Published online Dec 28, 2021. doi: 10.3748/wjg.v27.i48.8262
Peer-review started: March 25, 2021
First decision: June 26, 2021
Revised: July 2, 2021
Accepted: December 7, 2021
Article in press: December 7, 2021
Published online: December 28, 2021
Processing time: 273 Days and 14.1 Hours
Gastric cancer (GC) remains a serious oncological problem, ranking third in the structure of mortality from malignant neoplasms. Improving treatment outcomes for this pathology largely depends on understanding the pathogenesis and biological characteristics of GC, including the identification and characterization of diagnostic, prognostic, predictive, and therapeutic biomarkers. It is known that the main cause of death from malignant neoplasms and GC, in particular, is tumor metastasis. Given that angiogenesis is a critical process for tumor growth and metastasis, it is now considered an important marker of disease prognosis and sensitivity to anticancer therapy. In the presented review, modern concepts of the mechanisms of tumor vessel formation and the peculiarities of their morphology are considered; data on numerous factors influencing the formation of tumor microvessels and their role in GC progression are summarized; and various approaches to the classification of tumor vessels, as well as the methods for assessing angiogenesis activity in a tumor, are highlighted. Here, results from studies on the prognostic and predictive significance of tumor microvessels in GC are also discussed, and a new classification of tumor microvessels in GC, based on their morphology and clinical significance, is proposed for consideration.
Core Tip: In this review, data on the factors associated with the activation of angiogenesis in tumors, the mechanisms of tumor microvessel formation and the features of their morphology, methods for assessing the activity of angiogenesis in a tumor, and their role in the progression of gastric cancer (GC) are discussed. A new classification of tumor microvessels in GC based on their morphology and clinical significance is proposed. Considering the different types of tumor microvessels can have different sensitivities to antiangiogenic therapy, further study of their prognostic and predictive value is undoubtedly relevant.
- Citation: Senchukova MA. Issues of origin, morphology and clinical significance of tumor microvessels in gastric cancer. World J Gastroenterol 2021; 27(48): 8262-8282
- URL: https://www.wjgnet.com/1007-9327/full/v27/i48/8262.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i48.8262
Gastric cancer (GC) remains a serious oncological problem, ranking third in the structure of mortality from malignant neoplasms. The disease is biologically heterogeneous, and the oncogenic mechanisms remain poorly understood[1-3]. In this regard, a deep understanding of the pathogenesis and biological characteristics of GC, including the identification and characterization of diagnostic, prognostic, predictive, and therapeutic biomarkers, is important to improve the results of treatment.
Angiogenesis is a critical process for tumor growth and metastasis, including in GC. Currently, its assessment is considered an important marker of disease prognosis and sensitivity to anticancer therapy[4-9]. The study of angiogenesis is of fundamental importance, not only in terms of predicting disease outcome but also in determining tumor sensitivity to systemic therapy, such as chemotherapy, targeted therapy, and antiangiogenic therapy. In this case, not only is a quantitative assessment of angiogenesis of great importance but also an assessment of the functional adequacy of vessels, in view of the fact that vessels are the pathways for the delivery of anticancer drugs to tumor cells. In connection with the above, this review will discuss modern concepts of the mechanisms of tumor vessel formation and the peculiarities of their morphology, various approaches to the classification of tumor vessels and methods for assessing angiogenesis activity in tumors, and the results of studies on the prognostic and predictive significance of tumor microvessels in GC. Additionally, a new classification of tumor microvessels in GC, based on their morphology and clinical significance, is proposed for consideration.
The formation of new vessels is associated with the activation of various factors, and among them, vascular endothelial growth factor (VEGF), which is expressed by tumor cells, immune cells, tumor-associated fibroblasts, and endothelial cells (ECs), plays a special role. There are five subtypes of VEGF family proteins, namely, VEGF-A, -B, -C, -D, and placental growth factor, among which VEGF-A is a key protein responsible for the proliferation, survival, and mobilization of endothelial progenitor cells from the bone marrow into the peripheral circulation, as well as for the increased permeability of tumor vessels, which is important for the formation of tumor stroma[10-12]. VEGF-A affects the development of new blood vessels and survival of immature blood vessels[13], while VEGF-C and VEGF-D stimulate the formation, proliferation, and germination of lymphatic ECs[14]. It is believed that ECs of existing lymphatic vessels, bone marrow cells, myeloid progenitors, and finally differentiated macrophages can participate in the formation of tumor lymphatic vessels[15,16].
VEGF signaling is mediated through membrane tyrosine kinase receptors (VEGFR-1, -2 and -3) located on tumor cells and ECs[11,17,18], which leads to the activation of signal transducer and activator of transcription 3 (STAT3), phosphoinositide 3-kinase, extracellular signal-regulated kinase (ERK)/protein kinase B (AKT) and other signaling pathways[8,11,18,19]. An increase in VEGF expression attracts monocytes and macrophages to the tumor stroma, which promotes the activation of matrix metalloproteinases (MMPs) and cell adhesion molecules[20-23] to function in the degradation of the extracellular matrix and initiation of the processes of invasion, metastasis, and angiogenesis[24-26]. Along the invasive edge of the tumor, the active processes of formation and lysis of the extracellular matrix components proceed, which leads to the formation of channels that facilitate the formation of blood vessels, invasion, and metastasis of tumor cells[27].
The most powerful stimulant of tumor angiogenesis is hypoxia, which is constantly experienced by cells of growing neoplasms under conditions of insufficient blood supply. One of the key transcription factors responsible for the regulation of gene expression during hypoxia and ischemia is hypoxia-inducible factor-1 alpha (HIF-1α). HIF-1α expression is regulated by the activation of the nuclear factor-kappa B (NF-κB)/HIF-1α/VEGF pathway[28]. Thus, HIF-1α is the main regulator of transcription in the adaptive response to hypoxia, directly participating in the activation of the mechanisms of angiogenesis, invasion, and metastasis of malignant neoplasms, including GC[29].
It has been established that hypoxia can stimulate cells to secrete more exosomes and extracellular vesicles[30,31], containing pro-angiogenic cytokines[30]. Extracellular vesicles originating from cancer cells, under hypoxic conditions, directly transport VEGF or activate the VEGF pathway in ECs, which leads to tumor angiogenesis[31].
Modern technologies of RNA sequencing (RNAseq) have made it possible to create a complete annotation of microRNAs (miRNAs), which are expressed by two-dimensional cultured human ECs under normal[32] or hypoxic[33] conditions. It has been shown that miR-130a is a mediator of the hypoxic response in human primary endothelial colony-forming cells. Under hypoxic conditions of 1% O2, an increase in the expression and biological activity of miR-130a in ECs was observed, which led to the activation of VEGFR2 and STAT3 and the accumulation of HIF-1α. As a result, there was an increase in the clonogenic potential, proliferative and migratory capacity, and survival of ECs, as well as their ability for two-dimensional migration and tubulogenesis. EC tubulogenesis is also facilitated by the expression of miR-210 associated with hypoxia[34]. Interestingly, under conditions of normoxia, overexpression of miR-130a does not cause such effects[35].
It is important to note that HIF-1α can directly regulate the expression of many molecules associated with vasculogenic mimicry (VM), such as VEGF, twist-related protein, MMP2, and others[36]. The hypoxic microenvironment promotes VM by enhancing the differentiation of cancer stem cells, activating epithelial-endothelial transition (EMT), and remodeling the extracellular matrix[36,37].
In addition to VEGF and HIF-1α, many other proangiogenic factors are known. These include epidermal growth factor, main fibroblast growth factor, platelet growth factor, interleukin-1b (IL-1b), and hepatocyte growth factor (HGF), among others. Table 1 summarizes the role of the most studied factors associated with the activation of angiogenesis[38-67].
| Factor | Signaling pathways | Effects | Ref. |
| EGF and EGFR | p38 MAPK, HIF-1α, VEGF | Enhanced angiogenesis, increased VEGF expression, and MMP-1 | [38] |
| EGFR | EMT activation | [39] | |
| PI3K/Akt/mTOR | EMT activation | [40] | |
| Notch and MAPK | Enhanced ECs proliferation, vascular growth and development, increased vascular permeability, inhibition of apoptosis | [41] | |
| Increased expression level in GC patients with peritoneal metastases | [42] | ||
| PIGF | VEGF/VEGFR | A high level of PIGF in plasma is associated with enhanced ECs proliferation and decreased survival of GC patients | [4] |
| Angs (Ang-1, -2, -3, -4) | Ang/Tie | The formation of blood vessels from preexisting, maturation of blood vessels, migration, adhesion, and survival of ECs | [43] |
| Plasma Ang-2 level correlated with liver metastases in patients with GC | [44] | ||
| A high level of angiopoietin-like protein 2 in serum is associated with a high risk of early recurrence of GC | [45] | ||
| PDGF-β; PDGF-D; PDGF-BB and other | In the intestinal-type GC, higher MVD was correlated to overexpression, intensity, and proportion of PDGF-B, but not of VEGF-A. PDGF-B plays a more important role in angiogenesis in intestinal-type gastric carcinomas than VEGF-A | [46] | |
| STAT3, AKT, ERK1/2, mTOR and GSK-3β | PDGF-D promoted the migration, proliferation, adhesion, and tube formation of endothelial progenitor cells | [47] | |
| STAT3, AKT, ERK1/2, mTOR and GSK-3β | PDGF-BB could activate VEGF-A expression | [48] | |
| A high level of PDGFR-β gene expression in tumor is associated with decreased 5-year overall survival rate in GC patients | [49] | ||
| FGFs and FGFR | AKT and Notch | Increased VEGF expression | [50] |
| Snail | The effect of FGF-1 on ECs culture is associated with overexpression of Snai1, increased expression of CD31, CD34, and VWF, and formation of tubes | [51] | |
| WNT and Twist1 | EMT activation | [52] | |
| Serum FGF level was related to MVD, tumor size, infiltration degree, TNM staging, lymph node metastasis, and distant metastasis | [53] | ||
| High levels of FGF2 expression in the tumor is associated with advanced TNM stage and decreased survival of GC patients | [54] | ||
| Tryptase | AKT and ERK, PAR-2 and MAPK | The density of mast cells positive to tryptase is associated MVD in GC patients | [55-57] |
| IL-8 | Src/Vav2/Rac1/PAK1 | Induction of expression of VEGF-A, VEGFR-1, and VEGFR-2; stimulation of proliferation, survival, and migration of ECs, activation of MMP production | [58] |
| Stimulation of ECs migration | [59] | ||
| HER2 | Expression of HER2 (2+ and 3+) in gastric tumors is associated with an increase in MVD | [60] | |
| Expression of HER2 in a tumor is associated with an increase in MVD and a decrease in the survival rate of GC patients | [61] | ||
| ITGAX | PI3k/Akt | Overexpression of ITGAX in HUVEC is associated with induction of VEGF-A and VEGFR-2 expression, enhanced HUVEC proliferation, migration, and tube formation, as well as promoted angiogenesis and ovarian tumor growth | [62] |
| IGF2 and IGF1R | Enhances sprouting angiogenesis and affects tip cell phenotype | [63] | |
| MCU | MCU was related with the activation of EMT mechanisms and HIF-1α and VEGF expression. High level of MCU expression in the tumor was associated with the advanced TNM stage and decreased survival of GC patients | [64] | |
| Helicobacter pylori | Wnt/beta-catenin | VEGF and MVD levels were significantly higher in H. pylori-positive tissues | [65] |
| Epstein-Barr virus | PI3K/AKT/mTOR/HIF-1α | EBV is associated with the formation of vasculogenic mimicry | [66,67] |
When assessing the role of various factors in angiogenesis activation, it is important to understand that exosomes are the main mediators of the cross-interaction of tumor cells with ECs, immune cells, fibroblasts, and other stromal cells. Exosomes are involved in the transport of numerous proangiogenic biomolecules, such as VEGF, MMP, microRNAs, and long noncoding RNAs, among others. In addition, exosomes promote angiogenesis by suppressing the expression of factor-inhibiting HIF-1[68].
Currently, miRNAs that both activate and suppress the expression of genes responsible for angiogenesis have been identified. The activation of angiogenesis during hypoxia is associated with the upregulation of miR-26, miR-130a, miR-130b, miR-126, and miR-210[69]. MiR-135b, delivered by exosomes from stomach tumors to ECs, suppresses the expression of the forkhead box O1 protein and promotes angiogenesis in GC[70]. Exosomal miR-155, obtained from GC cells, promotes VEGF expression and the formation of EC tubes. In human umbilical vein endothelial cell culture, miR-155 increases cell proliferation, migration, and ring formation[71]. An oncogenic, long noncoding RNA MALAT1 regulates the expression of VE-cadherin, β-catenin, MMP 2 and 9, MT1-MMP, p-ERK, p-focal adhesion kinase (FAK), and p-paxillin, which have been recognized as classic markers of VM and angiogenesis[72]. IL-1α mRNA enhances the metastatic potential of GC by activating the IL-1α/VEGF signaling pathways[73].
The number of miRNAs associated with angiogenesis suppression is usually reduced in GC patients[74,75]. For example, miR-590 has been shown to inhibit the migration, invasion, and proliferation of GC cells in vivo and in vitro by targeting VEGFR1/2[75]. Likewise, overexpression of miR-1 in GC cells inhibited proliferation, migration, and formation of EC tubes by suppressing the expression of VEGF-A and endothelin 1[76].
It has been established that proangiogenic and pro-oncogenic pathways are linked to each other. In this context, the activation of these signaling pathways leads to a cascade of interrelated events: proliferation and migration of tumors and ECs, antiapoptosis, EMT, invasion, and tumor metastasis[8]. The most studied proangiogenic and pro-oncogenic signaling pathways are STAT3 and NF-κB. The STAT3 signaling pathway induces angiogenesis by activating VEGF expression[77]. Activation of the signaling pathways can be mediated not only by hypoxia but also by the expression of the cytokines IL-17A and IL-6. For example, the activation of the transcription factor STAT3 by IL-17A promoted an increase in the expression of VEGF and microvessel density (MVD) and was associated with a deterioration in the prognosis of GC[78]. In vitro IL-6 increased the levels of JKA, STAT3, p-STAT3, and VEGF-C proteins in GC cells, promoting growth, invasion, and lymphangiogenesis in GC[79]. Macrophages treated with lipopolysaccharides induced the production of tumor necrosis factor (TNF)-α, IL-6, IL-1β, and IL-8 and promoted the activation of the NF-κB and STAT3 signaling pathways[80]. These data are of particular interest since they can contribute to understanding the mechanisms of angiogenesis activation and factors of GC progression in patients with Helicobacter pylori and Epstein-Barr virus infections[65-67]. Inhibition of STAT3 decreased VEGF expression[81]. At the same time, it should be noted that in a number of studies, there were no correlations between STAT3 activation and the expression levels of VEGF, HIF-1α, β-catenin, and MVD[82].
NF-κB belongs to a group of transcription factors that form homo and heterodimers and increase or suppress the expression of many genes[83]. NF-κB activation occurs in response to various stimuli, including growth factors, cytokines, hormones, and microbial and chemical compounds, and leads to the synthesis of proangiogenic factors, such as IL-1, IL-8, TNF, IL-6, VEGF, MMP-2, and MMP-9[31].
Signaling pathways associated with the activation of angiogenesis, invasion, EMT, and metastasis also include ITGB1/FAK[84], Wnt/β-catenin[85], NF-κB-MMP-9/VEGF[86], ERK/AKT[11], and other pathways. Knock down of these pathways leads to a decrease in angiogenesis and metastasis.
It should be noted that the origin of tumor vessels is an important factor affecting their morphology, participation in tumor progression, and tumor sensitivity to antiangiogenic therapy. Currently, several methods of angiogenesis formation have been described, while different types of pathological vascularization can be observed simultaneously in the tumor stroma[87-89].
Sprouting angiogenesis is the growth of new capillary vessels from pre-existing vessels. This type of angiogenesis is characteristic of all malignant neoplasms, and its routine assessment is carried out by determining the expression of VEGF and MVD in the tumor and adjacent tissues[57,90-94].
The formation of "endothelial sprouts" occurs in several stages and in close interaction with the components of the extracellular matrix. Under the influence of angiogenesis mediators, the basement membrane of the vessels is destabilized, and ECs acquire the ability to proliferate, migrate, and invade. The release of MMPs causes degradation of the basement membrane and leads to directed migration and proliferation of ECs, which differentiate into tip and stalk cells. Within the germinating capillaries, tip cells express high levels of VEGFR2. In response to VEGF, tip cells form characteristic protrusions (filopodia) that are rich in actin. As a result of the polarization of moving ECs, the lumen of the vessel is formed, after which remodeling and maturation occur due to the recruitment of pericytes and synthesis of a new basement membrane[95,96].
It should be noted that the shape and number of this type of vessel depend on the density and composition of the extracellular matrix[97,98], the formation of which is influenced by the permeability of newly formed vessels. Their abnormal permeability increases the density of stromal cells, which leads to an increase in tissue hypoxia and interstitial hypertension, which promotes the entry of cancer cells into the blood and their further spread to distant organs with the formation of metastases[99].
Intussusceptive angiogenesis, this type of angiogenesis is an intravascular process that is invisible under standard light microscopy. It consists of the formation of new capillaries due to the formation of a septum inside their lumen[100-102]. Despite the fact that at present, its role in tumor progression has not been adequately studied, in a number of works, it was noted that in the process of radiation therapy or antiangiogenic therapy, there is a "switch" from sprouting angiogenesis to intussusceptive angiogenesis. The authors believe that the described "switch" can explain the development of tumor resistance to therapy and continued tumor growth after termination of treatment[103,104]. In GC, this type of angiogenesis has not been studied.
Vasculogenesis is a de novo process of blood vessel formation involving progenitor ECs or angioblasts[105]. Its induction in the postnatal period may be due to tissue hypoxia associated with tissue damage or tumor growth. Under physiological conditions, progenitor ECs rest, but under the influence of hypoxia, growth factors, and cytokines, they leave the bone marrow and travel into the peripheral blood, acquiring the ability to circulate, proliferate, and differentiate into mature ECs involved in the formation of new vessels. A number of studies have shown that the number of progenitor ECs in the blood of cancer patients is significantly higher than that in healthy individuals[106,107], and their high content is associated with advanced stages and poor prognosis of the disease[108], including GC[109].
Vessel co-option is a nonangiogenic type of tumor vascularization in which cancer cells use pre-existing blood vessels instead of inducing new blood vessel formation[90]. Thus, the development of a tumor can proceed without the formation of new vessels due to co-option with the vessels of the organ and VM[110]. Currently, vessel co-option, in which the perivascular arrangement of tumor cells is observed[111], is considered the main mechanism for the development of chemoresistance in malignant neoplasms[112].
High endothelial venules (HEVs) are also an example of vessel co-option. HEVs are located in sentinel lymph nodes and serve as a gateway for cancer cells to enter the bloodstream, thereby facilitating distant metastases[87]. HEVs are postcapillary venules characterized by active lymphocyte trafficking and are usually observed in secondary lymphoid organs, excluding the spleen. They are detected using the HEV-specific antibody MECA-79, which is associated with adhesion and transendothelial migration of lymphocytes along the HEV wall[113]. HEVs have been identified in lymphoid infiltrates in breast, ovary, lung, colon, and other carcinomas. In breast cancer and melanoma, high HEV density has been associated with a favorable prognosis, possibly due to an increase in tumor-infiltrating lymphocytes (TILs) and their phenotypes[114,115]. In GC, the number of CD8+ TILs was significantly higher in the HEV-positive group of patients than in the HEV-negative group (P = 0.027), whereas the levels of Foxp3+ and CD20+ TILs did not depend on the presence of HEVs. Overall survival was significantly greater only in the CD8+ TILs- and HEV-positive group. The other combinations were not associated with the survival of patients with GC[113]. However, in the CD8+ TILs and HEV-positive group, there were significantly fewer patients with lymph node metastases (45.7% and 68.0%, in the CD8+ TILs and HEV-positive group and CD8- TIL and HEV-negative group, respectively; P = 0.048). Therefore, it is not entirely clear whether this combination is a sign of a more favorable prognosis of GC or if an improvement in survival is associated with a lower node stage.
VM is the formation of a vessel-like network by tumor cells. This type of angiogenesis is closely associated with extracellular matrix deposition[116]. Originally, the term VM was used to describe the process by which tumor cells form a network of tubular structures with the ability to conduct fluids. Later, VM was understood as any fluid-conducting structures that do not contain ECs (that is, not blood vessels). It is believed that vasculogenesis occurs due to the ability of ECs to self-assemble into a three-dimensional vascular network under the influence of VEGF, FGF-2, and other activators of angiogenesis[117].
In addition to tumor cells, macrophages can take part in the formation of VM structures. Macrophages that form the vasculature have been found to express genes for a variety of cytokines, HIF-1α, and genes commonly associated with ECs, including PECAM-1, endoglin, VE-cadherin, and neuropilins-1, 2. In addition, during the cultivation of lymphatic ECs, tubule-like structures (tubulogenesis) were formed only when cocultivated with macrophages. Macrophages isolated from GC and from metastatic lymph nodes more intensively secrete lymphangiogenic factors, including inflammatory cytokines, MMPs, adhesion molecules, and VEGFs[118]. In GC, patients with PAS+ structures are predisposed to a higher histological class, metastases, distant relapses, and a decrease in overall and disease-free survival[119-121].
Interestingly, VM is associated with the overexpression of MMP-2, MMP-9, VEGF-A, and VEGFR-1 but not with VEGFR-2[122,123], while sprouting angiogenesis is characterized by the overexpression of MMP7, MMP9, and MMP13[124].
At the same time, a number of researchers have questioned the existence of VM in malignant tumors[125]. They argue that the PAS-positive structures observed in VM that do not contain ECs are nothing more than an “artifact”, forming as a result of the unstable structure of the tumor endothelium and accumulation of blood originating from microbleeds[125,126]. The reason for the disagreement is believed to be the lack of reliable markers of BM until recently, and the presence of filamentous PAS+ structures in the tumor stroma does not always indicate that these structures are hollow structures capable of performing circulatory functions[116].
In evaluating angiogenesis in malignant growth, it should be considered that tumor vessels have some morphological features distinguishing them from normal vessels:
Tumor vessels are often located chaotically. Tortuosity, the formation of vascular rings and pathological partitions, abnormal arteriovenous shunts, and vascular lacunae are typical. The size of the vessels varies from severe dilatation to sharp narrowing, with possible alternation of expanded and constricted areas[127-129]. Tumor vasculature often has bidirectional blood flow[42,130].
Some authors have noted the absence of pericytes in tumor vessels, which are cells that are functionally related to the vascular endothelium and extremely important for the stabilization and maturation of vascular structures[131,132].
Tumor vessels (mainly of the capillary type) are characterized by increased proliferation of ECs and have impaired endothelial linings and discontinuous basal membranes and abnormal processes[133-135].
Tumor vessels are characterized by increased permeability, which plays an important role in the activation of tumor angiogenesis[99,136].
In the lumen of blood and lymph vessels of the tumor, tumor emboli are often observed, the presence of which is an unfavorable prognostic factor[137-142].
These features determine the oxygen heterogeneity of tumor tissue, which affects the growth and metastasis of malignant tumors[143], as well as the sensitivity of tumor cells to chemotherapy and radiation therapy[144].
To assess the activity of angiogenesis, in vitro and in vivo models, as well as immunohistochemical and molecular genetic studies on clinical material, can be used[90,145,146].
Evaluation of the clinical significance of VEGF levels in the blood serum of GC patients showed that these signaling proteins can be used as prognostic, but not diagnostic, biomarkers[147]. Thus, the level of VEGF-C associated with lymphangiogenesis was significantly higher in the serum of GC patients than in the control group[148]. High VEGF-C levels were associated with poorly differentiated cancers, advanced stages, a higher density of lymphatic vessels in the tumor, and the presence of metastases to regional lymph nodes and distant organs[149,150]. In addition, high levels of the marker predicted a decrease in the survival rate of GC patients[148,149], especially in Caucasian patients[151]. However, in contrast, some authors noted lower serum levels of VEGF-C in patients with GC than in the control group[152].
A high level of VEGF-A and a low level of Ang-1 in serum were associated with a decrease in the overall survival of patients with GC, but the differences were not statistically significant. However, a 25% decrease in serum VEGF-A levels after two courses of chemotherapy (docetaxel, cisplatin, and fluorouracil), compared to baseline values, was associated with a better response to treatment and improved overall survival[4,153]. The predictive value of VEGF-A was also noted by other researchers[5]. At the same time, a high level of Ang-2 was associated with a decrease in the overall survival of patients with GC but did not predict the efficacy of bevacizumab alone or in combination with the initial VEGF level[154].
In tumor tissue, the level of VEGF-A expression positively correlated with tumor, node and metastasis (TNM) stage, tumor size, lymph node metastases, and lymphovascular invasion (LVI), as well as a decrease in overall survival[155]. Similar data were obtained by other authors[90-92]. In addition, a positive correlation of VEGF-A with the levels of circulating progenitor ECs and ECs was noted[91]. In turn, the level of VEGF-C expression in a tumor positively correlated with the presence of metastases, MVD, density of lymphatic vessels, and stage of GC but not with age, sex, or grade[156]. Interestingly, although no significant correlations were found between the levels of VEGF and VEGFR-2 expression in tumors, overexpression of VEGFR-2 was associated with a decrease in survival in intestinal GC but not in diffuse GC[157].
Evaluation of MVD is performed in vascular hotspots using panendothelial immunohistochemistry markers, such as von Willebrand factor, Ulex Europaeus, or antibodies against CD31, CD34 and, less commonly, VE-cadherin, αvβ3-integrin, CD105, or type IV collagen[158,159]. However, it should be noted that these markers do not allow differentiation between mature and immature vessels, which may be important for identifying vessel co-option[160]. In addition, interobserver variability in MVD scoring methods can affect study results, which can be reduced by applying strict scoring rules and consistent training of individual observers[161].
Comparative analysis of MVD in patients with normal gastric mucosa, gastric ulcers, and GC showed that MVD in GC was significantly higher than that in benign processes in the stomach. MVD also correlated with the expression of fibroblast activation protein (FAP) and HGF[53]. FAP, HGF, and MVD were significantly correlated with the depth of tumor invasion and TNM stage.
In GC, endocan-expressing MVD was associated with tumor size, Borrmann type, tumor differentiation, tumor invasion, lymph node metastases, TNM stage and VEGF and VEGFR2 expression. Patients with high levels of endocan-MVD had significantly lower overall survival[6]. Similar results in assessing MVD in patients with GC were obtained by other researchers[57,90,93,94]. However, in patients with a more aggressive diffuse type of GC, there was a decrease in the expression of MVD in the tumor compared with GC of the intestinal type, and this decrease was associated with advanced TNM stage of the disease. There were no differences in VEGF expression in GC of diffuse and intestinal types[162].
For the assessment of lymphatic vessel density, one should consider the fact that lymphatic vessels can play a dual role in malignant tumors[163,164] in that they can promote cancer metastasis, and their high density correlates with a decrease in patient survival[165,166]. Thus, in GC, high lymphatic vessel density was associated with metastases to the lymph nodes and LVI[9]. The presence of functional lymphatic vessels also enhances the antitumor immune response and facilitates the delivery of chemotherapeutic agents, enhancing their action[167,168]. Interestingly, in GC, vessels that stained for both the D2-40 antibody (a marker of lymphatic vessels) and factor VIII (a marker of blood vessels) were identified. The authors noted that MVD in the tumor was higher than in nontumor tissue, but there were no differences in MVD in mucosal carcinoma and submucosa-invasive carcinoma tissues[169].
In GC, upregulated expression of CD44 and CD133 correlated with high TNM stage, high depth of invasion, lymph node metastasis, vascular invasion, distant metastasis, and poor five-year overall survival[170].
When assessing LVI, it is important to exclude false-positive and false-negative cases of LVI, which is possible when using the immunohistochemical method of staining tumor tissue[171]. In a group of patients with LVI+/perineural invasion (PNI)+, the overall and relapse-free survival rates were significantly lower than in the group of patients who were LVI-/PNI-[137-140], including in patients with lymph node-negative GC[141,142] and in patients who received neoadjuvant chemotherapy[172]. Interestingly, adjuvant chemotherapy significantly improved overall and disease-free survival in PNI+ but not PNI- patients, and these results were not influenced by disease stage[173].
It is important to note that at present, extravascular mechanisms of tumor cell spread, including PNI, are being considered. Recently, the term angiotropism was introduced, which indicates the tendency of tumor cells to spread through continuous migration along the abluminal surfaces of vessels or other pathways to nearby or more distant sites without entering the vascular channels[174].
In patients with GC, the presence of VM was associated with poor overall and disease-free survival, high tumor grade, advanced stage, lymph node metastasis, deep tumor invasion, and distant metastasis[94,120,123,175-177]. Positive correlations were found between VM and the expression of the stem cell markers CD133 and Lgr5. The cancer stem cells responsible for the formation of VM are believed to be able to determine the chemotherapy and radioresistance of malignant neoplasms[94,175-177].
In experimental oncology, the migration ability of ECs[178-180], the three-dimensional model for calculating MVD[181,182], methods of three-dimensional spheroids for EC cocultivation with monocytes, fibroblasts and other cells of the tumor microenvironment, EC metabolism, identification of progenitor ECs and other methods of analysis are also used to assess angiogenesis. They can be reproduced both in vitro and in vivo. However, these methods are hardly applicable in wide clinical practice due to the need to perform laborious and complex manipulations using immunodeficient animals and expensive equipment. A detailed analysis of methods for assessing angiogenesis is presented in the "Consensus guidelines for the use and interpretation of angiogenesis assays"[117].
The unsatisfactory results of antiangiogenic therapy highlight the relevance of further studies on angiogenesis for disease prognosis and tumor response to therapy, as well as for the search of new directions in the treatment of malignant neoplasms[183]. It should be noted that at present, in clinical practice, preference is given to the quantitative assessment of angiogenesis, which include the determination of MVD, level of VEGF expression, and other markers, in GC[4-7,156]. At the same time, tumor vessels are known to be heterogeneous in their origin and morphology, and various types of vessels may differ not only in clinical significance but also in their sensitivity to antiangiogenic therapy[130,133,184-186].
Despite the fact that heterogeneity of tumor vessels has been confirmed by numerous studies, a standard classification of vessels has not yet been developed, which would consider not only morphological features but also the relationship with the clinical and morphological characteristics of the pathological process, long-term treatment results and sensitivity to therapy. The proposed classifications are aimed primarily at determining the sensitivity of malignant neoplasms to antiangiogenic therapy. Thus, Gee et al[187] proposed distinguishing tumor microvessels by their degree of maturity. The authors, depending on the size, perfusion, EC proliferation, and presence of pericytes, identified three types of microvessels: (1) highly proliferative, nonperfused EC sprouts emanating from functional vessels; (2) small, perfused vessels that, like angiogenic sprouts, were not covered by pericytes; and (3) larger vessels, which were predominantly pericyte-covered with quiescent ECs and few associated sprouts. Only type 1 and type 2 vessels were sensitive to anti-vascular agents[187,188].
Another classification of microvessels based on their morphological features was proposed by Nagy et al[130]. The researchers identified six types of microvessels, which, in their opinion, developed sequentially over time: mother vessels, glomeruloid microvascular proliferations, vascular malformations, capillaries, feeding arteries, and draining veins[99,130]. Only immature mother vessels and glomeruloid microvascular proliferations were sensitive to therapy with antiangiogenic drugs[185,186].
Furthermore, Kuczynski et al[184], in an investigation of vessels in hepatocellular carcinoma, identified five types of vessels: (1) tumor-embedded vessels, defined as CD31+ vessels bordered only by lamin A/C+ tumor cells; (2) connective tissue vessels, which were CD31+ vessels bordered by fibroblasts; (3) hepatocyte vessels, which were CD31+ vessels bordered by hepatocytes; (4) hepatic central veins; and (5) normal vessels of the portal triads. The authors considered the presence of vessel types 3 through 5 in the tumor as evidence for vessel co-option since these vessels were present in the structure of the normal liver and their presence was believed to be associated with resistance to sorafenib treatment.
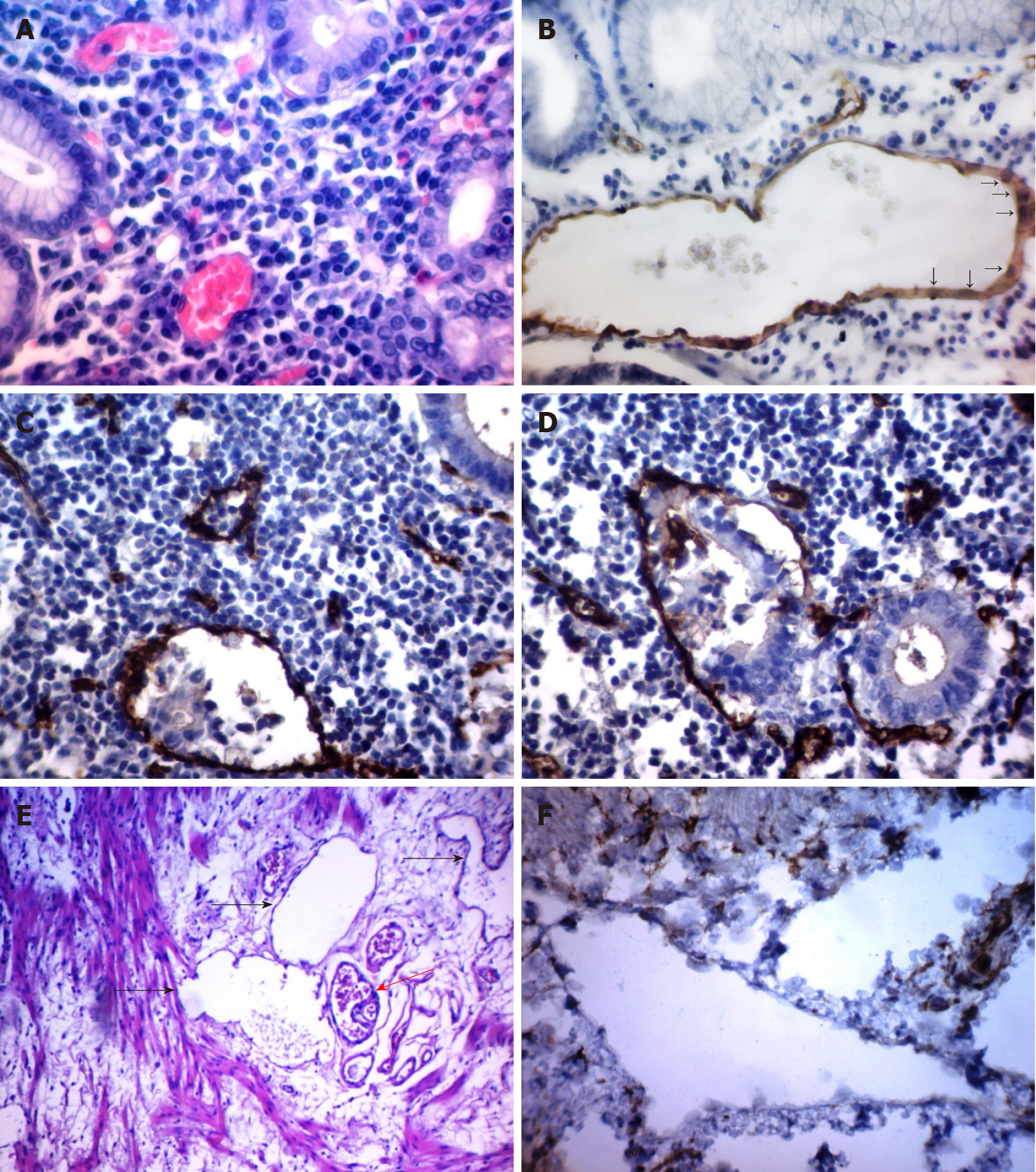
First, it should be noted that the above classifications took into account the degree of tumor microvessel maturity and their sensitivity to antiangiogenic therapy. These classifications do not allow distinction between tumor microvessels, depending on their prognostic significance. Considering that tumor microvessels have different origins and are heterogeneous in morphology, we set the goal of classifying them according to morphology and clinical significance. For this, we studied the features of tumor microvessel morphology in 73 patients with GC and compared the data obtained with the clinical characteristics and prognosis of the disease[189]. As a result of the study, five types of microvessels and structures with endothelial lining were identified (Figure 1).
Vessels 5–40 microns in diameter lined with EC with flat, hyperchromic nuclei. The correlations between the vessels of this type and the factors of GC progression were not revealed.
Large vessels of predominantly round or oval shape with a diameter of 40 microns or more that possessed clear, even contours and endothelial lining formed both by cells with flattened, hyperchromic nuclei and cells with large, pale nuclei with fine-netted chromatin structure. The cytoplasm of the lining cells was evenly stained by CD34. We also found no correlations between the vessels of this type and the factors of GC progression.
Vessels of an irregular shape with a diameter of 40 microns or more with indistinct, uneven contours. The endothelial lining of such vessels was formed by randomly located cells of irregular shape, unevenly accumulating the CD34 marker. In the lumen of such vessels, tumor emboli were often found.
Their characteristic feature was the chaotic arrangement of ECs with irregular shape, uneven contours, and uneven expression of CD34 markers. In GC, multiple, atypical, dilated capillaries and structures with partial endothelial linings were significantly more frequently observed at stages T3–4 (P = 0.001) and N2 (P = 0.001). With or without multiple structures with partial endothelial lining, the three-year overall survival was 52.7% and 93.9%, respectively (P = 0.0013), and the relapse-free survival was 32.4% and 87.7%, respectively (P = 0.0001).
Vessels located in the gastric submucosa adjacent to the tumor. The presence of these vessels was observed more often in patients with lymphatic metastases (P = 0.01) and in grade 3–4 tumors (P = 0.04) and was associated with a decrease in three-year relapse-free and overall survival (P = 0.049 and P = 0.008, respectively).
It should be noted that we changed the names of some vessels, which made it possible to more accurately characterize the features of their morphology. In particular, cavitary structures of type-1 were renamed structures with partial endothelial linings, and cavitary structures of type-1 were renamed dilated capillaries with weak expression of CD34. In further studies, it was shown that the proposed classification of tumor microvessels can be used for other localizations of malignant neoplasms[190,191].
Overall, angiogenesis plays a key role in tumor progression, affecting the growth and metastasis of malignant neoplasms. At the same time, the origin, degree of maturity, morphological features, and functionality of tumor microvessels are of decisive importance for the delivery of drugs to the tumor, and in addition, they determine the sensitivity of tumor microvessels to angiogenic therapy. Most of the proposed classifications of tumor microvessels are based on assessing the degree of their maturity and do not take into account the different roles of individual types of microvessels in tumor progression. In contrast to the classifications proposed by other authors, our classification considers not only the morphology of the vessels but also their clinical significance. We believe, however, that further studies are needed to understand angiogenesis mechanisms in GC and verify the hypotheses made regarding the role of different types of tumor vessels in the progression of GC and GC chemoresistance.
Provenance and peer review: Invited article; Externally peer reviewed
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Association of Oncologists of Russia.
Specialty type: Oncology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: da Costa AC S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Baniak N, Senger JL, Ahmed S, Kanthan SC, Kanthan R. Gastric biomarkers: a global review. World J Surg Oncol. 2016;14:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol. 2020;18:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 982] [Article Influence: 196.4] [Reference Citation Analysis (1)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55825] [Article Influence: 7975.0] [Reference Citation Analysis (132)] |
| 4. | Aktaş SH, Akbulut Yazici HO, Zengin N, Akgün HN, Üstüner Z, Içli F. A new angiogenesis prognostic index with VEGFA, PlGF, and angiopoietin1 predicts survival in patients with advanced gastric cancer. Turk J Med Sci. 2017;47:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Liu X, Guo W, Zhang W, Yin J, Zhang J, Zhu X, Liu T, Chen Z, Wang B, Chang J, Lv F, Hong X, Wang H, Wang J, Zhao X, Wu X, Li J. A multi-center phase II study and biomarker analysis of combined cetuximab and modified FOLFIRI as second-line treatment in patients with metastatic gastric cancer. BMC Cancer. 2017;17:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Chang Y, Niu W, Lian PL, Wang XQ, Meng ZX, Liu Y, Zhao R. Endocan-expressing microvessel density as a prognostic factor for survival in human gastric cancer. World J Gastroenterol. 2016;22:5422-5429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Nienhüser H, Schmidt T. Angiogenesis and Anti-Angiogenic Therapy in Gastric Cancer. Int J Mol Sci. 2017;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Hsieh HL, Tsai MM. Tumor progression-dependent angiogenesis in gastric cancer and its potential application. World J Gastrointest Oncol. 2019;11:686-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Sun Y, Yu X, Li M, Zou Z. Expression of CD44v6 and lymphatic vessel density in early gastric cancer tissues and their clinical significance. Pak J Med Sci. 2019;35:549-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Zecchin A, Kalucka J, Dubois C, Carmeliet P. How Endothelial Cells Adapt Their Metabolism to Form Vessels in Tumors. Front Immunol. 2017;8:1750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Caporarello N, Lupo G, Olivieri M, Cristaldi M, Cambria MT, Salmeri M, Anfuso CD. Classical VEGF, Notch and Ang signalling in cancer angiogenesis, alternative approaches and future directions (Review). Mol Med Rep. 2017;16:4393-4402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Chen S, Zhang X, Peng J, Zhai E, He Y, Wu H, Chen C, Ma J, Wang Z, Cai S. VEGF promotes gastric cancer development by upregulating CRMP4. Oncotarget. 2016;7:17074-17086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Yehya AHS, Asif M, Petersen SH, Subramaniam AV, Kono K, Majid AMSA, Oon CE. Angiogenesis: Managing the Culprits behind Tumorigenesis and Metastasis. Medicina (Kaunas). 2018;54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Vaahtomeri K, Karaman S, Mäkinen T, Alitalo K. Lymphangiogenesis guidance by paracrine and pericellular factors. Genes Dev. 2017;31:1615-1634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 15. | Gutierrez-Miranda L, Yaniv K. Cellular Origins of the Lymphatic Endothelium: Implications for Cancer Lymphangiogenesis. Front Physiol. 2020;11:577584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Ran S, Volk-Draper L. Lymphatic Endothelial Cell Progenitors in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1234:87-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Lian L, Li XL, Xu MD, Li XM, Wu MY, Zhang Y, Tao M, Li W, Shen XM, Zhou C, Jiang M. VEGFR2 promotes tumorigenesis and metastasis in a pro-angiogenic-independent way in gastric cancer. BMC Cancer. 2019;19:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 18. | Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front Immunol. 2018;9:978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 446] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 19. | Johnston PA, Grandis JR. STAT3 signaling: anticancer strategies and challenges. Mol Interv. 2011;11:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 333] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 20. | Li H, Huang N, Zhu W, Wu J, Yang X, Teng W, Tian J, Fang Z, Luo Y, Chen M, Li Y. Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer. 2018;18:579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 21. | Osinsky S, Bubnovskaya L, Ganusevich I, Kovelskaya A, Gumenyuk L, Olijnichenko G, Merentsev S. Hypoxia, tumour-associated macrophages, microvessel density, VEGF and matrix metalloproteinases in human gastric cancer: interaction and impact on survival. Clin Transl Oncol. 2011;13:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Zhou Y, Li G, Wu J, Zhang Z, Wu Z, Fan P, Hao T, Zhang X, Li M, Zhang F, Li Q, Lu B, Qiao L. Clinicopathological significance of E-cadherin, VEGF, and MMPs in gastric cancer. Tumour Biol. 2010;31:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Beamish JA, Juliar BA, Cleveland DS, Busch ME, Nimmagadda L, Putnam AJ. Deciphering the relative roles of matrix metalloproteinase- and plasmin-mediated matrix degradation during capillary morphogenesis using engineered hydrogels. J Biomed Mater Res B Appl Biomater. 2019;107:2507-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Wen YL, Li L. Correlation between matrix metalloproteinase-9 and vascular endothelial growth factor expression in lung adenocarcinoma. Genet Mol Res. 2015;14:19342-19348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Yang Q, Ye ZY, Zhang JX, Tao HQ, Li SG, Zhao ZS. Expression of matrix metalloproteinase-9 mRNA and vascular endothelial growth factor protein in gastric carcinoma and its relationship to its pathological features and prognosis. Anat Rec (Hoboken). 2010;293:2012-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Andreuzzi E, Capuano A, Poletto E, Pivetta E, Fejza A, Favero A, Doliana R, Cannizzaro R, Spessotto P, Mongiat M. Role of Extracellular Matrix in Gastrointestinal Cancer-Associated Angiogenesis. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020;11:5120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 1323] [Article Influence: 264.6] [Reference Citation Analysis (0)] |
| 28. | Nam SY, Ko YS, Jung J, Yoon J, Kim YH, Choi YJ, Park JW, Chang MS, Kim WH, Lee BL. A hypoxia-dependent upregulation of hypoxia-inducible factor-1 by nuclear factor-κB promotes gastric tumour growth and angiogenesis. Br J Cancer. 2011;104:166-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Li H, Jia Y, Wang Y. Targeting HIF-1α signaling pathway for gastric cancer treatment. Pharmazie. 2019;74:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 30. | King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 650] [Cited by in RCA: 802] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 31. | Kuriyama N, Yoshioka Y, Kikuchi S, Azuma N, Ochiya T. Extracellular Vesicles Are Key Regulators of Tumor Neovasculature. Front Cell Dev Biol. 2020;8:611039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Kuosmanen SM, Kansanen E, Sihvola V, Levonen AL. MicroRNA Profiling Reveals Distinct Profiles for Tissue-Derived and Cultured Endothelial Cells. Sci Rep. 2017;7:10943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Voellenkle C, Rooij Jv, Guffanti A, Brini E, Fasanaro P, Isaia E, Croft L, David M, Capogrossi MC, Moles A, Felsani A, Martelli F. Deep-sequencing of endothelial cells exposed to hypoxia reveals the complexity of known and novel microRNAs. RNA. 2012;18:472-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 34. | Jung KO, Youn H, Lee CH, Kang KW, Chung JK. Visualization of exosome-mediated miR-210 transfer from hypoxic tumor cells. Oncotarget. 2017;8:9899-9910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 35. | Guduric-Fuchs J, Pedrini E, Lechner J, Chambers SEJ, O'Neill CL, Mendes Lopes de Melo J, Pathak V, Church RH, McKeown S, Bojdo J, Mcloughlin KJ, Stitt AW, Medina RJ. miR-130a activates the VEGFR2/STAT3/HIF1α axis to potentiate the vasoregenerative capacity of endothelial colony-forming cells in hypoxia. Mol Ther Nucleic Acids. 2021;23:968-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Wei X, Chen Y, Jiang X, Peng M, Liu Y, Mo Y, Ren D, Hua Y, Yu B, Zhou Y, Liao Q, Wang H, Xiang B, Zhou M, Li X, Li G, Li Y, Xiong W, Zeng Z. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol Cancer. 2021;20:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 276] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 37. | Wang M, Zhao X, Zhu D, Liu T, Liang X, Liu F, Zhang Y, Dong X, Sun B. HIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment. J Exp Clin Cancer Res. 2017;36:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 38. | Kim D, Dai J, Park YH, Fai LY, Wang L, Pratheeshkumar P, Son YO, Kondo K, Xu M, Luo J, Shi X, Zhang Z. Activation of Epidermal Growth Factor Receptor/p38/Hypoxia-inducible Factor-1α Is Pivotal for Angiogenesis and Tumorigenesis of Malignantly Transformed Cells Induced by Hexavalent Chromium. J Biol Chem. 2016;291:16271-16281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Pei YF, Liu J, Cheng J, Wu WD, Liu XQ. Silencing of LAMC2 Reverses Epithelial-Mesenchymal Transition and Inhibits Angiogenesis in Cholangiocarcinoma via Inactivation of the Epidermal Growth Factor Receptor Signaling Pathway. Am J Pathol. 2019;189:1637-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | Huo FC, Zhu WT, Liu X, Zhou Y, Zhang LS, Mou J. Epidermal growth factor-like domain multiple 6 (EGFL6) promotes the migration and invasion of gastric cancer cells by inducing epithelial-mesenchymal transition. Invest New Drugs. 2021;39:304-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Martorana A, La Monica G, Lauria A. Quinoline-Based Molecules Targeting c-Met, EGF, and VEGF Receptors and the Proteins Involved in Related Carcinogenic Pathways. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 42. | Song H, Wang T, Tian L, Bai S, Chen L, Zuo Y, Xue Y. Macrophages on the Peritoneum are involved in Gastric Cancer Peritoneal Metastasis. J Cancer. 2019;10:5377-5387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 43. | Forma A, Tyczyńska M, Kędzierawski P, Gietka K, Sitarz M. Gastric carcinogenesis: a comprehensive review of the angiogenic pathways. Clin J Gastroenterol. 2021;14:14-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Hacker UT, Escalona-Espinosa L, Consalvo N, Goede V, Schiffmann L, Scherer SJ, Hedge P, Van Cutsem E, Coutelle O, Büning H. Evaluation of Angiopoietin-2 as a biomarker in gastric cancer: results from the randomised phase III AVAGAST trial. Br J Cancer. 2016;114:855-862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Toiyama Y, Tanaka K, Kitajima T, Shimura T, Imaoka H, Mori K, Okigami M, Yasuda H, Okugawa Y, Saigusa S, Ohi M, Inoue Y, Mohri Y, Goel A, Kusunoki M. Serum angiopoietin-like protein 2 as a potential biomarker for diagnosis, early recurrence and prognosis in gastric cancer patients. Carcinogenesis. 2015;36:1474-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Suzuki S, Dobashi Y, Hatakeyama Y, Tajiri R, Fujimura T, Heldin CH, Ooi A. Clinicopathological significance of platelet-derived growth factor (PDGF)-B and vascular endothelial growth factor-A expression, PDGF receptor-β phosphorylation, and microvessel density in gastric cancer. BMC Cancer. 2010;10:659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Zhang J, Zhang H, Chen Y, Fu J, Lei Y, Sun J, Tang B. Plateletderived growth factor D promotes the angiogenic capacity of endothelial progenitor cells. Mol Med Rep. 2019;19:125-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Cheng X, Jin Z, Ji X, Shen X, Feng H, Morgenlander W, Ou B, Wu H, Gao H, Ye F, Zhang Y, Peng Y, Liang J, Jiang Y, Zhang T, Qiu W, Lu X, Zhao R. ETS variant 5 promotes colorectal cancer angiogenesis by targeting platelet-derived growth factor BB. Int J Cancer. 2019;145:179-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 49. | Higuchi A, Oshima T, Yoshihara K, Sakamaki K, Aoyama T, Suganuma N, Yamamoto N, Sato T, Cho H, Shiozawa M, Yoshikawa T, Rino Y, Kunisaki C, Imada T, Masuda M. Clinical significance of platelet-derived growth factor receptor-β gene expression in stage II/III gastric cancer with S-1 adjuvant chemotherapy. Oncol Lett. 2017;13:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Xie F, Zhang X, Luo W, Ge H, Sun D, Liu P. Notch Signaling Pathway Is Involved in bFGF-Induced Corneal Lymphangiogenesis and Hemangiogenesis. J Ophthalmol. 2019;2019:9613923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Zhang YK, Wang H, Guo YW, Yue Y. Novel role of Snail 1 in promoting tumor neoangiogenesis. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Yashiro M, Matsuoka T. Fibroblast growth factor receptor signaling as therapeutic targets in gastric cancer. World J Gastroenterol. 2016;22:2415-2423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (2)] |
| 53. | Gao LM, Wang F, Zheng Y, Fu ZZ, Zheng L, Chen LL. Roles of Fibroblast Activation Protein and Hepatocyte Growth Factor Expressions in Angiogenesis and Metastasis of Gastric Cancer. Pathol Oncol Res. 2019;25:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Li Y, Guo XB, Wang JS, Wang HC, Li LP. Function of fibroblast growth factor 2 in gastric cancer occurrence and prognosis. Mol Med Rep. 2020;21:575-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Sammarco G, Gadaleta CD, Zuccalà V, Albayrak E, Patruno R, Milella P, Sacco R, Ammendola M, Ranieri G. Tumor-Associated Macrophages and Mast Cells Positive to Tryptase Are Correlated with Angiogenesis in Surgically-Treated Gastric Cancer Patients. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Micu GV, Stăniceanu F, Sticlaru LC, Popp CG, Bastian AE, Gramada E, Pop G, Mateescu RB, Rimbaş M, Archip B, Bleotu C. Correlations Between the Density of Tryptase Positive Mast Cells (DMCT) and that of New Blood Vessels (CD105+) in Patients with Gastric Cancer. Rom J Intern Med. 2016;54:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | Ammendola M, Sacco R, Zuccalà V, Luposella M, Patruno R, Gadaleta P, Zizzo N, Gadaleta CD, De Sarro G, Sammarco G, Oltean M, Ranieri G. Mast Cells Density Positive to Tryptase Correlate with Microvascular Density in both Primary Gastric Cancer Tissue and Loco-Regional Lymph Node Metastases from Patients That Have Undergone Radical Surgery. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Shi J, Wei PK. Interleukin-8: A potent promoter of angiogenesis in gastric cancer. Oncol Lett. 2016;11:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 59. | Ju L, Zhou Z, Jiang B, Lou Y, Guo X. Autocrine VEGF and IL-8 Promote Migration via Src/Vav2/Rac1/PAK1 Signaling in Human Umbilical Vein Endothelial Cells. Cell Physiol Biochem. 2017;41:1346-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 60. | Ciesielski M, Szajewski M, Pęksa R, Lewandowska MA, Zieliński J, Walczak J, Szefel J, Kruszewski WJ. The relationship between HER2 overexpression and angiogenesis in gastric cancer. Medicine (Baltimore). 2018;97:e12854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Li F, Meng G, Tan B, Chen Z, Ji Q, Wang X, Liu C, Niu S, Li Y, Liu Y. Relationship between HER2 expression and tumor interstitial angiogenesis in primary gastric cancer and its effect on prognosis. Pathol Res Pract. 2021;217:153280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Wang J, Yang L, Liang F, Chen Y, Yang G. Integrin alpha x stimulates cancer angiogenesis through PI3K/Akt signaling-mediated VEGFR2/VEGF-A overexpression in blood vessel endothelial cells. J Cell Biochem. 2019;120:1807-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 63. | Dallinga MG, Habani YI, Kayser RP, Van Noorden CJF, Klaassen I, Schlingemann RO. IGF-binding proteins 3 and 4 are regulators of sprouting angiogenesis. Mol Biol Rep. 2020;47:2561-2572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Wang X, Song X, Cheng G, Zhang J, Dong L, Bai J, Luo D, Xiong Y, Li S, Liu F, Sun Y, Wang X, Li Y, Huang Y. The Regulatory Mechanism and Biological Significance of Mitochondrial Calcium Uniporter in the Migration, Invasion, Angiogenesis and Growth of Gastric Cancer. Onco Targets Ther. 2020;13:11781-11794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Liu N, Zhou N, Chai N, Liu X, Jiang H, Wu Q, Li Q. Helicobacter pylori promotes angiogenesis depending on Wnt/beta-catenin-mediated vascular endothelial growth factor via the cyclooxygenase-2 pathway in gastric cancer. BMC Cancer. 2016;16:321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 66. | Xiang T, Lin YX, Ma W, Zhang HJ, Chen KM, He GP, Zhang X, Xu M, Feng QS, Chen MY, Zeng MS, Zeng YX, Feng L. Vasculogenic mimicry formation in EBV-associated epithelial malignancies. Nat Commun. 2018;9:5009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 67. | Kim HS, Won YJ, Shim JH, Kim HJ, Kim J, Hong HN, Kim BS. Morphological characteristics of vasculogenic mimicry and its correlation with EphA2 expression in gastric adenocarcinoma. Sci Rep. 2019;9:3414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 68. | Olejarz W, Kubiak-Tomaszewska G, Chrzanowska A, Lorenc T. Exosomes in Angiogenesis and Anti-angiogenic Therapy in Cancers. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 69. | Rosano S, Corà D, Parab S, Zaffuto S, Isella C, Porporato R, Hoza RM, Calogero RA, Riganti C, Bussolino F, Noghero A. A regulatory microRNA network controls endothelial cell phenotypic switch during sprouting angiogenesis. Elife. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 70. | Bai M, Li J, Yang H, Zhang H, Zhou Z, Deng T, Zhu K, Ning T, Fan Q, Ying G, Ba Y. miR-135b Delivered by Gastric Tumor Exosomes Inhibits FOXO1 Expression in Endothelial Cells and Promotes Angiogenesis. Mol Ther. 2019;27:1772-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 71. | Deng T, Zhang H, Yang H, Wang H, Bai M, Sun W, Wang X, Si Y, Ning T, Zhang L, Li H, Ge S, Liu R, Lin D, Li S, Ying G, Ba Y. Exosome miR-155 Derived from Gastric Carcinoma Promotes Angiogenesis by Targeting the c-MYB/VEGF Axis of Endothelial Cells. Mol Ther Nucleic Acids. 2020;19:1449-1459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 72. | Li Y, Wu Z, Yuan J, Sun L, Lin L, Huang N, Bin J, Liao Y, Liao W. Long non-coding RNA MALAT1 promotes gastric cancer tumorigenicity and metastasis by regulating vasculogenic mimicry and angiogenesis. Cancer Lett. 2017;395:31-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 73. | Gong Z, Ma J, Su H, Guo T, Cai H, Chen Q, Zhao X, Qi J, Du J. Interleukin-1 receptor antagonist inhibits angiogenesis in gastric cancer. Int J Clin Oncol. 2018;23:659-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 74. | Zhang C, Liang Y, Ma MH, Wu KZ, Zhang CD, Dai DQ. Downregulation of microRNA-376a in Gastric Cancer and Association with Poor Prognosis. Cell Physiol Biochem. 2018;51:2010-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 75. | Mei B, Chen J, Yang N, Peng Y. The regulatory mechanism and biological significance of the Snail-miR590-VEGFR-NRP1 axis in the angiogenesis, growth and metastasis of gastric cancer. Cell Death Dis. 2020;11:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 76. | Xie M, Dart DA, Guo T, Xing XF, Cheng XJ, Du H, Jiang WG, Wen XZ, Ji JF. MicroRNA-1 acts as a tumor suppressor microRNA by inhibiting angiogenesis-related growth factors in human gastric cancer. Gastric Cancer. 2018;21:41-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 77. | Zhu F, Wang KB, Rui L. STAT3 Activation and Oncogenesis in Lymphoma. Cancers (Basel). 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 78. | Wu X, Yang T, Liu X, Guo JN, Xie T, Ding Y, Lin M, Yang H. IL-17 promotes tumor angiogenesis through Stat3 pathway mediated upregulation of VEGF in gastric cancer. Tumour Biol. 2016;37:5493-5501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 79. | Zhao G, Zhu G, Huang Y, Zheng W, Hua J, Yang S, Zhuang J, Ye J. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol Rep. 2016;35:1787-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 80. | Zhou Y, Xia L, Liu Q, Wang H, Lin J, Oyang L, Chen X, Luo X, Tan S, Tian Y, Su M, Wang Y, Chen P, Wu Y, Liao Q. Induction of Pro-Inflammatory Response via Activated Macrophage-Mediated NF-κB and STAT3 Pathways in Gastric Cancer Cells. Cell Physiol Biochem. 2018;47:1399-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 81. | Zhang X, Tang J, Zhi X, Xie K, Wang W, Li Z, Zhu Y, Yang L, Xu H, Xu Z. miR-874 functions as a tumor suppressor by inhibiting angiogenesis through STAT3/VEGF-A pathway in gastric cancer. Oncotarget. 2015;6:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 82. | Krstić M, Stojanović NM, Stojnev S, Radenković G, Čukuranović Kokoris J, Mladenović B, Janković Veličković L. Interplay between STAT3, Cell Adhesion Molecules and Angiogenesis-Related Parameters in Gastric Carcinoma. Does STAT3 Really Have a Prognostic Value? Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Sokolova O, Naumann M. NF-κB Signaling in Gastric Cancer. Toxins (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 84. | Wang N, Chang LL. Maspin suppresses cell invasion and migration in gastric cancer through inhibiting EMT and angiogenesis via ITGB1/FAK pathway. Hum Cell. 2020;33:663-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 85. | Tang L, Wen JB, Wen P, Li X, Gong M, Li Q. Long non-coding RNA LINC01314 represses cell migration, invasion, and angiogenesis in gastric cancer via the Wnt/β-catenin signaling pathway by down-regulating KLK4. Cancer Cell Int. 2019;19:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 86. | Chen P, Zhao D, Wang W, Zhang Y, Yuan Y, Wang L, Wu Y. High expression of RELM-α correlates with poor prognosis and promotes angiogenesis in gastric cancer. Oncol Rep. 2015;34:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Qian CN, Pezzella F. Tumor vasculature: a sally port for inhibiting cancer cell spreading. Cancer Commun (Lond). 2018;38:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77:1745-1770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 1179] [Article Influence: 196.5] [Reference Citation Analysis (0)] |
| 89. | Zuazo-Gaztelu I, Casanovas O. Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front Oncol. 2018;8:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 190] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 90. | Zhang Y, Qu H. Expression and clinical significance of aquaporin-1, vascular endothelial growth factor and microvessel density in gastric cancer. Medicine (Baltimore). 2020;99:e21883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Li B, Nie Z, Zhang D, Wu J, Peng B, Guo X, Shi Y, Cai X, Xu L, Cao F. Roles of circulating endothelial progenitor cells and endothelial cells in gastric carcinoma. Oncol Lett. 2018;15:324-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 92. | Hafez NH, Tahoun NS. Expression of cyclooxygenase 2 and vascular endothelial growth factor in gastric carcinoma: Relationship with clinicopathological parameters. J Egypt Natl Canc Inst. 2016;28:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 93. | Hong WG, Ko YS, Pyo JS. Clinicopathological significance and prognostic role of microvessel density in gastric cancer: A meta-analysis. Pathol Res Pract. 2017;213:1459-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Zhou L, Yu L, Feng ZZ, Gong XM, Cheng ZN, Yao N, Wang DN, Wu SW. Aberrant Expression of Markers of Cancer Stem Cells in Gastric Adenocarcinoma and their Relationship to Vasculogenic Mimicry. Asian Pac J Cancer Prev. 2015;16:4177-4183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Hosseini F, Naghavi N. Modelling Tumor-induced Angiogenesis: Combination of Stochastic Sprout Spacing and Sprout Progression. J Biomed Phys Eng. 2017;7:233-256. [PubMed] |
| 96. | Palm MM, Dallinga MG, van Dijk E, Klaassen I, Schlingemann RO, Merks RM. Computational Screening of Tip and Stalk Cell Behavior Proposes a Role for Apelin Signaling in Sprout Progression. PLoS One. 2016;11:e0159478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Shamloo A, Mohammadaliha N, Heilshorn SC, Bauer AL. A Comparative Study of Collagen Matrix Density Effect on Endothelial Sprout Formation Using Experimental and Computational Approaches. Ann Biomed Eng. 2016;44:929-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 98. | Feng X, Tonnesen MG, Mousa SA, Clark RA. Fibrin and collagen differentially but synergistically regulate sprout angiogenesis of human dermal microvascular endothelial cells in 3-dimensional matrix. Int J Cell Biol. 2013;2013:231279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 99. | Dvorak HF. Tumor Stroma, Tumor Blood Vessels, and Antiangiogenesis Therapy. Cancer J. 2015;21:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 100. | Burri PH, Hlushchuk R, Djonov V. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev Dyn. 2004;231:474-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 231] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 101. | Díaz-Flores L, Gutiérrez R, Gayoso S, García MP, González-Gómez M, Díaz-Flores L Jr, Sánchez R, Carrasco JL, Madrid JF. Intussusceptive angiogenesis and its counterpart intussusceptive lymphangiogenesis. Histol Histopathol. 2020;35:1083-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 102. | Ali Z, Mukwaya A, Biesemeier A, Ntzouni M, Ramsköld D, Giatrellis S, Mammadzada P, Cao R, Lennikov A, Marass M, Gerri C, Hildesjö C, Taylor M, Deng Q, Peebo B, Del Peso L, Kvanta A, Sandberg R, Schraermeyer U, Andre H, Steffensen JF, Lagali N, Cao Y, Kele J, Jensen LD. Intussusceptive Vascular Remodeling Precedes Pathological Neovascularization. Arterioscler Thromb Vasc Biol. 2019;39:1402-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 103. | Hlushchuk R, Riesterer O, Baum O, Wood J, Gruber G, Pruschy M, Djonov V. Tumor recovery by angiogenic switch from sprouting to intussusceptive angiogenesis after treatment with PTK787/ZK222584 or ionizing radiation. Am J Pathol. 2008;173:1173-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 104. | Ribatti D. Tumor refractoriness to anti-VEGF therapy. Oncotarget. 2016;7:46668-46677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 105. | Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6624] [Cited by in RCA: 6349] [Article Influence: 226.8] [Reference Citation Analysis (1)] |
| 106. | Moschetta M, Mishima Y, Sahin I, Manier S, Glavey S, Vacca A, Roccaro AM, Ghobrial IM. Role of endothelial progenitor cells in cancer progression. Biochim Biophys Acta. 2014;1846:26-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 107. | Paprocka M, Kieda C, Kantor A, Bielawska-Pohl A, Dus D, Czekanski A, Heimrath J. Increased Endothelial Progenitor Cell Number in Early Stage of Endometrial Cancer. Int J Gynecol Cancer. 2017;27:947-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Yu M, Men HT, Niu ZM, Zhu YX, Tan BX, Li LH, Jiang J. Meta-Analysis of Circulating Endothelial Cells and Circulating Endothelial Progenitor Cells as Prognostic Factors in Lung Cancer. Asian Pac J Cancer Prev. 2015;16:6123-6128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 109. | Ha XQ, Zhao M, Li XY, Peng JH, Dong JZ, Deng ZY, Zhao HB, Zhao Y, Zhang YY. Distribution of endothelial progenitor cells in tissues from patients with gastric cancer. Oncol Lett. 2014;7:1695-1700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 110. | Pezzella F, Gatter KC. Evidence Showing That Tumors Can Grow Without Angiogenesis and Can Switch Between Angiogenic and Nonangiogenic Phenotypes. J Natl Cancer Inst. 2016;108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 111. | Lugassy C, Kleinman HK, Vermeulen PB, Barnhill RL. Angiotropism, pericytic mimicry and extravascular migratory metastasis: an embryogenesis-derived program of tumor spread. Angiogenesis. 2020;23:27-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 112. | Kuczynski EA, Reynolds AR. Vessel co-option and resistance to anti-angiogenic therapy. Angiogenesis. 2020;23:55-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 113. | Hong SA, Hwang HW, Kim MK, Lee TJ, Yim K, Won HS, Sun S, Kim EY, Ko YH. High Endothelial Venule with Concomitant High CD8+ Tumor-Infiltrating Lymphocytes Is Associated with a Favorable Prognosis in Resected Gastric Cancer. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 114. | Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, Rochaix P, Girard JP. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71:5678-5687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 386] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 115. | Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P, Garrido I, Girard JP. High endothelial venules (HEVs) in human melanoma lesions: Major gateways for tumor-infiltrating lymphocytes. Oncoimmunology. 2012;1:829-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 163] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 116. | Valdivia A, Mingo G, Aldana V, Pinto MP, Ramirez M, Retamal C, Gonzalez A, Nualart F, Corvalan AH, Owen GI. Fact or Fiction, It Is Time for a Verdict on Vasculogenic Mimicry? Front Oncol. 2019;9:680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 117. | Nowak-Sliwinska P, Alitalo K, Allen E, Anisimov A, Aplin AC, Auerbach R, Augustin HG, Bates DO, van Beijnum JR, Bender RHF, Bergers G, Bikfalvi A, Bischoff J, Böck BC, Brooks PC, Bussolino F, Cakir B, Carmeliet P, Castranova D, Cimpean AM, Cleaver O, Coukos G, Davis GE, De Palma M, Dimberg A, Dings RPM, Djonov V, Dudley AC, Dufton NP, Fendt SM, Ferrara N, Fruttiger M, Fukumura D, Ghesquière B, Gong Y, Griffin RJ, Harris AL, Hughes CCW, Hultgren NW, Iruela-Arispe ML, Irving M, Jain RK, Kalluri R, Kalucka J, Kerbel RS, Kitajewski J, Klaassen I, Kleinmann HK, Koolwijk P, Kuczynski E, Kwak BR, Marien K, Melero-Martin JM, Munn LL, Nicosia RF, Noel A, Nurro J, Olsson AK, Petrova TV, Pietras K, Pili R, Pollard JW, Post MJ, Quax PHA, Rabinovich GA, Raica M, Randi AM, Ribatti D, Ruegg C, Schlingemann RO, Schulte-Merker S, Smith LEH, Song JW, Stacker SA, Stalin J, Stratman AN, Van de Velde M, van Hinsbergh VWM, Vermeulen PB, Waltenberger J, Weinstein BM, Xin H, Yetkin-Arik B, Yla-Herttuala S, Yoder MC, Griffioen AW. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis. 2018;21:425-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 462] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 118. | Tauchi Y, Tanaka H, Kumamoto K, Tokumoto M, Sakimura C, Sakurai K, Kimura K, Toyokawa T, Amano R, Kubo N, Muguruma K, Yashiro M, Maeda K, Ohira M, Hirakawa K. Tumor-associated macrophages induce capillary morphogenesis of lymphatic endothelial cells derived from human gastric cancer. Cancer Sci. 2016;107:1101-1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 119. | Li M, Gu Y, Zhang Z, Zhang S, Zhang D, Saleem AF, Zhao X, Sun B. Vasculogenic mimicry: a new prognostic sign of gastric adenocarcinoma. Pathol Oncol Res. 2010;16:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 120. | Guo Q, Yuan Y, Jin Z, Xu T, Gao Y, Wei H, Li C, Hou W, Hua B. Association between Tumor Vasculogenic Mimicry and the Poor Prognosis of Gastric Cancer in China: An Updated Systematic Review and Meta-Analysis. Biomed Res Int. 2016;2016:2408645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 121. | Ren HY, Shen JX, Mao XM, Zhang XY, Zhou P, Li SY, Zheng ZW, Shen DY, Meng JR. Correlation Between Tumor Vasculogenic Mimicry and Poor Prognosis of Human Digestive Cancer Patients: A Systematic Review and Meta-Analysis. Pathol Oncol Res. 2019;25:849-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 122. | Seftor RE, Seftor EA, Koshikawa N, Meltzer PS, Gardner LM, Bilban M, Stetler-Stevenson WG, Quaranta V, Hendrix MJ. Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61:6322-6327. [PubMed] |
| 123. | Lv J, Sun B, Sun H, Zhang Y, Sun J, Zhao X, Gu Q, Dong X, Che N. Significance of Vasculogenic Mimicry Formation in Gastric Carcinoma. Oncol Res Treat. 2017;40:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 124. | Said AH, Raufman JP, Xie G. The role of matrix metalloproteinases in colorectal cancer. Cancers (Basel). 2014;6:366-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 125. | McDonald DM, Munn L, Jain RK. Vasculogenic mimicry: how convincing, how novel, and how significant? Am J Pathol. 2000;156:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 126. | Sood AK, Seftor EA, Fletcher MS, Gardner LM, Heidger PM, Buller RE, Seftor RE, Hendrix MJ. Molecular determinants of ovarian cancer plasticity. Am J Pathol. 2001;158:1279-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 127. | Less JR, Skalak TC, Sevick EM, Jain RK. Microvascular architecture in a mammary carcinoma: branching patterns and vessel dimensions. Cancer Res. 1991;51:265-273. [PubMed] |
| 128. | Mărgăritescu C, Simionescu C, Pirici D, Mogoantă L, Ciurea R, Stepan A. Immunohistochemical characterization of tumoral vessels in oral squamous cell carcinoma. Rom J Morphol Embryol. 2008;49:447-458. [PubMed] |
| 129. | Fukumura D, Duda DG, Munn LL, Jain RK. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation. 2010;17:206-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 302] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 130. | Nagy JA, Chang SH, Shih SC, Dvorak AM, Dvorak HF. Heterogeneity of the tumor vasculature. Semin Thromb Hemost. 2010;36:321-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 274] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 131. | Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 546] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 132. | Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 727] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 133. | Birau A, Ceausu RA, Cimpean AM, Gaje P, Raica M, Olariu T. Assessement of angiogenesis reveals blood vessel heterogeneity in lung carcinoma. Oncol Lett. 2012;4:1183-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 134. | Ribatti D, Nico B, Crivellato E, Vacca A. The structure of the vascular network of tumors. Cancer Lett. 2007;248:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 135. | Jiménez-Torres JA, Virumbrales-Muñoz M, Sung KE, Lee MH, Abel EJ, Beebe DJ. Patient-specific organotypic blood vessels as an in vitro model for anti-angiogenic drug response testing in renal cell carcinoma. EBioMedicine. 2019;42:408-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 136. | Nagy JA, Dvorak AM, Dvorak HF. Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb Perspect Med. 2012;2:a006544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 137. | Tekesin K, Emin Gunes M, Tural D, Akar E, Zirtiloglu A, Karaca M, Selcukbiricik F, Bayrak S, Ozet A. Clinicopathological characteristics, prognosis and survival outcome of gastric cancer in young patients: A large cohort retrospective study. J BUON. 2019;24:672-678. [PubMed] |
| 138. | Zhai Z, Zhu ZY, Zhang Y, Yin X, Han BL, Gao JL, Lou SH, Fang TY, Wang YM, Li CF, Yu XF, Ma Y, Xue YW. Prognostic significance of Borrmann type combined with vessel invasion status in advanced gastric cancer. World J Gastrointest Oncol. 2020;12:992-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 139. | De Franco L, Marrelli D, Voglino C, Vindigni C, Ferrara F, Di Mare G, Iudici L, Marini M, Roviello F. Prognostic Value of Perineural Invasion in Resected Gastric Cancer Patients According to Lauren Histotype. Pathol Oncol Res. 2018;24:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 140. | Gao S, Cao GH, Ding P, Zhao YY, Deng P, Hou B, Li K, Liu XF. Retrospective evaluation of lymphatic and blood vessel invasion and Borrmann types in advanced proximal gastric cancer. World J Gastrointest Oncol. 2019;11:642-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 141. | Du CY, Chen JG, Zhou Y, Zhao GF, Fu H, Zhou XK, Shi YQ. Impact of lymphatic and/or blood vessel invasion in stage II gastric cancer. World J Gastroenterol. 2012;18:3610-3616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 142. | Zhao LY, Chen XL, Wang YG, Xin Y, Zhang WH, Wang YS, Chen XZ, Yang K, Liu K, Xue L, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. A new predictive model combined of tumor size, lymph nodes count and lymphovascular invasion for survival prognosis in patients with lymph node-negative gastric cancer. Oncotarget. 2016;7:72300-72310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 143. | Bernabeu MO, Köry J, Grogan JA, Markelc B, Beardo A, d'Avezac M, Enjalbert R, Kaeppler J, Daly N, Hetherington J, Krüger T, Maini PK, Pitt-Francis JM, Muschel RJ, Alarcón T, Byrne HM. Abnormal morphology biases hematocrit distribution in tumor vasculature and contributes to heterogeneity in tissue oxygenation. Proc Natl Acad Sci U S A. 2020;117:27811-27819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 144. | Hughes VS, Wiggins JM, Siemann DW. Tumor oxygenation and cancer therapy-then and now. Br J Radiol. 2019;92:20170955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 145. | Liu M, Xie S, Zhou J. Use of animal models for the imaging and quantification of angiogenesis. Exp Anim. 2018;67:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 146. | Shimo T, Takigawa M. Cell Biological Assays for Measuring Angiogenic Activities of CCN Proteins. Methods Mol Biol. 2017;1489:239-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 147. | Macedo F, Ladeira K, Longatto-Filho A, Martins SF. Gastric Cancer and Angiogenesis: Is VEGF a Useful Biomarker to Assess Progression and Remission? J Gastric Cancer. 2017;17:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 148. | Wang TB, Deng MH, Qiu WS, Dong WG. Association of serum vascular endothelial growth factor-C and lymphatic vessel density with lymph node metastasis and prognosis of patients with gastric cancer. World J Gastroenterol. 2007;13:1794-7; discussion 1797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (12)] |
| 149. | Wang TB, Wang J, Wei XQ, Wei B, Dong WG. Serum vascular endothelial growth factor-C combined with multi-detector CT in the preoperative diagnosis of lymph node metastasis of gastric cancer. Asia Pac J Clin Oncol. 2012;8:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 150. | Zhao WX, Liu ZF, Li XL, Li Z. Correlations of serum homocysteine, VEGF and gastrin 17 with gastric cancer and precancerous lesions. Eur Rev Med Pharmacol Sci. 2019;23:4192-4198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 151. | Park DJ, Seo AN, Yoon C, Ku GY, Coit DG, Strong VE, Suh YS, Lee HS, Yang HK, Kim HH, Yoon SS. Serum VEGF-A and Tumor Vessel VEGFR-2 Levels Predict Survival in Caucasian but Not Asian Patients Undergoing Resection for Gastric Adenocarcinoma. Ann Surg Oncol. 2015;22 Suppl 3:S1508-S1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 152. | Tsirlis TD, Kostakis A, Papastratis G, Masselou K, Vlachos I, Papachristodoulou A, Nikiteas NI. Predictive significance of preoperative serum VEGF-C and VEGF-D, independently and combined with Ca19-9, for the presence of malignancy and lymph node metastasis in patients with gastric cancer. J Surg Oncol. 2010;102:699-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 153. | Cheng R, Yong H, Xia Y, Xie Q, Gao G, Zhou X. Chemotherapy regimen based on sorafenib combined with 5-FU on HIF-1α and VEGF expression and survival in advanced gastric cancer patients. Oncol Lett. 2017;13:2703-2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 154. | Han K, Claret L, Piao Y, Hegde P, Joshi A, Powell JR, Jin J, Bruno R. Simulations to Predict Clinical Trial Outcome of Bevacizumab Plus Chemotherapy vs. Chemotherapy Alone in Patients With First-Line Gastric Cancer and Elevated Plasma VEGF-A. CPT Pharmacometrics Syst Pharmacol. 2016;5:352-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 155. | Wei B, Tai Y, Tong H, Wen SL, Tang SH, Huan H, Huang ZY, Liu R, Tang YM, Yang JH, Tang CW, Gao JH. Correlations between VEGF-A expression and prognosis in patients with gastric adenocarcinoma. Int J Clin Exp Pathol. 2017;10:8461-8469. [PubMed] |
| 156. | Dai Y, Jiang J, Wang Y, Jin Z, Hu S. The correlation and clinical implication of VEGF-C expression in microvascular density and lymph node metastasis of gastric carcinoma. Am J Transl Res. 2016;8:5741-5747. [PubMed] |
| 157. | Li X, Zhu X, Wang Y, Wang R, Wang L, Zhu ML, Zheng L. Prognostic value and association of Lauren classification with VEGF and VEGFR-2 expression in gastric cancer. Oncol Lett. 2019;18:4891-4899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 158. | Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4014] [Cited by in RCA: 4088] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 159. | Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, Beliën JA, de Waal RM, Van Marck E, Magnani E, Weidner N, Harris AL, Dirix LY. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002;38:1564-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 347] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 160. | Rada M, Lazaris A, Kapelanski-Lamoureux A, Mayer TZ, Metrakos P. Tumor microenvironment conditions that favor vessel co-option in colorectal cancer liver metastases: A theoretical model. Semin Cancer Biol. 2021;71:52-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 161. | Marien KM, Croons V, Waumans Y, Sluydts E, De Schepper S, Andries L, Waelput W, Fransen E, Vermeulen PB, Kockx MM, De Meyer GR. Development and Validation of a Histological Method to Measure Microvessel Density in Whole-Slide Images of Cancer Tissue. PLoS One. 2016;11:e0161496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 162. | Pavlovic M, Gajovic N, Jurisevic M, Mitrovic S, Radosavljevic G, Pantic J, Arsenijevic N, Jovanovic I. Diverse Expression of IL-32 in Diffuse and Intestinal Types of Gastric Cancer. Gastroenterol Res Pract. 2018;2018:6578273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 163. | Oliver G, Kipnis J, Randolph GJ, Harvey NL. The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease. Cell. 2020;182:270-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 442] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 164. | Petrova TV, Koh GY. Organ-specific lymphatic vasculature: From development to pathophysiology. J Exp Med. 2018;215:35-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 165. | Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 606] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 166. | Wilczak W, Wittmer C, Clauditz T, Minner S, Steurer S, Büscheck F, Krech T, Lennartz M, Harms L, Leleu D, Ahrens M, Ingwerth S, Günther CT, Koop C, Simon R, Jacobsen F, Tsourlakis MC, Chirico V, Höflmayer D, Vettorazzi E, Haese A, Steuber T, Salomon G, Michl U, Budäus L, Tilki D, Thederan I, Fraune C, Göbel C, Henrich MC, Juhnke M, Möller K, Bawahab AA, Uhlig R, Adam M, Weidemann S, Beyer B, Huland H, Graefen M, Sauter G, Schlomm T. Marked Prognostic Impact of Minimal Lymphatic Tumor Spread in Prostate Cancer. Eur Urol. 2018;74:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 167. | Lund AW, Wagner M, Fankhauser M, Steinskog ES, Broggi MA, Spranger S, Gajewski TF, Alitalo K, Eikesdal HP, Wiig H, Swartz MA. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J Clin Invest. 2016;126:3389-3402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 168. | Song E, Mao T, Dong H, Boisserand LSB, Antila S, Bosenberg M, Alitalo K, Thomas JL, Iwasaki A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020;577:689-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 169. | Sasaki H, Morohashi S, Toba T, Seino H, Yoshizawa T, Hirai H, Haga T, Wu Y, Kijima H. Neoangiogenesis of gastric submucosa-invasive adenocarcinoma. Oncol Lett. 2018;16:3895-3900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 170. | Lu L, Wu M, Sun L, Li W, Fu W, Zhang X, Liu T. Clinicopathological and prognostic significance of cancer stem cell markers CD44 and CD133 in patients with gastric cancer: A comprehensive meta-analysis with 4729 patients involved. Medicine (Baltimore). 2016;95:e5163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 171. | Gresta LT, Rodrigues-Júnior IA, de Castro LP, Cassali GD, Cabral MM. Assessment of vascular invasion in gastric cancer: a comparative study. World J Gastroenterol. 2013;19:3761-3769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 172. | Woodham BL, Chmelo J, Donohoe CL, Madhavan A, Phillips AW. Prognostic Significance of Lymphatic, Venous and Perineural Invasion After Neoadjuvant Chemotherapy in Patients with Gastric Adenocarcinoma. Ann Surg Oncol. 2020;27:3296-3304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 173. | Tao Q, Zhu W, Zhao X, Li M, Shu Y, Wang D, Li X. Perineural Invasion and Postoperative Adjuvant Chemotherapy Efficacy in Patients With Gastric Cancer. Front Oncol. 2020;10:530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 174. | Bentolila LA, Prakash R, Mihic-Probst D, Wadehra M, Kleinman HK, Carmichael TS, Péault B, Barnhill RL, Lugassy C. Imaging of Angiotropism/Vascular Co-Option in a Murine Model of Brain Melanoma: Implications for Melanoma Progression along Extravascular Pathways. Sci Rep. 2016;6:23834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 175. | Prieto-Vila M, Yan T, Calle AS, Nair N, Hurley L, Kasai T, Kakuta H, Masuda J, Murakami H, Mizutani A, Seno M. iPSC-derived cancer stem cells provide a model of tumor vasculature. Am J Cancer Res. 2016;6:1906-1921. [PubMed] |
| 176. | Cojoc M, Mäbert K, Muders MH, Dubrovska A. A role for cancer stem cells in therapy resistance: cellular and molecular mechanisms. Semin Cancer Biol. 2015;31:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 290] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 177. | Lizárraga-Verdugo E, Avendaño-Félix M, Bermúdez M, Ramos-Payán R, Pérez-Plasencia C, Aguilar-Medina M. Cancer Stem Cells and Its Role in Angiogenesis and Vasculogenic Mimicry in Gastrointestinal Cancers. Front Oncol. 2020;10:413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 178. | Costa G, Harrington KI, Lovegrove HE, Page DJ, Chakravartula S, Bentley K, Herbert SP. Asymmetric division coordinates collective cell migration in angiogenesis. Nat Cell Biol. 2016;18:1292-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 179. | Hamm MJ, Kirchmaier BC, Herzog W. Sema3d controls collective endothelial cell migration by distinct mechanisms via Nrp1 and PlxnD1. J Cell Biol. 2016;215:415-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 180. | Williams SP, Gould CM, Nowell CJ, Karnezis T, Achen MG, Simpson KJ, Stacker SA. Systematic high-content genome-wide RNAi screens of endothelial cell migration and morphology. Sci Data. 2017;4:170009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 181. | Brassard-Jollive N, Monnot C, Muller L, Germain S. In vitro 3D Systems to Model Tumor Angiogenesis and Interactions With Stromal Cells. Front Cell Dev Biol. 2020;8:594903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 182. | Zhang L, Zheng F, Peng Z, Hu Z, Yang Z. A Feasible Method of Angiogenesis Assessment in Gastric Cancer Using 3D Microvessel Density. Stem Cells Int. 2018;2018:7813729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 183. | Angelucci A, Delle Monache S, Cortellini A, Di Padova M, Ficorella C. "Vessels in the Storm": Searching for Prognostic and Predictive Angiogenic Factors in Colorectal Cancer. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 184. | Kuczynski EA, Yin M, Bar-Zion A, Lee CR, Butz H, Man S, Daley F, Vermeulen PB, Yousef GM, Foster FS, Reynolds AR, Kerbel RS. Co-option of Liver Vessels and Not Sprouting Angiogenesis Drives Acquired Sorafenib Resistance in Hepatocellular Carcinoma. J Natl Cancer Inst. 2016;108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 185. | Sitohy B, Chang S, Sciuto TE, Masse E, Shen M, Kang PM, Jaminet SC, Benjamin LE, Bhatt RS, Dvorak AM, Nagy JA, Dvorak HF. Early Actions of Anti-Vascular Endothelial Growth Factor/Vascular Endothelial Growth Factor Receptor Drugs on Angiogenic Blood Vessels. Am J Pathol. 2017;187:2337-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 186. | Sitohy B, Nagy JA, Jaminet SC, Dvorak HF. Tumor-surrogate blood vessel subtypes exhibit differential susceptibility to anti-VEGF therapy. Cancer Res. 2011;71:7021-7028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 187. | Gee MS, Procopio WN, Makonnen S, Feldman MD, Yeilding NM, Lee WM. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am J Pathol. 2003;162:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 166] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 188. | Cascone T, Herynk MH, Xu L, Du Z, Kadara H, Nilsson MB, Oborn CJ, Park YY, Erez B, Jacoby JJ, Lee JS, Lin HY, Ciardiello F, Herbst RS, Langley RR, Heymach JV. Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor-resistant human lung adenocarcinoma. J Clin Invest. 2011;121:1313-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 189. | Senchukova M, Kiselevsky MV. The "cavitary" type of angiogenesis by gastric cancer. Morphological characteristics and prognostic value. J Cancer. 2014;5:311-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 190. | Senchukova MA, Nikitenko NV, Tomchuk ON, Zaitsev NV, Stadnikov AA. Different types of tumor vessels in breast cancer: morphology and clinical value. Springerplus. 2015;4:512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 191. | Senchukova MA, Makarova EV, Shurygina EI, Volchenko NN. Morphological Characteristics and Clinical Significance of Different Types of Tumor Vessels in Patients with Stages I-IIA of Squamous Cervical Cancer. J Oncol. 2020;2020:3818051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |