Published online Mar 28, 2022. doi: 10.3748/wjg.v28.i12.1226
Peer-review started: November 5, 2021
First decision: December 26, 2021
Revised: January 6, 2022
Accepted: February 23, 2022
Article in press: February 23, 2022
Published online: March 28, 2022
Processing time: 139 Days and 17.3 Hours
Hepatitis E virus (HEV) and hepatitis C virus (HCV) are both RNA viruses with a tropism for liver parenchyma but are also capable of extrahepatic manifestations. Hepatitis E is usually a viral acute fecal-oral transmitted and self-limiting disease presenting with malaise, jaundice, nausea and vomiting. Rarely, HEV causes a chronic infection in immunocompromised persons and severe fulminant hepatitis in pregnant women. Parenteral HCV infection is typically asymptomatic for decades until chronic complications, such as cirrhosis and cancer, occur. Despite being two very different viruses in terms of phylogenetic and clinical presentations, HEV and HCV show many similarities regarding possible transmission through organ transplantation and blood transfusion, pathogenesis (production of antinuclear antibodies and cryoglobulins) and response to treatment with some direct-acting antiviral drugs. Although both HEV and HCV are well studied individually, there is a lack of knowledge about coinfection and its consequences. The aim of this review is to analyze current literature by evaluating original articles and case reports and to hypothesize some interactions that can be useful for research and clinical practice.
Core Tip: Hepatitis E virus (HEV) and Hepatitis C virus (HCV) are both RNA viruses characterized by greater variability than DNA viruses and mainly infect the liver. Despite these similarities, the two viruses have different species barriers and disease progression. Coinfection with particular HCV and HEV types could aggravate hepatic and/or extrahepatic diseases, taking into account virus–host interactions between the two viruses during viral replication.
- Citation: Marascio N, Rotundo S, Quirino A, Matera G, Liberto MC, Costa C, Russo A, Trecarichi EM, Torti C. Similarities, differences, and possible interactions between hepatitis E and hepatitis C viruses: Relevance for research and clinical practice . World J Gastroenterol 2022; 28(12): 1226-1238
- URL: https://www.wjgnet.com/1007-9327/full/v28/i12/1226.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i12.1226
Viral hepatitis is a global public health problem, affecting more than 325 million people globally. In countries with poor health care standards, coinfection among hepatotropic viruses is possible due to multiple risk factors. This condition increases morbidity and mortality rates in infected patients[1]. Hepatitis E virus (HEV) could influence hepatic or extrahepatic symptoms in patients with chronic hepatitis C virus (HCV) infection[2,3]. Both the prevalence and spreading of HEV and HCV infections worldwide reflect different routes of transmission and high genomic variability[4,5], however coinfections or superinfections with the two viruses in the same individuals may occur, though a paucity of data exist in this respect.
A summary of virological and pathogenic characteristics of both viruses discussed through the text of this review are reported in the Table 1.
| Categories | Similarity | Difference | Interaction |
| Epidemiology | High prevalence in developing countries[1] | HEV infects humans and animals[6,7] | Co-infections or superinfections[2,3] |
| Genetic variability | RNA viruses (quasispecies), genotype classification, recombination events[4,5] | Replication rate, HEV has non-enveloped or quasi-enveloped virions[12,26] | None |
| Pathogenesis | Disease progression in immunocompromised patients[32,54] | Microbiota alteration, hepatic severity[23,33,83,84] | HEV could influence hepatic or extrahepatic symptoms in patients with chronic HCV infection[2,3] |
| Treatment | Choice of therapy[35,50] | PEG-IFN-α and RBV are still the therapies of choice for HEV[35] | DAA therapy can be effective against both viruses[50,53] |
| Prevention | Public health measures[58] | Vaccine availability[60,62] | Improvement of screening policies[35,50] |
HEV affects around 20 million people worldwide, and the infection is distributed in both developing and industrialized countries[1]. This enteric non-enveloped virus, belonging to the Hepeviridae family, Orthohepevirus genus, is classified into eight genotypes and 24 subtypes. HEV1 and HEV2 infect only humans in resource limited settings, such as Asia, Mexico, and sub-Saharan and Central Africa[6]. HEV3, emerging in Europe as a sporadic infection, and HEV4 infect both humans and animals. HEV4 shows a high prevalence in Asia[7]. In 2014, HEV5 and HEV6 were isolated from wild boars, while HEV7 (originally infecting dromedaries) was isolated from a human case for the first time[8,9]. Lastly, HEV8 was detected in Bactrian camels[10]. Of note, the nomenclature system of this virus is constantly changing due to frequent identification of novel strains in various animal species[11]. The main routes of transmission are fecal-oral and zoonotic (i.e., undercooked meat or close contact with animals). In industrialized countries, transmission is related to travelers returning from endemic areas and to blood transfusion or organ transplantation[12,13]. Human-to-human transmission was also described in men having sex with men[14], as well as HEV can infect newborns by vertical transmission[15]. Sero
HCV is also very widely disseminated throughout the world. Indeed, approximately 71 million people worldwide are infected by HCV, an enveloped virus belonging to the Flaviviridae family and Hepacivirus genus. In 2018, Borgia and colleagues identified the eighth genotype in patients from India[17]. The distributions of the genotypes and 86 subtypes are related to risk factors and geography across the world. In developing countries, HCV1 and HCV2 with high subtype diversity are prevalent. HCV3 is predominant in Europe, North America and Southeast Asia. In the Middle East and Central Africa, HCV4 is endemic, while HCV5 was found exclusively in South Africa[18]. HCV6 is present essentially in Japan and nearby areas. HCV7 is responsible for less than 1% of cases of HCV hepatitis. In industrialized countries, the most prevalent subtypes are HCV1a, 1b, 2c, 3a, and 4a[19,20]. HCV1b and 2c are mainly transmitted by blood transfusion and infect older population groups, whereas HCV1a, 3a and 4a are prevalent in intravenous drug users[21,22]. Low standards for healthcare procedures have allowed HCV spreading among patients in hemodialysis units[23]. After 1992, blood screening controlled the spread of this infection. Sexual and mother-to-infant (6%) transmissions increased in subjects coinfected with human immunodeficiency virus (HIV), while breastfeeding does not significantly increase the risk of transmission from mother to baby[24].
RNA viruses have high genetic plasticity, and they can rapidly generate a drug-resistant viral population or evade the host system under pressure. The key of this variability is the polymerase without proofreading activity[25]. During viral replication with a mutation rate ranging from 10-6 to 10-4 substitutions per nucleotide, the virus produces hundreds of progeny (quasispecies), which differ by one or a few nucleotides in the genomic sequence. The fitness of quasispecies reflects Darwinian evolution and natural selection allows the spread of a better adapted viral population[26]. HEV and HCV are both positive-sense single-stranded (ss) RNA viruses, even if the organization and length of the genome are different.
The HEV genome (7.2 kb) contains three open reading frames (ORFs) between the 5′UTR- and 3′-UTR (polyA-tract) regions. ORF1 encodes enzymes, including RNA-dependent RNA polymerase (RdRp) and non-structural proteins. ORF2 and ORF3 encode for capsid protein and a multifunctional phosphoprotein, respectively. ORF4 is directly involved during replication[27]. By contrast, the HCV genome (9.6 kb), containing one ORF between the 5’-3’UTRs, encodes three structural (C, core) proteins, envelope glycoproteins 1 and 2 (E1 and E2), and finally seven non-structural (p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B) proteins. In particular, NS5B encodes the polymerase enzyme[28].
HEV genetic characteristics make it suitable for infecting humans and animals through various transmission routes, since it is maintained in the environment[12]. Virions are present in two different forms, non-enveloped excreted in the feces of humans or animals and quasi-enveloped coming from blood. Quasi-enveloped virions bind cells in a less effective way, showing minor infectivity[12]. The high similarity among HEV3 and HEV4 strains isolated from humans and animals demonstrated that adaptation is not necessary for infection. On the other hand, HEV1 does not have zoonotic reservoirs, as experimentally, intra-species transmission failed to infect the progeny in pig, rat or goat. Species barriers of HEV1 appear to be related to genetic elements carried on the ORF1 non-structural protein[11]. As far as HCV is concerned, the barrier between species may be responsible for the unique targeting of humans by this virus. However, endemic circulation in an area of the world where human, ape and monkey populations overlap and the discovery of viruses closely related to HCV in animals suggested a zoonotic origin[29].
Recombination events increased the genetic variability for both HCV and HEV viruses. Among HEV genotypes, as well as fragment of human genes and HEV strains, recombination is possible. In particular, two insertions of the ORF1 hypervariable domain on the human RPS17 gene (ribosomal protein S17) increased replication in hepatoma cells[27]. Likewise, during HCV superinfection, recombination events (inter-genotype or inter-subtype), using different breakpoints within the viral genome were identified. The first circulating form was HCV2k/1b with a mapped breakpoint in the NS2 gene. At present, seven inter-genotypes (2k/1b, 21/6p, 2b/1b, 2/5, 2b/6w, 3a/1b, and 2a/1a) and three inter-subtypes (1b/1a, 1a/1c, and 4a/4d) recombinant forms (RFs) are known[30].
The incubation period of HEV infection ranges from 2 to 10 wk[31]. HEV determines acute hepatitis with a very low incidence (1%-4%), varying severities, which resolves in 2–3 mo[32]. One of the most serious outcomes is fulminant hepatitis (FH), which is characterized by hepatic parenchyma necrosis, renal failure or coma[33,34]. The wide spectrum of clinical illness could indeed be related to the infecting genotype[31]. In 2015, Smith and Simmonds reviewed published papers for causal association between FH and genomic variability[34]. The correlation appears to be related to epidemiological factors, namely, restricted geographical areas and time span of collected isolates[34]. The majority of people acquiring infection do not have severe consequences. However, HEV1 and HEV2 are the principal genotypes related to severe disease and mortality and HEV1 was the principal responsible for outbreaks in some countries of Asia and Africa between 1987 and 2015[15]. Several studies reported FH to be related to specific nucleotide substitutions in the HEV1, HEV3 and HEV4 genomes. For instance, the U3148 and C5907 substitutions in HEV3 and HEV4 strains were significantly associated with FH[27]. However, HEV3 hardly progresses to acute liver failure[35]. Extra-hepatic manifestations such as membranoproliferative glomerulonephritis and cryoglobulinemia are not rare in HEV infected patients[13] and it was suggested that in severely immunocompromised patients HEV could be implicated in development of hepatic cancer[36]. Also, common neurological disorders in the course of HEV infection were found such as nerve root, plexus disorders and meningoencephalitis[37,38].
HEV can also cause chronic infection, lasting a year or more in immunosuppressed individuals[32], which has only been observed for HEV3 and HEV4[39]. Comparison of HEV3 isolates between blood donors and patients with hepatitis showed just one polymorphism difference (leucine to phenylalanine ORF2 substitution) in sequences from the first category. Anyway, there is no evidence of pathogenesis related with substitutions occurring in virus genomes[33]. Interestingly, the fast progression to liver fibrosis has been associated with slow quasispecies diversification during one year of chronic infection[40].
In contrast to HEV, HCV frequently (50%-80%) causes chronic hepatitis, which is associated with liver cirrhosis, steatosis and hepatocellular carcinoma (HCC)[40]. The variability of genotypes/subtypes was associated with pathogenetic significance. HCV1b hypervariable region 1 (HVR1) of E2 protein displays significantly higher genetic variability than HCV3. HCV3 establishes hepatic chronic infection in less cases compared to other HCV types, particularly HCV1b. The hypervariable E2 region of HCV1b displays low evolutionary dynamics during the course of infection, generating few viral variants, which could provide a fitness advantage under immune system and therapy pressures[41], while the lower variability of HCV3 results in a lower chance to establish chronic infection[41]. On the other hand, HCV3 core protein expression is able to induce more intracellular lipid accumulation causing steatosis more than other genotypes[40]. Indeed, HCV3 infection is associated with steatosis more frequently than HCV1. Some amino acid substitutions in HCV3 core proteins upregulate the sterol regulatory element binding protein-1 (SREBP-1), inducing intracellular lipid accumulation[41].
Usually, acute HEV infection does not require antiviral therapy[35]. Ribavirin (RBV) monotherapy may be considered in cases of severe acute hepatitis or chronic infection in solid-organ transplant recipients. PEGylated-interferon-a (PEG-IFN-α) was effectively administered to patients after liver transplant or hemodialysis[35], although IFN can cause several side effects[34]. RBV therapy with or without PEG-IFN-a is contraindicated during pregnancy[15]. Sustained virological response (SVR) is achieved only in 78% of chronic patients treated with ribavirin for a median period of three months, probably because of viral mutants[35]. Deep sequencing detected the Y1320H, K1383N and G1634R polymerase substitutions on HEV3 isolates from patients who relapsed or failed RBV therapy[35,42]. Clearly, RBV increases viral heterogeneity, leading to the emergence of different viral populations[35].
PEG-IFN-α and RBV were the standard of care to treat HCV until 2011. Direct-acting antiviral (DAA) drugs quickly changed the landscape of infection, as patients achieved a high SVR rate (95%-99%). Five pan-drug combinations are available right now to treat HCV: Sofosbuvir (SOF), sofosbuvir/velpatasvir (SOF/VEL), sofosbuvir/velpatasvir/voxilaprevir (SOF/VEL/VOX), glecaprevir/pibrentasvir (GLE/PIB) and grazoprevir/elbasvir (GZR/EBR)[43]. DAA drugs determined direct pressure on the viral genome, producing quasispecies with resistance associated substitutions (RASs) on NS3/4A, NS5A and NS5B target regions escaping therapy[44]. Several RASs on all target regions after treatment with first-/second-generation and IFN-free regimens in specific HCV types were reported[44-46]. Additionally, natural polymorphisms carried on specific subtypes can confer resistance to NS5A inhibitors. In the last EASL guidelines, experts recommended to detect resistance on NS5A (from 24 to 93 amino acid positions) for subtypes 1l, 4r, 3b, 3g, 6u, and 6v prior to first-line treatment[43]. Indeed, patients who failed therapy displayed NS5A RAS at baseline in the same rate of virological failure[47]. The HCV RFs have been reported in few cases around the world, thus pathogenesis and therapy efficacy are not well characterized. Two patients infected by RF 2b/1b achieved viral clearance with an interferon-free regimen[48]. In contrast, a patient infected by the same RF failed two different interferon-free regimens[49].
Of note, new DAA therapies for HCV had an indirect effect on HEV in coinfected patients. SOF is approved for the treatment of chronic HCV infection but can also inhibit HEV replication in vitro (especially if co-administered with RBV) and could be an interesting treatment option in coinfected individuals[50], but clinical universal efficacy has not yet been demonstrated[35]. A SOF based DAA regimen excludes occult HCV or HEV infection in patients who received a liver or renal transplant[51] and successful treatment was reported in some cases of HEV/HCV coinfection. Biliotti and colleagues reported viral clearance of HCV3 and HEV3 in one infected patient after therapy with SOF plus RBV[52]. In a subject infected through liver transplantation, the combination of SOF, daclatasvir (DCV) and RBV led both to HCV-RNA undetectability 6 wk after the initiation of therapy and to HEV-RNA undetectability at 12 wk after initiation of therapy[53]. In another immunosuppressed patient affected by both HCV and HEV infections, SOF in combination with DCV reduced HCV-RNA to undetectable levels after 4 wk of treatment but did not have a significant effect on serum HEV-RNA levels[54]. Lastly, one patient treated for 12 wk with SOF/DCV/RBV and tenofovir cleared HCV and HEV without risk of HBV reactivation[55]. In clinical practice, detection of HCV and potential HEV genome substitutions may be useful to predict treatment failure[25,44].
In 2016, a new molecular mechanism against HCV and HEV was proposed by Wang and colleagues[56]. INF-γ and TNF-α play essential roles in infections by intracellular agents and show a synergistic effect in experimentally transfected cells with HCV or HEV by activating NF-kB signaling. Antiviral activity is related to innate immune responses. Cooperation between INF-γ and TNF-α, activating signaling cascades, protects against HCV and HEV infection[56].
Prevention of infections is possible through public health measures and screening policies. In endemic areas for HEV, it is important to wash hands frequently, drink bottled water and eat fruits and vegetables washed with safe water[57]. In areas with low endemicity and zoonotic transmission, simple hygiene measures and cooking meat well done can be fundamental to reduce transmission[30]. HCV and HEV may share the same route of transmission, and blood transfusion and organ transplantation can be dangerous for recipient patients and their immunosuppressed status[57,58]. Tests to detect anti-HCV antibodies are standardized. Additionally, HCV core antigen and molecular assay are used to identify patients with ongoing viral infection[43]. On the other hand, a HEV diagnosis needs a combination of an antibody test and molecular assay due to the specificity of the assay being suboptimal and anti-HEV IgM not being a really robust marker[35].
However, vaccines are the best protection against viral infections. HEV genotypes represent one single serotype, with a serological cross-reactivity, thus one vaccine should protect against all types, despite genetic heterogeneity[30]. In China, a vaccine based on the ORF2 protein had high efficiency in a large human population and has been licensed, but is not available elsewhere at this moment[59]. However, mutations on the ORF changed the structure of the ORF2 protein, reducing the protective efficacy of the vaccine. For preventive purposes, naturally attenuated viral variants carrying substitutions in the polymerase region could be used in the future[27]. Very recently, Chen and colleagues evaluated the safety and efficacy of immunization with an accelerated HEV239 vaccine (Hecolin®). Protective antibodies, produced within 21 d, can be useful during an ongoing HEV outbreak or for travelers and humanitarian workers moving to endemic areas in a short time[60]. At present, HEV Vaccine Working Group by the WHO’s Strategic Advisory Group of Experts (SAGE) considered the use of Hecolin® for the general populations residing in endemic areas during outbreaks as quickly as possible. However, due to the lack of data about immunogenicity and safety, the Working Group did not recommend the routine use of this vaccine for specific risk groups, such as pregnant women, patients with chronic liver disease and immunocompromised persons[1]. In the next future, human and animal vaccinations should be associated, considering the One Health concept, for preventing transmission and improving public health[57].
In contrast to HEV vaccine, the HCV vaccine is still under development since there are several limitations, such as easy culture systems not being available, animal models for testing, and viral genetic diversity (genotypes, subtypes and quasispecies). The extraordinary variability of HCV determines several opportunities to select, within and between infected individuals, viral variants escaping the immune response[61]. In 2017, University of Oxford in collaboration with other industries developed a candidate vaccine using the entire HCV NS3-5B protein. At present, the vaccine is in phase 1 (EudraCT Number 2016-000983-41) to assess the safety and effectiveness of the immune response against the virus in healthy volunteers. The estimated completion of the study is August 2022[62]. Eradication of HCV by 2030 is the goal of the World Health Organization, and the organization must consider improvements in screening policies and hope for an effective vaccine.
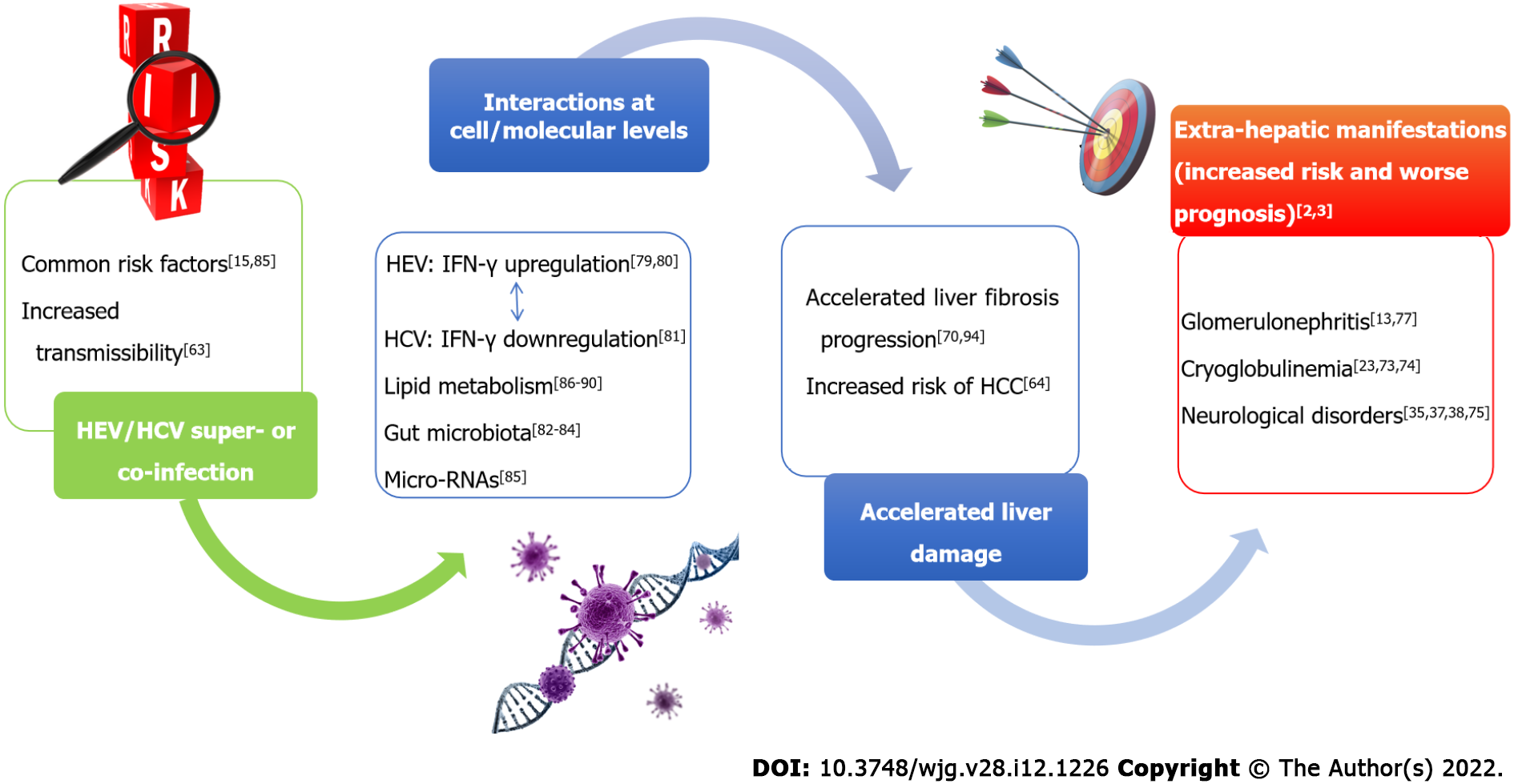
A schematic overview of HEV/HCV possible interactions is reported in Figure 1.
Co-infections or superinfections of HEV with HCV may be due to a common parenteral route of transmission. Moreover, it was hypothesized that alteration of the intestinal mucosa associated with chronic liver damage due to HCV facilitates HEV translocation from the gut to the liver of patients infected through the oral route[63]. There is a lack of studies investigating the prevalence of possible coinfections with HCV and HEV. At present, it is impossible to provide reliable estimates of the actual prevalence, since information came from few studies and case reports. Future studies, including adequately large sample size, should be planned to estimate the actual prevalence of coinfections. Moreover, the main limitation of the epidemiological surveys conducted so far is that only antibody tests were used[58,64]. Detection of anti-HEV immunoglobulins is related to specificity and sensitivity of commercial kits, among which discordant results were reported in the literature[65]. In 2016, Norder and coworkers[66] evaluated the performance of five commercial assays to determine IgM and IgG levels against HEV. IgM titer was detected by a sensitive HEV IgM/HEV IgG test after the onset of symptoms, providing concordant results in 99% samples from patients with suspected HEV infection. By contrast, recomWell™ HEV IgG/IgM (Mikrogen Diagnostik, Neuried, Germany) and DS-EIA-ANTI-HEV-G/M™ (DSI Srl, Milan, Italy) tests were found to be less specific. In conclusion, investigating the actual rate of coinfections and the effect of both viruses on liver disease progression would require more accurate serological assays and more studies using direct detection of HCV and HEV RNA by molecular tests.
Hepatic damage: Infections due to HEV and HCV, even if occurring at different times, can lead to a worse clinical course[58,67]. In fact, serum IgG directed against HEV were associated with a faster evolution towards more severe degrees of fibrosis in patients with chronic HCV infection[58]. Coexistence of the two viruses appeared to be associated with accelerated progression of liver damage as evidenced by the reduced number of platelets, increased transaminases and prolonged prothrombin times observed in patients with chronic HCV hepatitis with HEV exposure during their lifetime (IgG-positive) when compared to HCV mono-infected patients[68]. It is possible that HEV infection in patients infected by HCV with a significant degree of liver fibrosis, accelerates liver damage to such an extent that liver decompensation and death may occur more frequently[69]. These considerations point to the importance of treating HCV and preventing HEV superinfection (either primary prevention or vaccine strategies) in patients affected by chronic HCV infection, a situation which may be particularly frequent or problematic in resource-limited settings. In patients with HCV related HCC, HEV seroprevalence was 11% (compared to 6% in the healthy population), while it reached 42% in patients who underwent liver transplantation for chronic HCV infection[67]. In 2005, Elhendawy and coauthors reported HCV/HEV coinfections in 71.4% of chronic hepatitis patients and in 96.1% of cirrhotic patients with or without HCC, suggesting a possible relationship between the two viruses on progression of liver disease[67]. Recently, the prevalence of HEV infection among adults with chronic liver disease, from 2011 and 2018, was evaluated and anti-HEV IgG positivity was found in 8.6% of HCV chronic positive patients, with a high prevalence in the oldest individuals compared to young age groups[68]. Also, possible effects of HEV infection in increasing the risk of liver cancer over HCV-induced subclinical liver injury[70] further emphasizes the importance of treatment and preventative strategies for these two viruses to reduce overlap in the same individuals.
Extra-hepatic diseases: Since both viruses may be responsible for extra-hepatic diseases, several studies described these manifestations and correlated them with genetic features[38]. Importantly, HCV does not infect only hepatic cells, and the virus has been found in peripheral blood mononuclear cells, T cells, and monocytes, as well as in B cells and macrophages of colonic tissue. HCV replicates within carotid plaques induce arterial inflammation, probably through the pro-inflammatory cytokine interleukin 1β regardless of viral type[71]. The extrahepatic infection, demonstrated by cell lines producing HCV2a virions, could explain the late relapses observed in clinical trials[72]. Both acute and chronic hepatitis E infections are associated with antinuclear antibodies and cryoglobulinemia in the serum of patients that is similar to untreated HCV infection. The cryoglobulin concentration correlates with the viral load rather than with the degree of inflammation[73]. Serum cryoglobulins in the serum of patients affected by HCV infection are associated with a worse degree of steatosis and fibrosis, and it is not known if the same can happen in HEV infection[74]. Likewise, the risk of evolution to lymphoproliferative diseases associated with HEV cryoglobulinemia with or without HCV cryoglobulinemia is unknown. Furthermore, insulin resistance and metabolic syndrome have already been related to HCV infection, as well as HEV infection recently, which can contribute to the progression of fibrosis in patients with chronic liver disease[3]. As far as HEV is concerned, the neurological disease Guillain-Barre syndrome did not appear to be genotype specific[38], but HEV1 was associated with neurological injury[35], as well as HCV[75]. Moreover, HEV1 and HEV3 were found to be responsible for acute pancreatitis, which has already been described for major hepatitis viruses, in a large number of reports or case control studies[39]. In 2012, a causal link between HEV3 and renal injury was reported[76]. Additionally, mechanisms inducing glomerular disease were found to be similar to those induced by HCV[77]. HCV increased the risk of chronic kidney disease, inducing glomerular injury through the high viral load related to HCV1 or HCV2[23].
It is known that HEV inhibits production of type I IFNs[78], while it induces upregulation of IFN-γ by natural killer (NK) or natural killer T lymphocytes[79,80]. The core and some non-structural proteins of HCV (NS3, NS5A and NS5B) were demonstrated to alter the function of dendritic cells (DCs) in vitro, resulting in impaired CD4+ and CD8+ T-cell responses to the virus. Also, patients with chronic HCV infection have reduced interleukin-12 and IFN-γ levels compared to those who cleared the virus[81]. Therefore, at least in principle, HEV could counteract chronicity of HCV through IFN-γ upregulation, but interactions between the two viruses via cytokine cross-talk may be complex and not well demonstrated or easy to predict.
Interestingly, liver health is related to the composition of gut microbiota. This is influenced by enteric virome, with whom is in continuous and dynamic equilibrium, and by viruses chronically infecting host tissues[82]. The number of studies on the gut-liver axis and hepatitis infections is presently very low, but microbiota alteration is related to liver disease. HCV-positive people had lower bacterial diversity
Lastly, at molecular level, microRNAs (miRNAs) play a pivotal role in the progression of liver diseases[82]. The roles of the miRNAs are still under study, but it was already speculated that miR-628-3p, miR-194, miR-151-3p, miR-512-3p, miR-335 and miR-590 are potentially involved in HEV/HCV coinfection[85].
Studies in animal models highlighted the ability of HCV to determine changes in the expression of genes that regulate the lipid metabolism[86]. The role of statins in inhibiting viral replication was subsequently proven[87]. Interestingly, not all statins show an inhibitory effect on HCV replication, suggesting an anti-viral mechanism independent from 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase[88]. However, the capability of fluvastatin in lowering HCV RNA in people with chronic hepatitis C appears to be modest, variable, and often fleeting[89]. In contrast, patients treated with statins who are chronically infected with HEV show significantly higher viral loads than chronically infected patients without statin administration and this underlines the possible impact of lipid metabolism on HEV replication[90], while treatment with proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, such as alirocumab, determines a poor antiviral activity against HEV. These observations led to the hypothesis that the antiviral activity of these molecules is related to their ability to determine an increase in intracellular cholesterol, which is greater for statins than for PCSK9 inhibitors[90]. Possible indirect interactions between the two viruses through their influence on lipid metabolism merit determination.
The interactions between the two viruses could be even promoted by immune-suppression induced by HIV, which may facilitate HEV transmission[91]. High prevalence of IgG anti-HEV antibodies (> 15%) was found in people living with HIV (PLWH) affected by HCV chronic infection, in particular if CD4+ T-cell count was below 350 cells/mm3[92]. In endemic rural areas, HEV/HCV coinfection also occurred frequently among pregnant women, inducing a significant worsening of biochemical liver indices than women with negative HCV serology[2]. HCV pathogenesis during pregnancy is poorly understood, and it was related to preterm delivery, placental abruption, and low birth weight in a large cohort of infected women[93]. HEV replicates in the human placenta, among pregnant women, the fatality rate being around 20% and up to 30% in the third trimester. HEV infection determines fulminant hepatic failure, membrane rupture and spontaneous abortions[27].
Since HEV/HCV coinfection is a novel topic, several clinical and research questions remain summarized in Table 2.
| No. | Research perspectives for the next future |
| 1 | In vitro and in vivo studies to define pathogenic interactions during HEV/HCV coinfection. Cell lines model could explain interactions between viral proteins and cellular pathway responsible for liver fibrosis progression, liver steatosis and insulin resistance encountered in patients |
| 2 | Studies to understand relationships among immune phenomena (autoantibodies, cryoglobulins and autoimmune diseases) in patients infected by both viruses, and their correlated in terms of T- and B-cell responses and human leukocyte antigen type |
| 3 | Clinical trials to test safety and effectiveness of DAA in co-infected patients and new therapies. At present, data on DAA treatment is limited to in vitro studies or very few case reports |
| 4 | In vitro studies to evaluate genetic interactions between HEV and HCV during viral replication. Permissive cell lines, infected simultaneously by the two viruses, could show whether there is an interference or synergy between them during viral progeny production |
As previously discussed, seroprevalence studies demonstrated that the lifetime risk of HEV infection in patients affected by chronic HCV hepatitis is not rare. Although the prevalence of HEV/HCV coinfection is not known, it is reasonable to speculate that in resource limited settings where HEV is a frequent cause of acute hepatitis, superinfections with this virus in patients with chronic HCV infection is quite frequent[94], and the consequences in terms of worsening liver damage and liver decompensation merit to be further investigated. By contrast, since HEV infection is a much rarer cause of chronic liver disease than HCV, chronic co-infections with both viruses are less frequently observed unless in immune-compromised individuals.
Immune phenomena are described for both viruses, and physicians should be aware that patients with autoantibodies and cryoglobulins could be tested for both acute and chronic HEV or HCV infection. However, to the best of our knowledge, no one has described immune alterations in patients affected by HEV/HCV coinfection. We propose, given the relative rarity of the infection, that physicians (who diagnose coinfection) also screen for immune phenomena.
Some DAA drugs, such as SOF, are active against both HEV and HCV in vitro, but a regime with SOF and DCV failed to clear HEV RNA in a coinfected patient who did not tolerate ribavirin[54]. Our limited knowledge is based on too few cases being described[52,53,55], and it is not possible to get definitive conclusions on the use of DDA drugs in coinfected patients. It is desirable that researchers focus on in vitro studies to better define possible pathogenetic interactions determined by the two viruses. People at risk of HEV or HCV infection (such as transfused or transplanted patients) should be screened regularly to identify coinfected patients. Also, PLWH should be screened for HEV in cases of unexpected elevations of liver enzymes, with or without HCV co-infection.
HEV and HCV are both RNA viruses characterized by greater variability than DNA viruses and mainly infect the liver. Despite these similarities, the two viruses have different species barriers and disease progression. However, coinfection in endemic areas can be a serious public health problem, especially for immunosuppressed individuals or pregnant women. The evolutionary behavior of RNA viruses is responsible for its pathogenesis and antiviral success in infected hosts, as well as vaccine design[26]. Coinfection with particular HCV and HEV types could aggravate hepatic and/or extrahepatic diseases, taking into account viruses–host interaction and the possible genetic interaction between the two viruses during viral replication. At present, the prevention of infections is mainly related to screening policies and public health measures.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Karaksy H, Mukhopadhyay A S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | World Health Organization. Hepatitis. [cited 15 October 2021] Available from: https://www.who.int/health-topics/hepatitis. |
| 2. | Gad YZ, Mousa N, Shams M, Elewa A. Seroprevalence of subclinical HEV infection in asymptomatic, apparently healthy, pregnant women in Dakahlya Governorate, Egypt. Asian J Transfus Sci. 2011;5:136-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Bricks G, Senise JF, Pott-Jr H, Grandi G, Carnaúba-Jr D, de Moraes HAB, Granato CFH, Castelo A. Previous hepatitis E virus infection, cirrhosis and insulin resistance in patients with chronic hepatitis C. Braz J Infect Dis. 2019;23:45-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Echeverría N, Moratorio G, Cristina J, Moreno P. Hepatitis C virus genetic variability and evolution. World J Hepatol. 2015;7:831-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (2)] |
| 5. | Waqar S, Sharma B, Koirala J. Hepatitis E. 2021 Jul 18. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 6. | Nelson KE, Labrique AB, Kmush BL. Epidemiology of Genotype 1 and 2 Hepatitis E Virus Infections. Cold Spring Harb Perspect Med. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Dalton HR, Izopet J. Transmission and Epidemiology of Hepatitis E Virus Genotype 3 and 4 Infections. Cold Spring Harb Perspect Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Smith DB, Simmonds P; Members Of The International Committee On The Taxonomy Of Viruses Hepeviridae Study Group; Jameel S, Emerson SU, Harrison TJ, Meng XJ, Okamoto H, Van der Poel WHM, Purdy MA. Consensus proposals for classification of the family Hepeviridae. J Gen Virol. 2015;96:1191-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Woo PC, Lau SK, Teng JL, Tsang AK, Joseph M, Wong EY, Tang Y, Sivakumar S, Xie J, Bai R, Wernery R, Wernery U, Yuen KY. New hepatitis E virus genotype in camels, the Middle East. Emerg Infect Dis. 2014;20:1044-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 10. | Woo PC, Lau SK, Teng JL, Cao KY, Wernery U, Schountz T, Chiu TH, Tsang AK, Wong PC, Wong EY, Yuen KY. New Hepatitis E Virus Genotype in Bactrian Camels, Xinjiang, China, 2013. Emerg Infect Dis. 2016;22:2219-2221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 11. | Pavio N, Meng XJ, Doceul V. Zoonotic origin of hepatitis E. Curr Opin Virol. 2015;10:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (3)] |
| 12. | Fenaux H, Chassaing M, Berger S, Gantzer C, Bertrand I, Schvoerer E. Transmission of hepatitis E virus by water: An issue still pending in industrialized countries. Water Res. 2019;151:144-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Thakur V, Ratho RK, Kumar S, Saxena SK, Bora I, Thakur P. Viral Hepatitis E and Chronicity: A Growing Public Health Concern. Front Microbiol. 2020;11:577339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Lanini S, Garbuglia AR, Lapa D, Puro V, Navarra A, Pergola C, Ippolito G, Capobianchi MR. Epidemiology of HEV in the Mediterranean basin: 10-year prevalence in Italy. BMJ Open. 2015;5:e007110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Pérez-Gracia MT, Suay-García B, Mateos-Lindemann ML. Hepatitis E and pregnancy: current state. Rev Med Virol. 2017;27:e1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 16. | Meng XJ, Wiseman B, Elvinger F, Guenette DK, Toth TE, Engle RE, Emerson SU, Purcell RH. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol. 2002;40:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 363] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 17. | Borgia SM, Hedskog C, Parhy B, Hyland RH, Stamm LM, Brainard DM, Subramanian MG, McHutchison JG, Mo H, Svarovskaia E, Shafran SD. Identification of a Novel Hepatitis C Virus Genotype From Punjab, India: Expanding Classification of Hepatitis C Virus Into 8 Genotypes. J Infect Dis. 2018;218:1722-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 18. | Ciccozzi M, Equestre M, Costantino A, Marascio N, Quirino A, Lo Presti A, Cella E, Bruni R, Liberto MC, Focà A, Pisani G, Zehender G, Ciccaglione AR. Hepatitis C virus genotype 4d in Southern Italy: reconstruction of its origin and spread by a phylodynamic analysis. J Med Virol. 2012;84:1613-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Marascio N, Ciccozzi M, Equestre M, Lo Presti A, Costantino A, Cella E, Bruni R, Liberto MC, Pisani G, Zicca E, Barreca GS, Torti C, Focà A, Ciccaglione AR. Back to the origin of HCV 2c subtype and spreading to the Calabria region (Southern Italy) over the last two centuries: a phylogenetic study. Infect Genet Evol. 2014;26:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Marascio N, Costantino A, Taffon S, Lo Presti A, Equestre M, Bruni R, Pisani G, Barreca GS, Quirino A, Trecarichi EM, Costa C, Mazzitelli M, Serapide F, Matera G, Torti C, Liberto MC, Ciccaglione AR. Phylogenetic and Molecular Analyses of More Prevalent HCV1b Subtype in the Calabria Region, Southern Italy. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Marascio N, Mazzitelli M, Scarlata GGM, Giancotti A, Barreca GS, Lamberti AG, Divenuto F, Costa C, Trecarichi EM, Matera G, Liberto MC, Torti C. HCV antibody prevalence and genotype evolution in a teaching hospital, Calabria Region, southern Italy over a decade (2008-2018). Open Microbiol J. 2020;14:84-90. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Pawlotsky JM. Hepatitis C virus genetic variability: pathogenic and clinical implications. Clin Liver Dis. 2003;7:45-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Goel A, Bhadauria DS, Aggarwal R. Hepatitis C virus infection and chronic renal disease: A review. Indian J Gastroenterol. 2018;37:492-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Cauda R. Handbook on Selected Topics in Tropical Diseases. Quaderni del CeSI. eBook 2020. |
| 25. | Figlerowicz M, Alejska M, Kurzyńska-Kokorniak A, Figlerowicz M. Genetic variability: the key problem in the prevention and therapy of RNA-based virus infections. Med Res Rev. 2003;23:488-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Lauring AS, Andino R. Quasispecies theory and the behavior of RNA viruses. PLoS Pathog. 2010;6:e1001005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 698] [Cited by in RCA: 587] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 27. | van Tong H, Hoan NX, Wang B, Wedemeyer H, Bock CT, Velavan TP. Hepatitis E Virus Mutations: Functional and Clinical Relevance. EBioMedicine. 2016;11:31-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Marascio N, Torti C, Liberto M, Focà A. Update on different aspects of HCV variability: focus on NS5B polymerase. BMC Infect Dis. 2014;14 Suppl 5:S1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Simmonds P. The origin of hepatitis C virus. Curr Top Microbiol Immunol. 2013;369:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | González-Candelas F, López-Labrador FX, Bracho MA. Recombination in hepatitis C virus. Viruses. 2011;3:2006-2024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | 31vAggarwal R. Hepatitis e: epidemiology and natural history. J Clin Exp Hepatol. 2013;3:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Lanini S, Ustianowski A, Pisapia R, Zumla A, Ippolito G. Viral Hepatitis: Etiology, Epidemiology, Transmission, Diagnostics, Treatment, and Prevention. Infect Dis Clin North Am. 2019;33:1045-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 33. | Smith DB, Ijaz S, Tedder RS, Hogema B, Zaaijer HL, Izopet J, Bradley-Stewart A, Gunson R, Harvala H, Kokki I, Simmonds P. Variability and pathogenicity of hepatitis E virus genotype 3 variants. J Gen Virol. 2015;96:3255-3264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Smith DB, Simmonds P. Hepatitis E virus and fulminant hepatitis--a virus or host-specific pathology? Liver Int. 2015;35:1334-1340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | European Association for the Study of the Liver. Clinical Practice Guidelines Panel: Chair: EASL Governing Board representative: Panel members: EASL recommendations on treatment of hepatitis C: Final update of the series☆. J Hepatol. 2020;73:1170-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 779] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 36. | Klöhn M, Schrader JA, Brüggemann Y, Todt D, Steinmann E. Beyond the Usual Suspects: Hepatitis E Virus and Its Implications in Hepatocellular Carcinoma. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Jha AK, Kumar G, Dayal VM, Ranjan A, Suchismita A. Neurological manifestations of hepatitis E virus infection: An overview. World J Gastroenterol. 2021;27:2090-2104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Fritz-Weltin M, Niedermeier L, Frommherz E, Isenmann N, Csernalabics B, Boettler T, Neumann-Haefelin C, Endres D, Panning M, Berger B. Hepatitis E virus and Bell's palsy. Eur J Neurol. 2022;29:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Lhomme S, Abravanel F, Dubois M, Sandres-Saune K, Rostaing L, Kamar N, Izopet J. Hepatitis E virus quasispecies and the outcome of acute hepatitis E in solid-organ transplant patients. J Virol. 2012;86:10006-10014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Khaliq S, Jahan S, Pervaiz A. Sequence variability of HCV Core region: important predictors of HCV induced pathogenesis and viral production. Infect Genet Evol. 2011;11:543-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (3)] |
| 41. | Janiak M, Perlejewski K, Grabarczyk P, Kubicka-Russel D, Zagordi O, Berak H, Osuch S, Pawełczyk A, Bukowska-Ośko I, Płoski R, Laskus T, Caraballo Cortés K. Hepatitis C virus (HCV) genotype 1b displays higher genetic variability of hypervariable region 1 (HVR1) than genotype 3. Sci Rep. 2019;9:12846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Debing Y, Ramière C, Dallmeier K, Piorkowski G, Trabaud MA, Lebossé F, Scholtès C, Roche M, Legras-Lachuer C, de Lamballerie X, André P, Neyts J. Hepatitis E virus mutations associated with ribavirin treatment failure result in altered viral fitness and ribavirin sensitivity. J Hepatol. 2016;65:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 43. | AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:1477-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 479] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 44. | Marascio N, Quirino A, Barreca GS, Galati L, Costa C, Pisani V, Mazzitelli M, Matera G, Liberto MC, Focà A, Torti C. Discussion on critical points for a tailored therapy to cure hepatitis C virus infection. Clin Mol Hepatol. 2019;25:30-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Marascio N, Pavia G, Strazzulla A, Dierckx T, Cuypers L, Vrancken B, Barreca GS, Mirante T, Malanga D, Oliveira DM, Vandamme AM, Torti C, Liberto MC, Focà A; The SINERGIE-UMG Study Group. Detection of Natural Resistance-Associated Substitutions by Ion Semiconductor Technology in HCV1b Positive, Direct-Acting Antiviral Agents-Naïve Patients. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Di Maio VC, Cento V, Lenci I, Aragri M, Rossi P, Barbaliscia S, Melis M, Verucchi G, Magni CF, Teti E, Bertoli A, Antonucci F, Bellocchi MC, Micheli V, Masetti C, Landonio S, Francioso S, Santopaolo F, Pellicelli AM, Calvaruso V, Gianserra L, Siciliano M, Romagnoli D, Cozzolongo R, Grieco A, Vecchiet J, Morisco F, Merli M, Brancaccio G, Di Biagio A, Loggi E, Mastroianni CM, Pace Palitti V, Tarquini P, Puoti M, Taliani G, Sarmati L, Picciotto A, Vullo V, Caporaso N, Paoloni M, Pasquazzi C, Rizzardini G, Parruti G, Craxì A, Babudieri S, Andreoni M, Angelico M, Perno CF, Ceccherini-Silberstein F; HCV Italian Resistance Network Study Group. Multiclass HCV resistance to direct-acting antiviral failure in real-life patients advocates for tailored second-line therapies. Liver Int. 2017;37:514-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 47. | Marascio N, Pavia G, Romeo I, Talarico C, Di Salvo S, Reale M, Marano V, Barreca GS, Fabiani F, Perrotti N, De Siena M, Giancotti F, Gravina T, Alcaro S, Artese A, Torti C, Liberto MC, Focà A. Real-life 3D therapy failure: Analysis of NS5A 93H RAS plus 108 K polymorphism in complex with ombitasvir by molecular modeling. J Med Virol. 2018;90:1257-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Uribe-Noguez LA, Ocaña-Mondragón A, Mata-Marín JA, Cázares-Cortázar A, Ribas-Aparicio RM, Gómez-Torres ME, Gaytán-Martínez J, Martínez-Rodríguez ML. Case report: Identification of recombinant HCV genotype 1b-2b by viral sequencing in two patients with treatment failure, who responded to re-treatment with sofosbuvir and daclatasvir. J Infect Chemother. 2018;24:928-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Kurata H, Uchida Y, Kouyama JI, Naiki K, Nakazawa M, Ando S, Nakao M, Motoya D, Sugawara K, Inao M, Imai Y, Nakayama N, Tomiya T, Mochida S. Chronic hepatitis caused by hepatitis C virus showing a discrepancy between serogroup and genotype because of intergenotypic 2b/1b recombination: A pitfall in antiviral therapy with direct-acting antivirals. Hepatol Res. 2018;48:E372-E378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Dao Thi VL, Debing Y, Wu X, Rice CM, Neyts J, Moradpour D, Gouttenoire J. Sofosbuvir Inhibits Hepatitis E Virus Replication In Vitro and Results in an Additive Effect When Combined With Ribavirin. Gastroenterology. 2016;150:82-85.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 51. | Del Bello A, Abravanel F, Alric L, Lavayssiere L, Lhomme S, Bellière J, Izopet J, Kamar N. No evidence of occult hepatitis C or E virus infections in liver-transplant patients with sustained virological response after therapy with direct acting agents. Transpl Infect Dis. 2019;21:e13093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Biliotti E, Franchi C, Spaziante M, Garbuglia AR, Volpicelli L, Palazzo D, De Angelis M, Esvan R, Taliani G. Autochthonous acute hepatitis E: treatment with sofosbuvir and ribavirin. Infection. 2018;46:725-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | De Martin E, Antonini TM, Coilly A, Pittau G, Vibert E, Duclos-Vallée JC, Samuel D, Roque-Afonso AM. HCV and HEV recurrence after liver transplantation: one antiviral therapy for two viruses. Transpl Int. 2017;30:318-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Donnelly MC, Imlach SN, Abravanel F, Ramalingam S, Johannessen I, Petrik J, Fraser AR, Campbell JD, Bramley P, Dalton HR, Hayes PC, Kamar N, Simpson KJ. Sofosbuvir and Daclatasvir Anti-Viral Therapy Fails to Clear HEV Viremia and Restore Reactive T Cells in a HEV/HCV Co-Infected Liver Transplant Recipient. Gastroenterology. 2017;152:300-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 55. | Wahid B. Successful treatment of HBV, HCV, & HEV, with 12-week long use of tenofovir, sofosbuvir, daclatasvir, and ribavirin: A case report. J Infect Public Health. 2020;13:149-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Wang W, Xu L, Brandsma JH, Wang Y, Hakim MS, Zhou X, Yin Y, Fuhler GM, van der Laan LJ, van der Woude CJ, Sprengers D, Metselaar HJ, Smits R, Poot RA, Peppelenbosch MP, Pan Q. Convergent Transcription of Interferon-stimulated Genes by TNF-α and IFN-α Augments Antiviral Activity against HCV and HEV. Sci Rep. 2016;6:25482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Spina A, Beversluis D, Irwin A, Chen A, Nassariman JN, Ahamat A, Noh I, Oosterloo J, Alfani P, Sang S, Lenglet A, Taylor DL. Learning from water treatment and hygiene interventions in response to a hepatitis E outbreak in an open setting in Chad. J Water Health. 2018;16:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Koning L, Charlton MR, Pas SD, Heimbach JK, Osterhaus AD, Watt KD, Janssen HL, de Knegt RJ, van der Eijk AA. Prevalence and clinical consequences of Hepatitis E in patients who underwent liver transplantation for chronic Hepatitis C in the United States. BMC Infect Dis. 2015;15:371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP, Wang YJ, Ai X, Hu YM, Tang Q, Yao X, Yan Q, Xian YL, Wu T, Li YM, Miao J, Ng MH, Shih JW, Xia NS. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 555] [Article Influence: 37.0] [Reference Citation Analysis (1)] |
| 60. | Chen Z, Lin S, Duan J, Luo Y, Wang S, Gan Z, Yi H, Wu T, Huang S, Zhang Q, Lv H. Immunogenicity and safety of an accelerated hepatitis E vaccination schedule in healthy adults: a randomized, controlled, open-label, phase IV trial. Clin Microbiol Infect. 2019;25:1133-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Bailey JR, Barnes E, Cox AL. Approaches, Progress, and Challenges to Hepatitis C Vaccine Development. Gastroenterology. 2019;156:418-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 175] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 62. | Capone S, Naddeo M, D'Alise AM, Abbate A, Grazioli F, Del Gaudio A, Del Sorbo M, Esposito ML, Ammendola V, Perretta G, Taglioni A, Colloca S, Nicosia A, Cortese R, Folgori A. Fusion of HCV nonstructural antigen to MHC class II-associated invariant chain enhances T-cell responses induced by vectored vaccines in nonhuman primates. Mol Ther. 2014;22:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Krain LJ, Nelson KE, Labrique AB. Host immune status and response to hepatitis E virus infection. Clin Microbiol Rev. 2014;27:139-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 64. | Elhendawy M, Abo-Ali L, Abd-Elsalam S, Hagras MM, Kabbash I, Mansour L, Atia S, Esmat G, Abo-ElAzm AR, El-Kalla F, Kobtan A. HCV and HEV: two players in an Egyptian village, a study of prevalence, incidence, and co-infection. Environ Sci Pollut Res Int. 2020;27:33659-33667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 65. | Mansuy JM, Bendall R, Legrand-Abravanel F, Sauné K, Miédouge M, Ellis V, Rech H, Destruel F, Kamar N, Dalton HR, Izopet J. Hepatitis E virus antibodies in blood donors, France. Emerg Infect Dis. 2011;17:2309-2312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 289] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 66. | Norder H, Karlsson M, Mellgren Å, Konar J, Sandberg E, Lasson A, Castedal M, Magnius L, Lagging M. Diagnostic Performance of Five Assays for Anti-Hepatitis E Virus IgG and IgM in a Large Cohort Study. J Clin Microbiol. 2016;54:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 67. | Kyvernitakis A, Taremi M, Blechacz B, Hwang J, Jiang Y, Mahale P, Torres HA. Impact of hepatitis E virus seropositivity on chronic liver disease in cancer patients with hepatitis C virus infection. Hepatol Res. 2015;45:1146-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Wong RJ, Cheung R, Gish RG, Chitnis AS. Prevalence of hepatitis E infection among adults with concurrent chronic liver disease. J Viral Hepat. 2021;28:1643-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 69. | Choi JW, Son HJ, Lee SS, Jeon H, Cho JK, Kim HJ, Cha RR, Lee JM, Jung WT, Lee OJ. Acute hepatitis E virus superinfection increases mortality in patients with cirrhosis. BMC Infect Dis. 2022;22:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 70. | Amougou Atsama M, Atangana PJA, Noah Noah D, Moundipa PF, Pineau P, Njouom R. Hepatitis E virus infection as a promoting factor for hepatocellular carcinoma in Cameroon: Preliminary Observations. Int J Infect Dis. 2017;64:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 71. | Adinolfi LE, Rinaldi L, Nevola R. Chronic hepatitis C, atherosclerosis and cardiovascular disease: What impact of direct-acting antiviral treatments? World J Gastroenterol. 2018;24:4617-4621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 72. | Hetta HF, Mekky MA, Khalil NK, Mohamed WA, El-Feky MA, Ahmed SH, Daef EA, Medhat A, Nassar MI, Sherman KE, Shata MTM. Extra-hepatic infection of hepatitis C virus in the colon tissue and its relationship with hepatitis C virus pathogenesis. J Med Microbiol. 2016;65:703-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 73. | Horvatits T, Schulze Zur Wiesch J, Polywka S, Buescher G, Lütgehetmann M, Hussey E, Horvatits K, Peine S, Haag F, Addo MM, Lohse AW, Weiler-Normann C, Pischke S. Significance of Anti-Nuclear Antibodies and Cryoglobulins in Patients with Acute and Chronic HEV Infection. Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Saadoun D, Asselah T, Resche-Rigon M, Charlotte F, Bedossa P, Valla D, Piette JC, Marcellin P, Cacoub P. Cryoglobulinemia is associated with steatosis and fibrosis in chronic hepatitis C. Hepatology. 2006;43:1337-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 75. | Santoro L, Manganelli F, Briani C, Giannini F, Benedetti L, Vitelli E, Mazzeo A, Beghi E; HCV Peripheral Nerve Study Group. Prevalence and characteristics of peripheral neuropathy in hepatitis C virus population. J Neurol Neurosurg Psychiatry. 2006;77:626-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 76. | Kamar N, Weclawiak H, Guilbeau-Frugier C, Legrand-Abravanel F, Cointault O, Ribes D, Esposito L, Cardeau-Desangles I, Guitard J, Sallusto F, Muscari F, Peron JM, Alric L, Izopet J, Rostaing L. Hepatitis E virus and the kidney in solid-organ transplant patients. Transplantation. 2012;93:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 77. | D'Amico G. Renal involvement in hepatitis C infection: cryoglobulinemic glomerulonephritis. Kidney Int. 1998;54:650-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 175] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 78. | Lhomme S, Migueres M, Abravanel F, Marion O, Kamar N, Izopet J. Hepatitis E Virus: How It Escapes Host Innate Immunity. Vaccines (Basel). 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Nan Y, Yu Y, Ma Z, Khattar SK, Fredericksen B, Zhang YJ. Hepatitis E virus inhibits type I interferon induction by ORF1 products. J Virol. 2014;88:11924-11932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 80. | Todt D, François C, Anggakusuma, Behrendt P, Engelmann M, Knegendorf L, Vieyres G, Wedemeyer H, Hartmann R, Pietschmann T, Duverlie G, Steinmann E. Antiviral Activities of Different Interferon Types and Subtypes against Hepatitis E Virus Replication. Antimicrob Agents Chemother. 2016;60:2132-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 81. | Irshad M, Khushboo I, Singh S. Hepatitis C virus (HCV): a review of immunological aspects. Int Rev Immunol. 2008;27:497-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 82. | Focà A, Liberto MC, Quirino A, Marascio N, Zicca E, Pavia G. Gut inflammation and immunity: what is the role of the human gut virome? Mediators Inflamm. 2015;2015:326032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 83. | Inoue T, Nakayama J, Moriya K, Kawaratani H, Momoda R, Ito K, Iio E, Nojiri S, Fujiwara K, Yoneda M, Yoshiji H, Tanaka Y. Gut Dysbiosis Associated With Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 84. | Wu J, Huang F, Ling Z, Liu S, Liu J, Fan J, Yu J, Wang W, Jin X, Meng Y, Cao H, Li L. Altered faecal microbiota on the expression of Th cells responses in the exacerbation of patients with hepatitis E infection. J Viral Hepat. 2020;27:1243-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 85. | McGowan K, Simpson KJ, Petrik J. Expression Profiles of Exosomal MicroRNAs from HEV- and HCV-Infected Blood Donors and Patients: A Pilot Study. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 86. | Bigger CB, Guerra B, Brasky KM, Hubbard G, Beard MR, Luxon BA, Lemon SM, Lanford RE. Intrahepatic gene expression during chronic hepatitis C virus infection in chimpanzees. J Virol. 2004;78:13779-13792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 87. | Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci U S A. 2005;102:2561-2566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 406] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 88. | Ikeda M, Abe K, Yamada M, Dansako H, Naka K, Kato N. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 89. | Bader T, Fazili J, Madhoun M, Aston C, Hughes D, Rizvi S, Seres K, Hasan M. Fluvastatin inhibits hepatitis C replication in humans. Am J Gastroenterol. 2008;103:1383-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 90. | Glitscher M, Martín DH, Woytinek K, Schmidt B, Tabari D, Scholl C, Stingl JC, Seelow E, Choi M, Hildt E. Targeting Cholesterol Metabolism as Efficient Antiviral Strategy Against the Hepatitis E Virus. Cell Mol Gastroenterol Hepatol. 2021;12:159-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 91. | Pisanti FA, Coppola A, Galli C. Association between hepatitis C and hepatitis E viruses in southern Italy. Lancet. 1994;344:746-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 92. | Vázquez-Morón S, Berenguer J, González-García J, Jiménez-Sousa MÁ, Canorea I, Guardiola JM, Crespo M, Quereda C, Sanz J, Carrero A, Hontañón V, Avellón A, Resino S. Prevalence of hepatitis E infection in HIV/HCV-coinfected patients in Spain (2012-2014). Sci Rep. 2019;9:1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 93. | Le Campion A, Larouche A, Fauteux-Daniel S, Soudeyns H. Pathogenesis of hepatitis C during pregnancy and childhood. Viruses. 2012;4:3531-3550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 94. | Zitelli PMY, Gomes-Gouvêa M, Mazo DF, Singer JDM, Oliveira CPMS, Farias AQ, Pinho JR, Tanigawa RY, Alves VAF, Carrilho FJ, Pessoa MG. Hepatitis E virus infection increases the risk of diabetes and severity of liver disease in patients with chronic hepatitis C virus infection. Clinics (Sao Paulo). 2021;76:e3270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |