Published online Feb 7, 2021. doi: 10.3748/wjg.v27.i5.442
Peer-review started: October 12, 2020
First decision: November 23, 2020
Revised: December 8, 2020
Accepted: January 6, 2021
Article in press: January 6, 2021
Published online: February 7, 2021
Processing time: 108 Days and 19.5 Hours
Fistula and intraabdominal abscess are common complications of Crohn’s disease (CD), but complex rectal fistula with abscess formation is rare. Tumor necrosis factor antagonists combined with percutaneous drainage or surgical intervention is optimal treatment for fistulizing CD with intraabdominal abscess. There is no study showing the efficacy of vedolizumab in such complicated condition.
A 47-year-old man has decompensated liver cirrhosis, Child B. He suffered from abdominal pain, bloody diarrhea, fever, and body weight loss. CD with rectoprostatic fistula, rectopresacral fistula, presacral abscess and cyto-megalovirus (CMV) infection were noted. He received antibiotics, anti-viral therapy, transverse colostomy and vedolizumab treatment. Six months later, he had deep remission and complete fistula tracts closure.
Early vedolizumab and stool diversion are effective and safe in treating CD with complex rectal fistula with abscess formation.
Core Tip: Fistulas and intraabdominal abscess are common complications of Crohn’s disease (CD) and tumor necrosis factor antagonists combined with percutaneous drainage or surgical intervention is the optimal treatment for fistulizing CD with intraabdominal abscess. However, no previous study has reported the efficacy of vedolizumab in this complicated situation. This 47-year-old male presented with CD with complex fistulas and presacral abscess. He received vedolizumab with transverse colostomy and the follow-up sigmoidoscopy 6 mo later showed mucosal healing without any visible fistula tracts. We think early vedolizumab treatment with stool diversion are effective and safe in treating CD with complex fistulas and abscess formation.
- Citation: Yeh H, Kuo CJ, Wu RC, Chen CM, Tsai WS, Su MY, Chiu CT, Le PH. Vedolizumab in Crohn’s disease with rectal fistulas and presacral abscess: A case report. World J Gastroenterol 2021; 27(5): 442-448
- URL: https://www.wjgnet.com/1007-9327/full/v27/i5/442.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i5.442
Fistula affects up to 50% Crohn’s disease (CD) patients within 20 years of initial diagnosis[1,2]. It includes perianal, rectovaginal, enterocutaneous and internal fistula, but no rectoprostatic fistula or rectopresacral fistula were reported. The treatment of fistula usually requires a combination of medical and surgical approach[3]. As far as medical treatment is concerned, tumor necrosis factor (TNF) antagonists are the most effective to treat fistulizing CD[4]. However, some studies also noted the beneficial effect of vedolizumab for fistulizing CD[5,6]. We presented the patient of CD, complicated with rectoprostatic fistula, rectopresacral fistula and presacral abscess. He had complete fistula closure and deep remission after transverse colostomy and vedolizumab treatment for six months.
Low abdominal pain and intermittent bloody stool for 6 mo.
A 47-year-old man has decompensated liver cirrhosis, Child B, hepatitis C virus (HCV) and alcoholism related, complicated with hypoalbuminemia, hyperbilirubinemia, coagulopathy, thrombocytopenia, splenomegaly and esophageal varices, Form 1. He complained low abdominal pain and intermittent bloody stool for 6 mo. Appendicitis was diagnosed in local hospital, and he received appendectomy on 16th November 2019. However, he suffered from progressive low abdominal pain, bloody stool, dizziness, and intermittent fever up to 38 °C for four days. He also mentioned body weight loss 20 kg within one year. He was brought to our emergent department.
Decompensated liver cirrhosis, Child B, hepatitis C virus infection and alcoholism.
Appendicitis was diagnosed in local hospital, and he received appendectomy on 16th November 2019.
Lab data revealed hemoglobin 10.1 g/dL, platelet 66000/μL, white blood cell 7700/μL, segment 68.7%, lymphocyte 23.7%, international normalized ratio (INR) 1.5, aspartate aminotransferase (AST) 55 U/L, alanine aminotransferase (ALT) 95 U/L, total bilirubin 2.1 mg/dL, albumin 2.38 g/dL and CRP 22.81 mg/L. Cytomegalovirus (CMV) immunoglobulin (Ig) M, CMV DNA, Epstein-Barr virus (EBV)-VCA IgM, EBV DNA, human immunodeficiency virus (HIV) antibody (Ab), amebic Ab, Clostridium difficile toxin, culture for Salmonella, Shigella and Campylobacter were all negative. Positive CMV IgG, EBV-VCA IgG, stool pus cell and occult blood were noted.
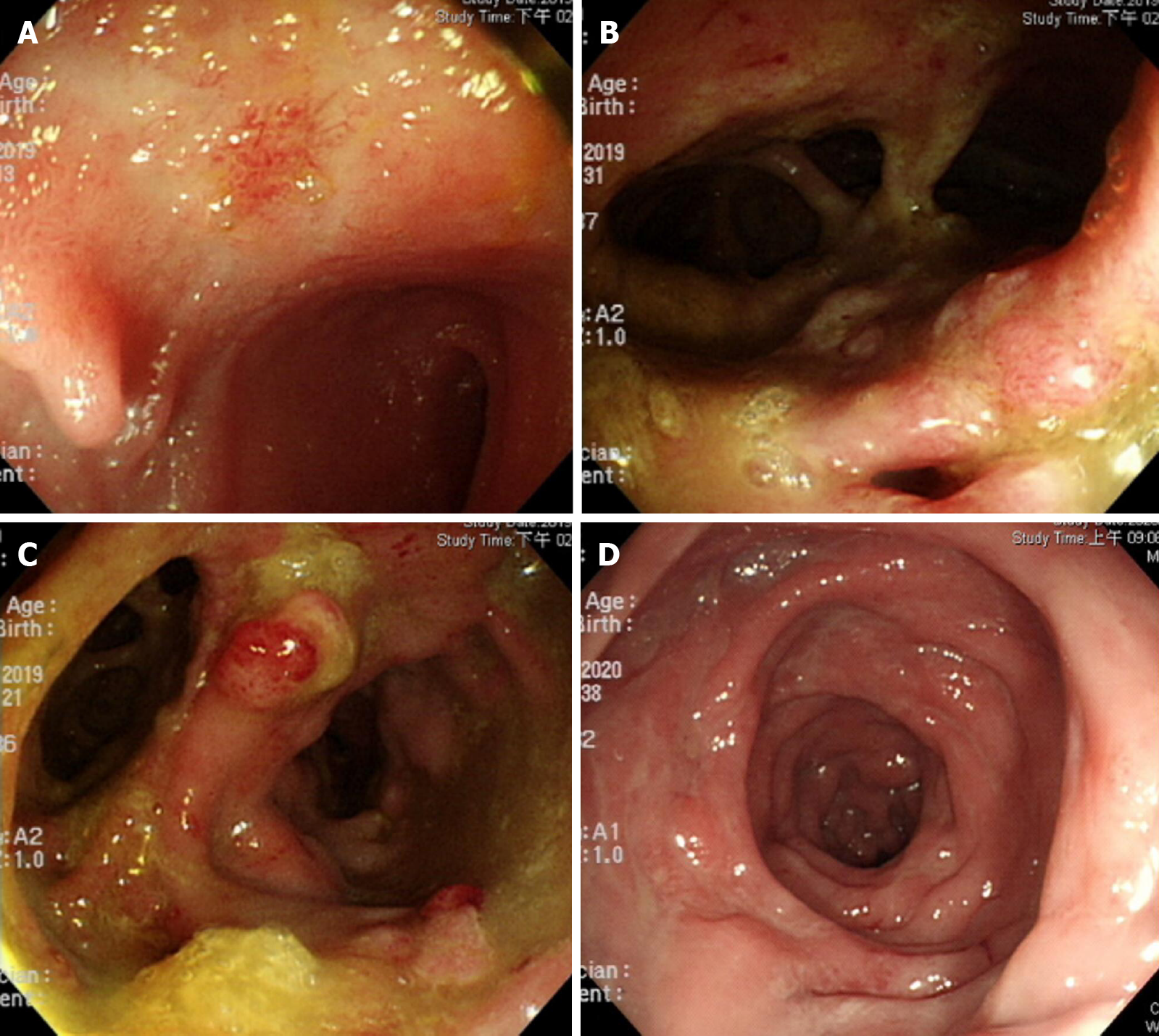
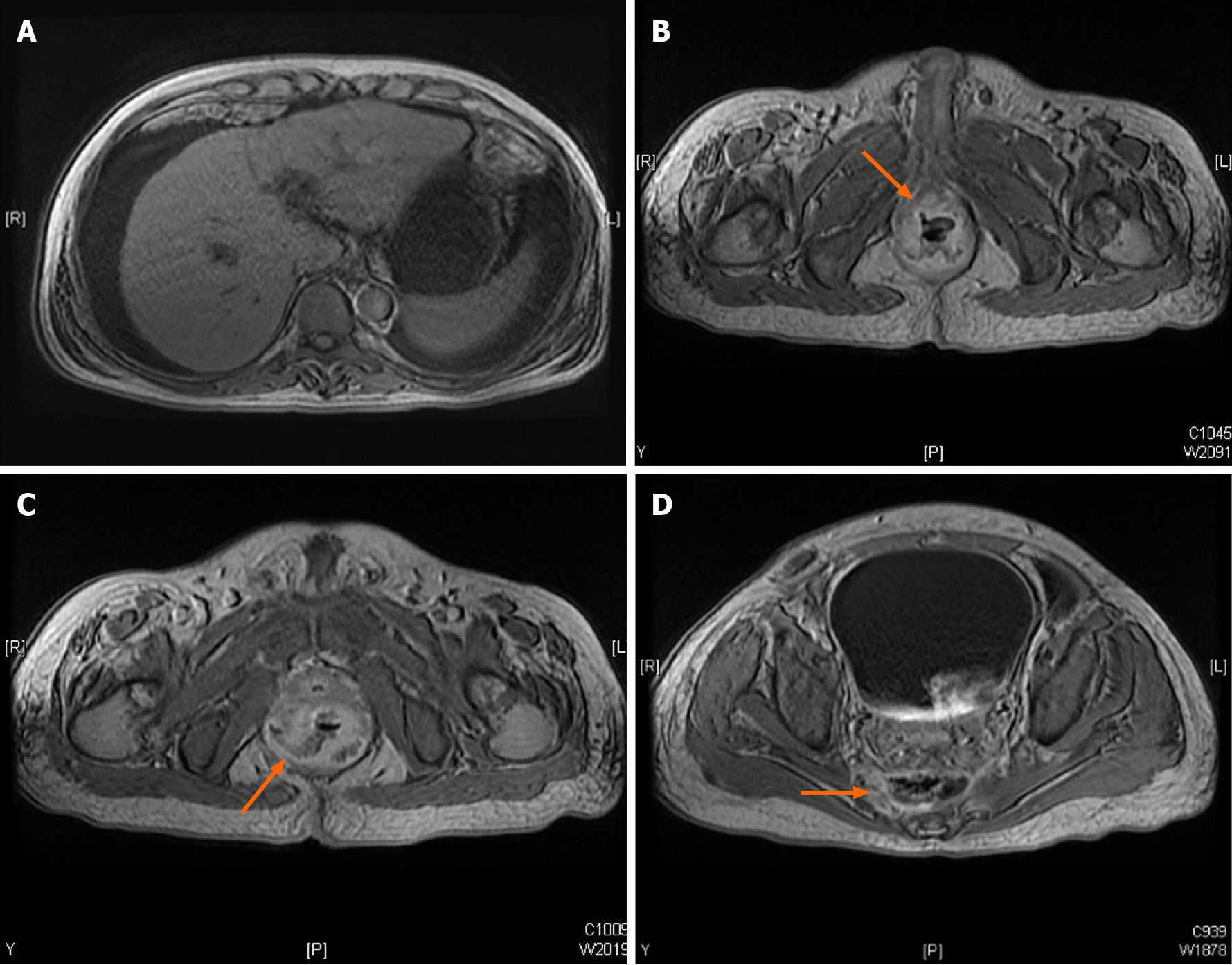
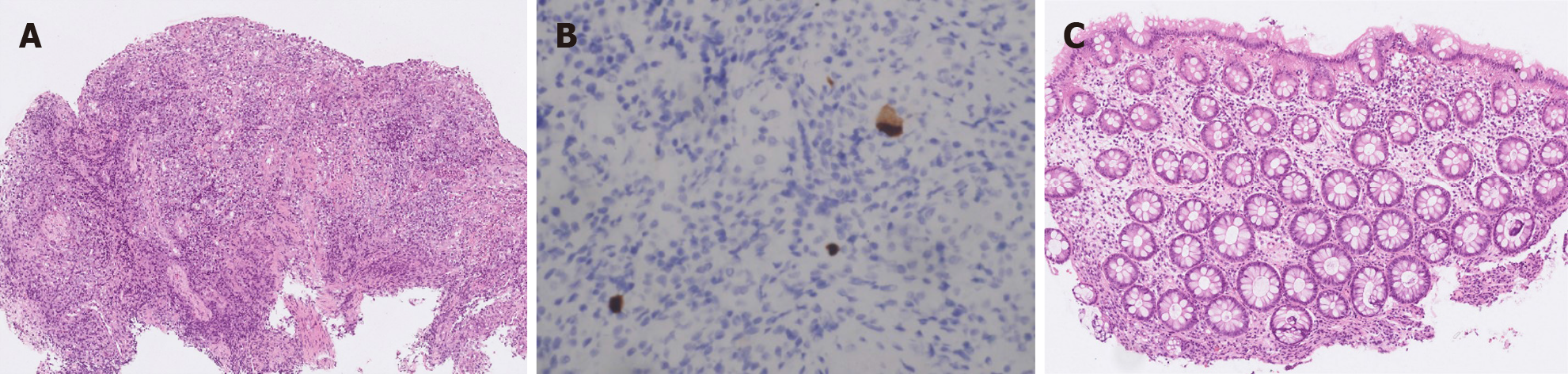
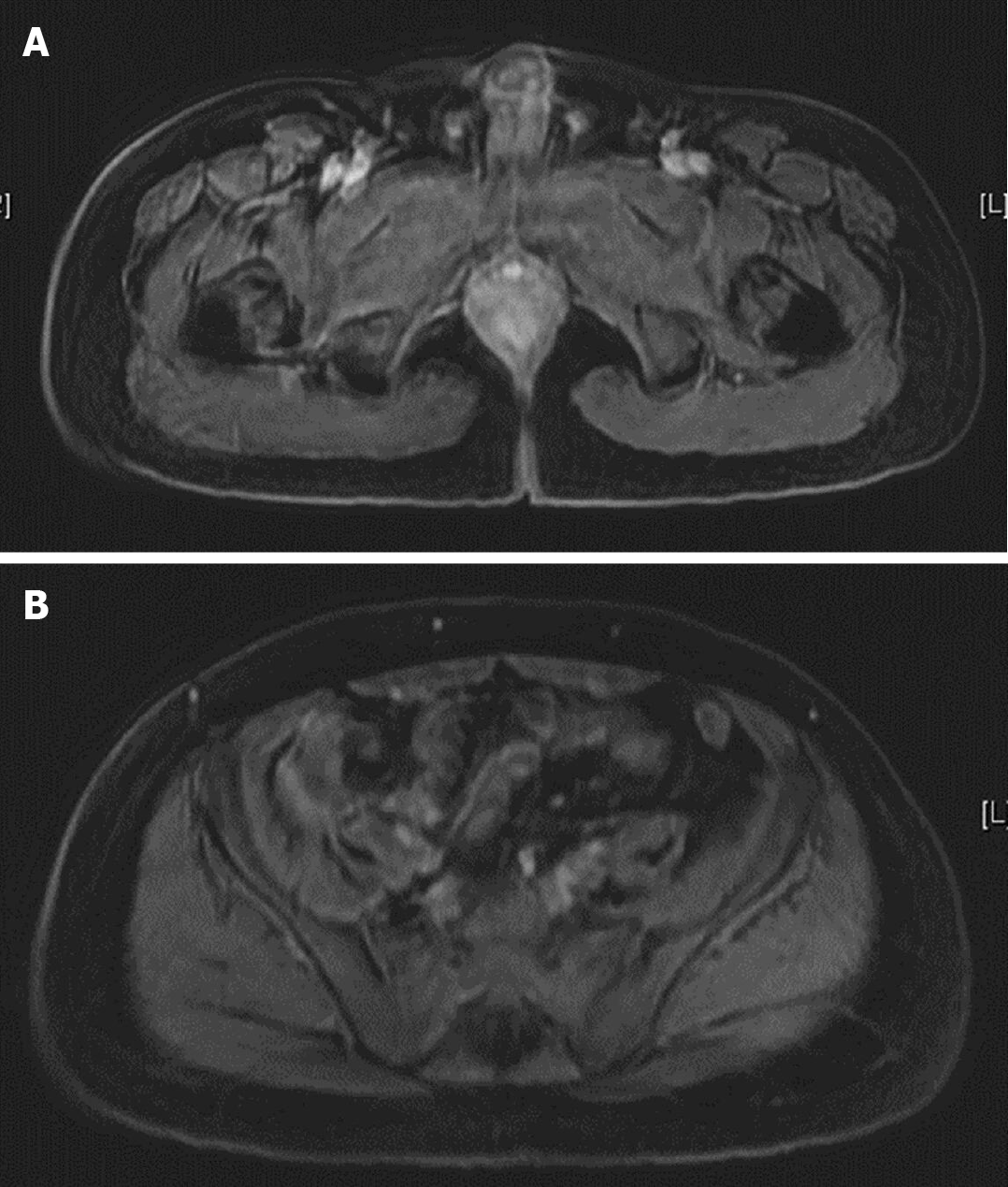
Colonoscopy showed terminal ileal shallow ulcer (Figure 1A) and multiple complex rectal fistula tracts (Figure 1B and C) on 10th December 2019. Magnetic resonance imaging (MRI) noted decompensated liver cirrhosis with ascites (Figure 2A), rectoprostatic fistula (Figure 2B), rectopresacral fistula (Figure 2C) and presacral abscess (Figure 2D) on 21th December 2019. Pathology revealed acute on chronic inflammation with granulation tissue, compactable with CD (Figure 3A). Besides, there was positive result of CMV immunohistochemistry (IHC) staining (Figure 3B), which was performed with a monoclonal antibody directed against the CMV pp65 antigen (Novocastra™ lyophilized mouse monoclonal antibody; Leica Microsystems, Wetzlar, Germany).
He was diagnosed to have CD with CMV infection based on endoscopy and pathological findings. The CD activity index (CDAI) was 526 points and Harvey-Bradshaw index (HBI) was 22 points. He also had rectoprostatic fistula, rectopresacral fistula and presacral abscess diagnosed by MRI findings.
He refused percutaneous abscess fine needle aspiration, and we kept Tazocin for abscess treatment for 27 d. Because of positive CMV IHC staining result, he also received intravenous ganciclovir for 17 d and then valganciclovir oral treatment for two months. Transverse colostomy was performed for stool diversion on 25th December 2019. He couldn’t tolerant azathioprine due to pancytopenia and vedolizumab (300 mg) was prescript since 22th January 2020.
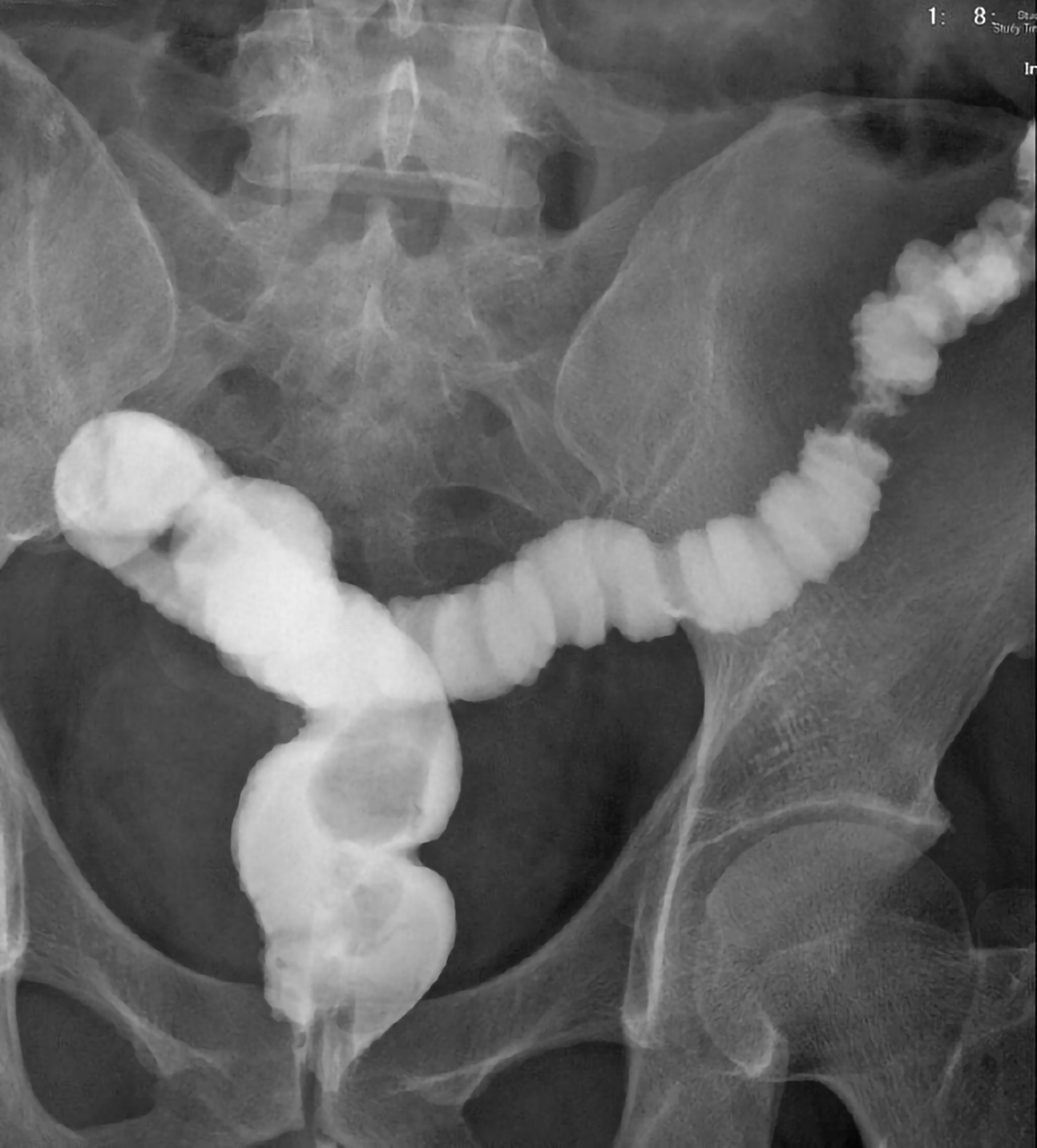
Follow-up sigmoidoscopy showed mucosal healing without any fistula tract (Figure 1D) on 9th July 2020. The pathologist reported minimal inflammatory activity (Figure 3C). Lower gastrointestinal series mentioned no more fistula tract (Figure 4) on 21th July 2020. There was no more rectoprostatic or rectopresacral fistula (Figure 5A) and presacral abscess (Figure 5B) in MRI. After vedolizumab treatment for 6 mo, the CDAI was 42 points and HBI score was 0 point. His body weight also increased 20 kg, back to the same level before the episode.
Fistulizing CD results in not only high morbidity but also impairs health-related quality of life[4]. Biologics combined with surgical intervention seems to be the best solution. Although Infliximab has the strongest evidence in fistulizing CD treatment[7,8], vedolizumab also showed its efficacy in some studies[5,6]. However, vedolizumab has better safety profiles (less severe adverse events and infections) in real world studies[9,10].
Intra-abdominal abscess occurs in up to 20% of patients with CD[11,12]. Adequate percutaneous drainage combined with early adalimumab treatment achieves up to 74% successful rate[13]. In this case, it was difficult to drain the presacral abscess and patient refused, too. Therefore, we chose vedolizumab with transverse colostomy in treating the complex rectal fistula and presacral abscess without abscess drainage.
This patient received vedolizumab treatment one month after the diagnosis. Earlier initiation of biological treatment shortly after diagnosis (less than one year) in patients with moderately to severely active CD improved the long-term clinical outcomes[14]. Besides, early stool diversion with transverse colostomy and early anti-viral treatment for CMV infection were crucial to achieve the good outcome in this case.
Vedolizumab with loop transverse colostomy was effective in treating CD with complex rectal fistulas with presacral abscess. Besides, early biological and anti-CMV treatments also lead to the favorable outcome.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Taiwan Association of the Study of Small Intestinal Disease.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Funel N, Makhlouf NA S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
| 1. | Siegmund B, Feakins RM, Barmias G, Ludvig JC, Teixeira FV, Rogler G, Scharl M. Results of the Fifth Scientific Workshop of the ECCO (II): Pathophysiology of Perianal Fistulizing Disease. J Crohns Colitis. 2016;10:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Schwartz DA, Loftus EV Jr, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 712] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 3. | Gecse KB, Bemelman W, Kamm MA, Stoker J, Khanna R, Ng SC, Panés J, van Assche G, Liu Z, Hart A, Levesque BG, D'Haens G; World Gastroenterology Organization; International Organisation for Inflammatory Bowel Diseases IOIBD; European Society of Coloproctology and Robarts Clinical Trials; World Gastroenterology Organization International Organisation for Inflammatory Bowel Diseases IOIBD European Society of Coloproctology and Robarts Clinical Trials. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn's disease. Gut. 2014;63:1381-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 277] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 4. | Vavricka SR, Rogler G. Fistula treatment: The unresolved challenge. Dig Dis. 2010;28:556-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 5. | Schwartz D, Peyrin-Biroulet L, Lasch K, Adsul S, Danese S. P476 Efficacy and safety of 2 vedolizumab IV regimens in patients with perianal fistulising Crohn’s disease: results of the ENTERPRISE study. J Crohns Colitis. 2020;14:S418-S419. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Feagan BG, Schwartz D, Danese S, Rubin DT, Lissoos TW, Xu J, Lasch K. Efficacy of Vedolizumab in Fistulising Crohn's Disease: Exploratory Analyses of Data from GEMINI 2. J Crohns Colitis. 2018;12:621-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 7. | Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, Schaible TF, van Deventer SJ. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1969] [Cited by in RCA: 1839] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 8. | Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE, Rachmilewitz D, Rutgeerts P, Wild G, Wolf DC, Marsters PA, Travers SB, Blank MA, van Deventer SJ. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1581] [Cited by in RCA: 1553] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 9. | Yarur A, Mantzaris G, Silverberg M, Walshe M, Zezos P, Stein D, Bassel M, Lissoos T, Lopez C, Natsios A, Radulescu G, Patel H, Demuth D, Bressler B. P573 Real-world effectiveness and safety of vedolizumab and anti-TNF in biologic-naive ulcerative colitis patients: Results from the EVOLVE study. J Crohns Colitis. 2019;13:S400-S401. [DOI] [Full Text] |
| 10. | Helwig U, Mross M, Schubert S, Hartmann H, Brandes A, Stein D, Kempf C, Knop J, Campbell-Hill S, Ehehalt R. Real-world clinical effectiveness and safety of vedolizumab and anti-tumor necrosis factor alpha treatment in ulcerative colitis and Crohn's disease patients: a German retrospective chart review. BMC Gastroenterol. 2020;20:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Hurst RD, Molinari M, Chung TP, Rubin M, Michelassi F. Prospective study of the features, indications, and surgical treatment in 513 consecutive patients affected by Crohn's disease. Surgery. 1997;122:661-7; discussion 667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Yamaguchi A, Matsui T, Sakurai T, Ueki T, Nakabayashi S, Yao T, Futami K, Arima S, Ono H. The clinical characteristics and outcome of intraabdominal abscess in Crohn's disease. J Gastroenterol. 2004;39:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Pineton de Chambrun G, Pariente B, Seksik P, Altwegg R, Vuitton L, Stefasnescu C, Nancey S, Aubourg A, Serrero M, Peyrin-Biroulet L, Filippi J, Viennot S, Abitbol V, Boualit M, Boureille A, Moreau J, Buisson A, Roblin X, Nachury M, Zappa M, Lambert J, Bouhnik Y; GETAID-MICA study group. Adalimumab for patients with Crohn's disease complicated by intra-abdominal abscess: a multicentre, prospective, observational cohort study. J Crohns Colitis. 2019;13:S616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Panaccione R, Löfberg R, Rutgeerts P, Sandborn WJ, Schreiber S, Berg S, Maa JF, Petersson J, Robinson AM, Colombel JF. Efficacy and Safety of Adalimumab by Disease Duration: Analysis of Pooled Data From Crohn's Disease Studies. J Crohns Colitis. 2019;13:725-734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |