was not included in our treatment strategy for patients with recurrent ICC. Due to the lack of reliable evidence-based medical data, the NCCN practice guidelines[17] did not recommend radiotherapy as routine treatment for recurrent ICC.
Among the 6 patients with recurrent ICC in our centre, one patient was treated with a tumour immune checkpoint inhibitor combined with targeted therapy after RFA; this patient was still alive without disease progression at the time of submission. PD-L1 expression was found in interstitial cells in 30% of ICC patients[38]. In the tumour microenvironment of connective tissue hyperplasia and immune system deficiency in ICC, the clinical efficacy of a single drug PD-1/PD-L1 inhibitor in tumour suppression is poor[39]. Targeted therapy combined with immunotherapy is being explored[40]. Targeted drugs can induce the death of tumour cells, leading to the release of their own antigens, which are then taken up by antigen-presenting cells to activate specific T cells. However, they also upregulate inhibitory factors such as CTLA-4 and PD-1. Therefore, the combination of PD-1 inhibitors can strengthen the killing effect, reduce the attack of nontumour antigens, and reduce the adverse reactions of immunotherapy[41]. The combination of tumour immune checkpoint inhibitors and targeted therapy is still a hotspot in the field of tumour therapy.
There are several limitations to this study. First, this is a retrospective study, and there may be selection and detection bias in patients with recurrent ICC. Second, ICC is a rare disease. Although the clinical study lasted for 8 years, the number of patients with recurrence is small, and there are not enough randomized controlled trials of recurrent patients. Finally, this is a single-centre study, so multicentre and prospective trials are needed to confirm our results.
The prognosis of patients with recurrence after ICC-related hepatectomy is poor. Alcohol consumption and DFS < 6 mo are independent risk factors in terms of the cumulative survival of patients with recurrence, while treatment after recurrence is an independent protective factor. We propose that multimodality therapy should be developed to improve long-term outcomes through the combined approach of local therapy, chemotherapy, targeted therapy, and immunotherapy.
Intrahepatic cholangiocarcinoma (ICC) is a highly malignant tumour originating from intrahepatic bile duct epithelial cells. Recurrence is very common after hepatectomy.
There are few reports on the clinical features and prognostic factors of recurrent ICC, and the treatment strategies for recurrent ICC have not been fully clarified.
The objective of this study was to analyze the prognostic factors of recurrent ICC and to explore treatment strategies.
We retrospectively analyzed all ICC patients who underwent hepatectomy at the First Affiliated Hospital of Zhengzhou University between January 2013 and August 2021. We summarized the clinical characteristics of patients with recurrent ICC and assessed prognostic factors by univariate and multivariate analyses.
Recurrence occurred in 54 of 103 patients with ICC after hepatectomy during the study period. The median OS of patients with recurrent ICC was 4 mo, and the cumulative OS rates at 1, 2, and 3 years after recurrence were 16.1%, 6.7%, and 3.4%, respectively. Multivariate analysis of cumulative survival by the Cox proportional risk model showed that alcohol consumption [hazard ratio (HR) = 4.64, 95% confidence interval (CI): 1.53-14.04, P = 0.007], DFS < 6 mo (HR = 3.47, 95%CI: 1.59-7.60, P = 0.002) and treatment after recurrence (HR = 0.21, 95%CI: 0.08-0.55, P = 0.001) were independent factors for recurrence. Patients who received multimodality therapy had higher survival rates than those who did not (P = 0.026).
The prognosis of recurrent patients is related to alcohol consumption, DFS < 6 mo and treatment after recurrence. Active and effective multidisciplinary treatment is beneficial to improve the prognosis of patients.
Multicentre prospective studies are needed to evaluate the efficacy of multidisciplinary treatment in recurrent ICC.
We are very grateful to the following doctors for their selfless help in the process of data collection, statistical analysis and clinical treatment: Wu TC, Yang H, Li JJ.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kordzaia D, Georgia; Saengboonmee C, Thailand S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72:353-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 63793] [Article Influence: 15948.3] [Reference Citation Analysis (174)] |
| 3. | Sirica AE, Gores GJ, Groopman JD, Selaru FM, Strazzabosco M, Wei Wang X, Zhu AX. Intrahepatic Cholangiocarcinoma: Continuing Challenges and Translational Advances. Hepatology. 2019;69:1803-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 224] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 4. | Zhang H, Yang T, Wu M, Shen F. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. 2016;379:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 5. | Zhou R, Lu D, Li W, Tan W, Zhu S, Chen X, Min J, Shang C, Chen Y. Is lymph node dissection necessary for resectable intrahepatic cholangiocarcinoma? HPB (Oxford). 2019;21:784-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Hu LS, Zhang XF, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Koerkamp BG, Itaru E, Pawlik TM. Recurrence Patterns and Timing Courses Following Curative-Intent Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2019;26:2549-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 7. | Doussot A, Gonen M, Wiggers JK, Groot-Koerkamp B, DeMatteo RP, Fuks D, Allen PJ, Farges O, Kingham TP, Regimbeau JM, D'Angelica MI, Azoulay D, Jarnagin WR. Recurrence Patterns and Disease-Free Survival after Resection of Intrahepatic Cholangiocarcinoma: Preoperative and Postoperative Prognostic Models. J Am Coll Surg. 2016;223:493-505.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 8. | Yoh T, Hatano E, Seo S, Okuda Y, Fuji H, Ikeno Y, Taura K, Yasuchika K, Okajima H, Kaido T, Uemoto S. Long-Term Survival of Recurrent Intrahepatic Cholangiocarcinoma: The Impact and Selection of Repeat Surgery. World J Surg. 2018;42:1848-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Cholangiocarcinoma Working Group. Italian Clinical Practice Guidelines on Cholangiocarcinoma - Part I: Classification, diagnosis and staging. Dig Liver Dis. 2020;52:1282-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Ohira M, Kobayashi T, Hashimoto M, Tazawa H, Abe T, Oshita A, Kohashi T, Irei T, Oishi K, Ohdan H; Hiroshima Surgical study group of Clinical Oncology (HiSCO). Prognostic factors in patients with recurrent intrahepatic cholangiocarcinoma after curative resection: A retrospective cohort study. Int J Surg. 2018;54:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 1067] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 12. | Cholangiocarcinoma Working Group. Italian Clinical Practice Guidelines on Cholangiocarcinoma - Part II: Treatment. Dig Liver Dis. 2020;52:1430-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Bartsch F, Paschold M, Baumgart J, Hoppe-Lotichius M, Heinrich S, Lang H. Surgical Resection for Recurrent Intrahepatic Cholangiocarcinoma. World J Surg. 2019;43:1105-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2389] [Article Influence: 477.8] [Reference Citation Analysis (3)] |
| 15. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4348] [Article Influence: 543.5] [Reference Citation Analysis (4)] |
| 16. | Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, OʼRourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schön MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619-629. |
| 17. | Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 558] [Article Influence: 139.5] [Reference Citation Analysis (0)] |
| 18. | Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17:669-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 339] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 19. | Li MX, Bi XY, Li ZY, Huang Z, Han Y, Zhao JJ, Zhao H, Cai JQ. Impaction of surgical margin status on the survival outcome after surgical resection of intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Surg Res. 2016;203:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Zhang XF, Chen Q, Kimbrough CW, Beal EW, Lv Y, Chakedis J, Dillhoff M, Schmidt C, Cloyd J, Pawlik TM. Lymphadenectomy for Intrahepatic Cholangiocarcinoma: Has Nodal Evaluation Been Increasingly Adopted by Surgeons over Time? J Gastrointest Surg. 2018;22:668-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Zhang XF, Xue F, Dong DH, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Koerkamp BG, Itaru E, Lv Y, Pawlik TM. Number and Station of Lymph Node Metastasis After Curative-intent Resection of Intrahepatic Cholangiocarcinoma Impact Prognosis. Ann Surg. 2021;274:e1187-e1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 22. | Chan KM, Tsai CY, Yeh CN, Yeh TS, Lee WC, Jan YY, Chen MF. Characterization of intrahepatic cholangiocarcinoma after curative resection: outcome, prognostic factor, and recurrence. BMC Gastroenterol. 2018;18:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Addeo P, Jedidi I, Locicero A, Faitot F, Oncioiu C, Onea A, Bachellier P. Prognostic Impact of Tumor Multinodularity in Intrahepatic Cholangiocarcinoma. J Gastrointest Surg. 2019;23:1801-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Gupta A, Dixon E. Epidemiology and risk factors: intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 25. | Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006;387:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 26. | Park HM, Yun SP, Lee EC, Lee SD, Han SS, Kim SH, Park SJ. Outcomes for Patients with Recurrent Intrahepatic Cholangiocarcinoma After Surgery. Ann Surg Oncol. 2016;23:4392-4400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Si A, Li J, Xing X, Lei Z, Xia Y, Yan Z, Wang K, Shi L, Shen F. Effectiveness of repeat hepatic resection for patients with recurrent intrahepatic cholangiocarcinoma: Factors associated with long-term outcomes. Surgery. 2017;161:897-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Li C, Kang D, Sun X, Liu Y, Wang J, Gao P. The Effect of C-X-C Motif Chemokine 13 on Hepatocellular Carcinoma Associates with Wnt Signaling. Biomed Res Int. 2015;2015:345413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Tonouchi A, Ohtsuka M, Ito H, Kimura F, Shimizu H, Kato M, Nimura Y, Iwase K, Hiwasa T, Seki N, Takiguchi M, Miyazaki M. Relationship between pancreatic secretory trypsin inhibitor and early recurrence of intrahepatic cholangiocarcinoma following surgical resection. Am J Gastroenterol. 2006;101:1601-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Chen YL, Jeng YM, Hsu HC, Lai HS, Lee PH, Lai PL, Yuan RH. Expression of insulin-like growth factor II mRNA-binding protein 3 predicts early recurrence and poor prognosis in intrahepatic cholangiocarcinoma. Int J Surg. 2013;11:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Zhang XF, Beal EW, Bagante F, Chakedis J, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Koerkamp BG, Itaru E, Pawlik TM. Early vs late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br J Surg. 2018;105:848-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 174] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 32. | Yamashita YI, Shirabe K, Beppu T, Eguchi S, Nanashima A, Ohta M, Ueno S, Kondo K, Kitahara K, Shiraishi M, Takami Y, Noritomi T, Okamoto K, Ogura Y, Baba H, Fujioka H. Surgical management of recurrent intrahepatic cholangiocarcinoma: predictors, adjuvant chemotherapy, and surgical therapy for recurrence: A multi-institutional study by the Kyushu Study Group of Liver Surgery. Ann Gastroenterol Surg. 2017;1:136-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Kitano Y, Yamashita YI, Nakagawa S, Okabe H, Imai K, Chikamoto A, Baba H. Effectiveness of surgery for recurrent cholangiocarcinoma: A single center experience and brief literature review. Am J Surg. 2020;219:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Wang L, Lin ZG, Ke Q, Lou JY, Zheng SG, Bi XY, Wang JM, Guo W, Li FY, Wang J, Zheng YM, Li JD, Cheng S, Zhou WP, Zeng YY. Adjuvant transarterial chemoembolization following radical resection for intrahepatic cholangiocarcinoma: A multi-center retrospective study. J Cancer. 2020;11:4115-4122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Wu L, Tsilimigras DI, Farooq A, Hyer JM, Merath K, Paredes AZ, Mehta R, Sahara K, Shen F, Pawlik TM. Potential survival benefit of radiofrequency ablation for small solitary intrahepatic cholangiocarcinoma in nonsurgically managed patients: A population-based analysis. J Surg Oncol. 2019;120:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, Kwak EL, Allen JN, Clark JW, Goyal L, Murphy JE, Javle MM, Wolfgang JA, Drapek LC, Arellano RS, Mamon HJ, Mullen JT, Yoon SS, Tanabe KK, Ferrone CR, Ryan DP, DeLaney TF, Crane CH, Zhu AX. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol. 2016;34:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 331] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 37. | Sumiyoshi T, Shima Y, Okabayashi T, Negoro Y, Shimada Y, Iwata J, Matsumoto M, Hata Y, Noda Y, Sui K, Sueda T. Chemoradiotherapy for Initially Unresectable Locally Advanced Cholangiocarcinoma. World J Surg. 2018;42:2910-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | Kitano Y, Yamashita YI, Nakao Y, Itoyama R, Yusa T, Umezaki N, Tsukamoto M, Yamao T, Miyata T, Nakagawa S, Okabe H, Imai K, Chikamoto A, Ishiko T, Baba H. Clinical Significance of PD-L1 Expression in Both Cancer and Stroma Cells of Cholangiocarcinoma Patients. Ann Surg Oncol. 2020;27:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE, Schell MJ, Zhou JM, Mahipal A, Kim BH, Kim DW. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020;6:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 321] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 40. | Wang D, Lin J, Yang X, Long J, Bai Y, Mao Y, Sang X, Seery S, Zhao H. Combination regimens with PD-1/PD-L1 immune checkpoint inhibitors for gastrointestinal malignancies. J Hematol Oncol. 2019;12:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 41. | Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1718] [Cited by in RCA: 1779] [Article Influence: 177.9] [Reference Citation Analysis (0)] |