Published online Oct 21, 2021. doi: 10.3748/wjg.v27.i39.6673
Peer-review started: April 7, 2021
First decision: May 27, 2021
Revised: July 7, 2021
Accepted: September 2, 2021
Article in press: September 2, 2021
Published online: October 21, 2021
Processing time: 195 Days and 15.7 Hours
Uncontrolled growth and loss of control over basic metabolic functions, leading to invasive proliferation and metastases, are the salient traits of malignant tumors in general and colorectal cancer in particular. Invasion and metastases hinder effective tumor treatment. While surgical techniques and radiotherapy can be used to remove tumor focus, only chemotherapy can eliminate dispersed neoplastic cells. However, the efficacy of the latter method is limited in the advanced stages of the disease. Therefore, recognition of the mechanisms involved in neoplastic cell spreading is indispensable for developing effective therapies.
To use a number of biomarkers involved in cancer progression and identify a panel that could be used for effective early diagnosis.
We recruited 185 patients with colorectal adenocarcinoma (98 men, 87 women with median age 63). Thirty-five healthy controls were sex and age-matched. Dukes’ staging was as follows: A = 22, B = 52, C = 72, D = 39. We analyzed patients' blood serum before surgery. We determined: (1) Cathepsin B (CB) with Barrett's method (fluorogenic substrate); (2) Leukocytic elastase (LE) in a complex with alpha 1 trypsin inhibitor (AAT) using the immunoenzymatic MERCK test; (3) Total sialic acid (TSA) with the colorimetric periodate-resorcinol method; (4) Lipid-bound sialic acid (LASA) with the colorimetric Taut's method; and (5) The antitrypsin activity (ATA) employing the colorimetric test.
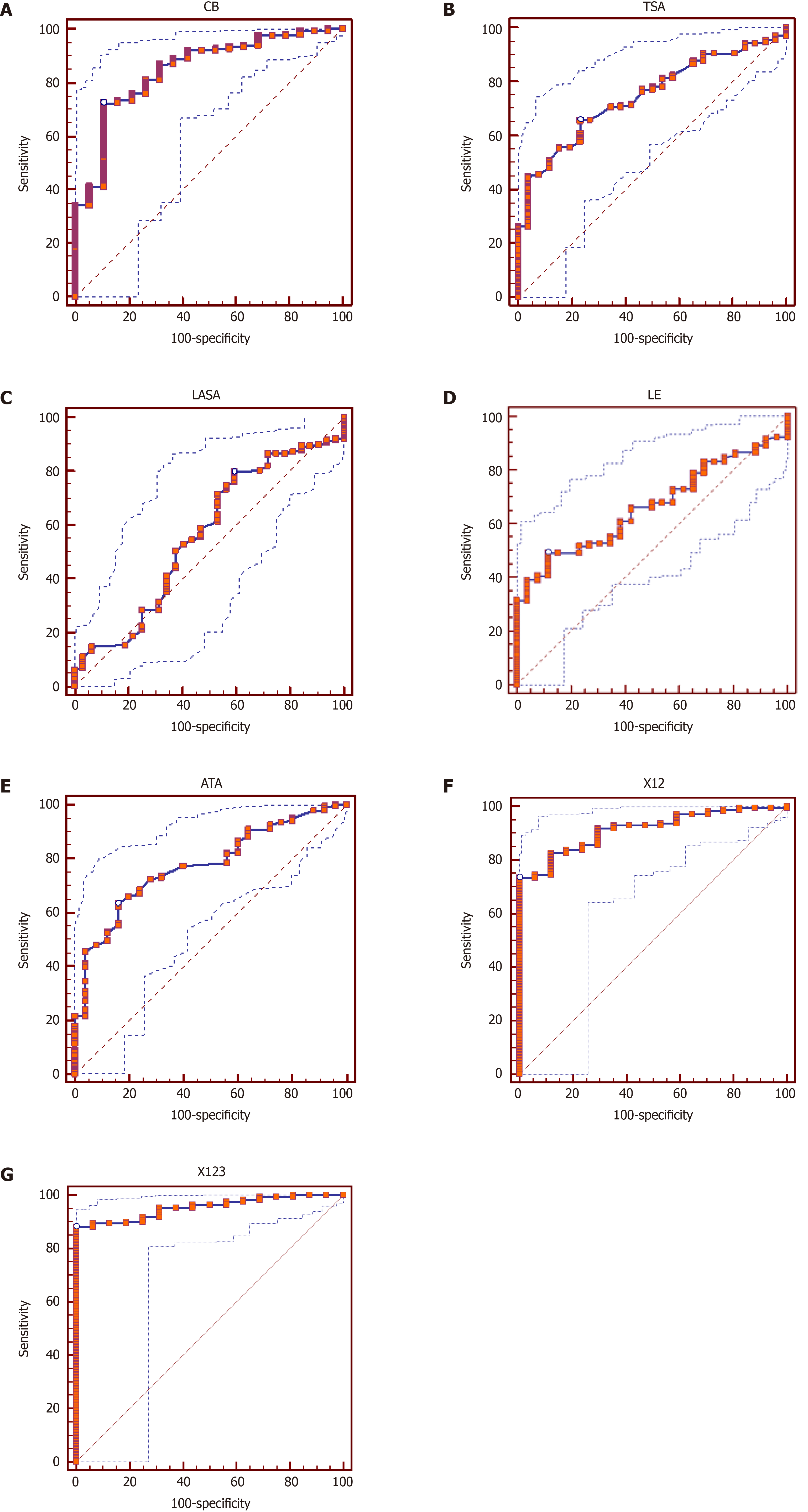
In patients, the values of the five biochemical parameters were as follows: CB = 16.1 ± 8.8 mU/L, LE = 875 ± 598 µg/L, TSA = 99 ± 31 mg%, LASA = 0.68 ± 0.33 mg%, and ATA = 3211 ± 1504 U/mL. Except for LASA, they were significantly greater than those of controls: CB = 11.4 ± 6.5 mU/L, LE = 379 ± 187 µg/L, TSA = 71.4 ± 15.1 mg%, LASA = 0.69 ± 0.28 mg%, and ATA = 2016 ± 690 U/mL. For CB and LASA, the differences between the four Dukes’ stages and controls were not statistically significant. The inter-stage differences for CB and LASA were also absent. The receiver operating characteristic (ROC) analysis revealed the potential diagnostic value of CB, TSA, and ATA. The area under ROC, sensitivity, and specificity for these three parameters were: 0.85, 72%, 90%; 0.75, 66%, 77%; and 0.77, 63%, 84%, respectively. The sensitivity and specificity for the three-parameter panel CB-TSA-ATA were equal to 88.2% and 100%, respectively.
The increased value of CB, TSA, and ATA parameters are associated with tumor biology, invasion, and metastasis of colorectal cancer. The presented evidence suggests the potential value of the CB-TSA-ATA biochemical marker panel in early diagnostics.
Core Tip: We searched for biomarkers applicable to the early detection of colorectal adenocarcinoma. Five parameters were determined in sera of patients and healthy individuals: Cathepsin B activity, total sialic acids concentration, lipid-associated sialic acids concentration, elastase concentration, and alpha 1 antitrypsin activity. We performed receiver operating characteristic analysis for single and multiple parameters. While the sensitivity and specificity were not very high for single parameters, the combined analysis of cathepsin B, alpha 1 antitrypsin, and total sialic acids concentration yielded 88% sensitivity and 100% specificity. We believe that this set of markers can be useful in clinical practice.
- Citation: Sebzda T, Gnus J, Dziadkowiec B, Latka M, Gburek J. Diagnostic usefulness of selected proteases and acute phase factors in patients with colorectal adenocarcinoma. World J Gastroenterol 2021; 27(39): 6673-6688
- URL: https://www.wjgnet.com/1007-9327/full/v27/i39/6673.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i39.6673
The process of neoplastic invasion consists of two main stages: Penetration of tissues surrounding cancer and creation of metastases in places distant from the original location. During migration, cancer-transformed cells encounter anatomical barriers: The basement membrane and connective tissue. Cathepsins play an important role in the process of overcoming them.
Under normal conditions, cathepsins do not occur extracellularly or appear outside the cell only in small quantities. Their main function is to participate in processes related to the "turn-over" of endogenous proteins and degradation of exogenous proteins absorbed in the process of endocytosis[1-4]. In smaller concentrations, e.g., in extralysosomal spaces, the enzymes can catalyze — by means of limited proteolysis — posttranslational processes of conversion of many peptides and proteins, including growth factors and hormones such as albumins, insulins, endorphins, and enkephalins[5,6]. The release of cathepsins from cancer cells and their expression in the plasma membrane of cancer cells facilitates the dissolution of the basement membrane and participation in the proteolytic metastatic cascade. In clinical practice, a significant increase in the activity of cathepsin B (CB) is observed in the serum of patients with malignant tumors. This phenomenon occurs regardless of the location of the tumor and is closely related to the severity of ovarian, cervical, breast, laryngeal and colorectal cancer. It was also found that the activation of CB, which takes place with the participation of elastase coming from the neutrophil intumescence and tumor tissue, has an impact on its invasiveness and metastatic capacity. Studies have also confirmed that an increase in cathepsin expression in colorectal tumor tissue homogenates may be a sensitive marker for cancer progression[7-16].
Leukocytic elastase (LE) belongs to the group of serine proteases. It is located mainly in the azurophilic granules of neutrophils, where it is an active component of the phagocytic system along with other hydrolyses and reactive oxygen species. The enzyme is also cytochemically detected in the nuclear membrane, the Golgi complex, endoplasmic reticulum, and mitochondria[17,18]. It also participates in remodeling and tissue repair processes and modulates the activity of cytokines and their receptors (e.g., mitogen-activated protein kinase 3). It can degrade elements of connective tissue by hydrolysis of elastin, various types of collagen, and other extracellular matrix proteins such as fibronectin, laminin, or proteoglycans. The physiological regulation of the activity and prevention of potentially destructive effects of elastase in pathological states is caused by protein inhibitors present in the blood serum. They include alfa 1 trypsin inhibitor (AAT), alpha 2-macroglobulin (alpha-2-MG), a secretory leukocytic protease inhibitor (SLPI), and elaphin[19-21]. In a healthy organism, LE remains in balance with them, and after the secretion from the cells, it is directly bound by inhibitors. The inhibitor molecules are thermally very stable and do not undergo proteolysis. Increased levels of LE in blood plasma, most often determined as LE-AAT complexes, have been found in many associated inflammatory conditions and are therefore considered acute-phase factors. Many authors regard the concentration of the above complexes to be a measure of the activity of the inflammatory process itself and be a marker of stimulation of neutrophils in the inflammatory focus. Lowering the level of AAT, caused by genetic or environmental factors, enables uncontrolled elastase activity and leads to many serious pathological conditions. This is caused by the enzyme-inhibitor imbalance and overexpression of the enzyme or a decrease in inhibitor concentration.
Sialic acid (N-acetyloneuraminic acid, NANA) is an organic compound from the sugar group, a derivative of neuraminic acid. It is a sugar component of glycoproteins and glycolipids. It occupies a terminal position in carbohydrate glycoprotein residues and has an important function in cell physiology as well as in the metabolism and maintenance of the proper concentration of glycoproteins in serum. It is also part of the ligand for the selectin and lectin receptors on leukocytes, T lymphocytes, platelets, and endothelium. It plays a key role in the immune response and hemostasis[22].
In the blood of patients suffering from metabolic disorders (e.g., diabetes mellitus, atherosclerosis), a clear increase in the glycoprotein fraction is observed, while the sialic acid content remains low. In the case of cancer, the level of glycoproteins that contain normal or increased amounts of sialic acid increases in the blood. Increased sialisation is associated with the development of a neoplastic tumor and malignant cell metastasis. An increase in sialic acid concentration is observed in the case of malignant melanoma, lung, larynx, breast, ovary, prostate, liver, or colorectal cancer. It has been shown that the sialyltransferase activity in blood serum taken from people with cancer is increased and reaches its highest concentration at advanced stages of cancer development. The main areas of protein glycosylation include the endoplasmic reticulum and Golgi apparatus[23]. During the transformation of normal cells into cancer cells, there are significant differences in the biosynthesis of the sugar parts of proteins and membrane lipids. These changes are primarily of a qualitative nature. The external part of the plasma membrane of cancer cells has an increased number of sialic acid molecules compared to normal cells. Glycoproteins rich in sialic acids are more frequently found in case of metastatic cancer. The increased level of sialoglycoproteins and sialoglycolipids in neoplasms is mainly due to increased disintegration of cancer cells, increased synthesis and secretion of glycoconjugates (glycoproteins and glycolipids) containing sialic acid[24-32].
Recently, many works have been published on the activity of proteolytic enzymes and their inhibitors in blood serum. However, they do not solve all the problems related to the diagnostic and prognostic usefulness of these parameters in neoplastic diseases.
In this study, a statistically consistent group of colorectal cancer patients was collected: Women and men. The division of colorectal cancer into the colon and rectal cancer, histopathological criteria (adenocarcinoma), a clinical division system based on Dukes’ cancer staging were considered.
The study presented below has both basic and diagnostic-clinical nature. The results of the study may be used in the future in the complementary diagnosis of colorectal adenocarcinoma to determine the severity of the disease and in further monitoring of patients under outpatient control and prognosis.
One hundred and eighty-five patients were recruited from the Lower Silesian Oncology Center and the Provincial Specialist Hospital in Wroclaw. The study material presented in this paper was blood serum from patients with colorectal adenocarcinoma. The patients' blood serum was examined before surgery.
The examined patients were evaluated in terms of their age, sex, location of neoplastic lesions (colon, sigmoid colon, rectum), histopathological differentiation of neoplastic cells (G), and their clinical stages were based on the Dukes’ classification. The characteristics of the examined patients are summarized in Table 1.
| Number of patients with colorectal cancer | 185 |
| Age, median (age range) | 63 (18-86) |
| Sex, n (%) | |
| Men | 98 (55) |
| Women | 87 (45) |
| Anatomical location, n (%) | |
| Colon | 77 (42) |
| Sigmoid colon | 37 (20) |
| Rectum | 71 (38) |
| Histological differentiation of cells, n (%) | |
| G1 | 8 (4) |
| G2 | 103 (56) |
| G3 | 74 (40) |
| Division of patients according to Dukes’ classification, n (%) | |
| A | 22 (12) |
| B | 52 (28) |
| C | 72 (39) |
| D | 39 (21) |
| Number of patients in the control group | 35 |
| Age, median (age range) | 61 (19-85) |
| Sex, n (%) | |
| Men | 19 (54) |
| Women | 16 (46) |
The following tests were performed in blood serum according to the methods given below: (1) CB was determined with the use of fluorogenic substrate using the Barrett method[33]; (2) LE in a complex with AAT was determined immunoenzymatically using the MERCK test; (3) Total sialic acid (TSA) was determined colorimetrically using the periodate-resorcinol method, according to Jourdian et al[34]; (4) Lipid-bound sialic acid (LASA) was determined colorimetrically using Tautu et al[35]; and (5) The antitrypsin activity (ATA) in blood plasma (in the study referred to as antitrypsin capacity — ATA) was determined colorimetrically against trypsin using the method proposed by Warwas et al[36] and Dietz et al[37].
The examined continuous features were characterized by the distribution parameters of these features, i.e., mean value (M), standard deviation (SD), and the number of patients (n). For the analysis of the statistical material, the following were used: For continuous features — single-factor analysis of variance (ANOVA), using Tukey's post hoc tests and multi-factor analysis of variance (MANOVA). The t-Student's t-test was also used for dependent samples. For features deviating from the normal distribution, which were also characterized by a median value, non-parametric tests were used: For independent samples — the Mann-Whitney U test, and for dependent samples — the Wilcoxon test. For categorized or dichotomous features, the χ2 test and the non-parametric Kruskal-Wallis test were used. The relationship between these features was also studied by determining the Spearman correlation coefficient. The receiver operating characteristic (ROC) curve method was used to determine the threshold values of clinical markers (continuous variables) with optimal precision. The significance threshold P for all statistical tests was set to 0.05. The statistical analysis was conducted using Statistica v. 10.
Table 2 presents the results of biochemical parameters examined, their M, SD, n, including the group of colorectal cancer patients and the control group of healthy individuals.
| No. | Biochemical parameters tested | Groups | ABCD | K | Significance level P value |
| 1 | CB, mU/L | M | 16.1 | 11.4 | < 0.050 |
| n | 185 | 35 | |||
| SD | 8.8 | 6.5 | |||
| 2 | LE, µg/L | M | 875.1 | 379.1 | < 0.001 |
| n | 51 | 30 | |||
| SD | 597.9 | 187.3 | |||
| 3 | TSA, mg% | M | 98.9 | 71.4 | < 0.001 |
| n | 71 | 31 | |||
| SD | 30.8 | 15.1 | |||
| 4 | LASA, mg% | M | 0.68 | 0.69 | NS |
| n | 68 | 35 | |||
| SD | 0.33 | 0.28 | |||
| 5 | ATA, U/mL | M | 3211.4 | 2015.9 | < 0.001 |
| n | 74 | 29 | |||
| SD | 1504.1 | 689.6 |
In the group of patients with colorectal cancer, differences were observed in the values of examined biochemical parameters in blood serum compared to the control group. Despite clear changes in the levels and activity of the studied factors, not in all groups, these differences were statistically significant. Relevance was observed between patient groups suffering from colorectal cancers and the control group for CB, LE, TSA, and ATA.
Table 3 presents statistically significant differences between the examined groups of patients according to Dukes’ classification and the control group. There were no significant differences for CB and LASA (P > 0.05), while the differences for other parameters were: LE (P < 0.001), TSA (P < 0.001), ATA (P < 0.01).
| No. | Biochemical parameters tested | Groups | A | B | C | D | K | Significance level P value |
| 1 | CB, mU/L | M | 19.9 | 15.4 | 16.8 | 15.5 | 11.4 | NS |
| n | 22 | 52 | 72 | 39 | 35 | |||
| SD | 19.3 | 9.4 | 8.4 | 7.6 | 6.5 | |||
| 2 | LE, µg/L | M | 386.1 | 929.9 | 602.1 | 1129.5 | 379.1 | < 0.001 |
| n | 5 | 16 | 15 | 15 | 30 | |||
| SD | 190.2 | 637.5 | 389.6 | 651.7 | 187.3 | |||
| 3 | TSA, mg% | M | 67.8 | 100.8 | 92.9 | 107.2 | 71.4 | < 0.001 |
| n | 6 | 21 | 24 | 20 | 31 | |||
| SD | 7.4 | 39.3 | 25.1 | 26.3 | 15.1 | |||
| 4 | LASA, mg% | M | 0.44 | 0.8 | 0.6 | 0.6 | 0.7 | NS |
| n | 6 | 21 | 24 | 21 | 37 | |||
| SD | 0.12 | 0.51 | 0.23 | 0.20 | 0.31 | |||
| 5 | ATA, U/mL | M | 3100.0 | 2925.0 | 3152.1 | 3576.2 | 2015.9 | < 0.010 |
| n | 2 | 21 | 24 | 21 | 29 | |||
| SD | 424.3 | 1294.5 | 1684.7 | 1548.8 | 689.6 |
Table 4 presents a comparison of statistically significant differences between individual parameters in the patient groups (A, B, C, D, according to Dukes’ classification) and control group. There were statistically significant differences for all groups except for group A. Statistically significant differences between patient groups were also being observed. For LE, the most statistically significant difference was observed between groups C and D (P < 0.002). For TSA, the difference was most strongly pronounced for A and D (P < 0.05). There were no stage differences for ATA.
| Patients’ Groups | |||||
| LE | A | B | C | D | K |
| A | NS | NS | NS | NS | NS |
| B | NS | NS | P < 0.05 | NS | P < 0.001 |
| C | NS | P < 0.05 | NS | P < 0.002 | P < 0.004 |
| D | NS | NS | P < 0.002 | NS | P < 0.001 |
| K | NS | P < 0.001 | P < 0.004 | NS | NS |
| TSA | A | B | C | D | K |
| A | NS | NS | NS | NS | NS |
| B | NS | NS | NS | NS | P < 0.001 |
| C | NS | NS | NS | NS | P < 0.003 |
| D | P < 0.05 | NS | NS | NS | P < 0.001 |
| K | NS | P < 0.001 | P < 0.003 | P < 0.001 | NS |
| ATA | A | B | C | D | K |
| A | NS | NS | NS | NS | NS |
| B | NS | NS | NS | NS | P < 0.02 |
| C | NS | NS | NS | NS | P < 0.002 |
| D | NS | NS | NS | NS | P < 0.001 |
| K | NS | P < 0.02 | P < 0.002 | P < 0.001 | NS |
In Table 5 the correlations between in main parameters are summarized. The values of all the above-mentioned correlation coefficients were positive except for CB and ATA and LASA and ATA. The highest correlations were observed between CB and ATA; LE and TSA; TSA and ATA (P < 0.001).
| Studied parameters | CB, mU/L | LE, μg/L | TSA, mg% | LASA, mg% | ATA, U/mL |
| CB, mU/L | -0.24 (187), P < 0.001 | ||||
| LE, μg/L | 0.37 (140), P < 0.001 | 0.17 (141), P < 0.05 | |||
| TSA, mg% | 0.37 (140), P < 0.001 | 0.19 (212), P < 0.01 | 0.33 (209), P < 0.001 | ||
| LASA, mg% | 0.17 (141), P < 0.05 | 0.19 (212), P < 0.006 | -0.14 (210), P < 0.05 | ||
| ATA, U/mL | -0.24 (187), P < 0.001 | 0.33 (209), P < 0.001 | -0.14 (210), P < 0.05 |
No statistically significant differences in the examined parameters in respect to histopathological differentiation of tumor cells (G1, G2, G3) were observed (data not shown).
The examined biochemical factors in the blood serum were also examined using ROC analysis. The analyses are summarized in Figure 1. The highest diagnostic value was observed for a single value for CB in blood serum, with a threshold value of 11.22 mU/L. The sensitivity of the method was 72%, and its specificity was 90%. Then, respectively: for TSA, with a threshold value of 75.34 mg% — 66% and 77%; for LASA with a threshold value of 0.562mg% — 80% and 41%; for LE with a threshold value of 543 µg/L — 49% and 89% and ATA in blood serum with a threshold value of 2400 U/mL — 63% and 84%.
The analysis of the ROC curves of the two associated parameters has shown that the connection between CB and TSA gives a sensitivity of 73% and a specificity of 100%, with a value under the curve reaching 95%.
Combining three parameters for the ROC curve: CB, TSA, and ATA, in blood serum, gives a sensitivity of 88% and a specificity of 100%, respectively, with a value of area under the curve of 95%.
In conclusion, ROC analysis has a high diagnostic value and can be helpful, especially in the combined analysis (biomarker panel determination).
The ability of the tumor to invade and metastasize is associated, among other pathogenic issues, with an increase in the expression of cysteine peptides. The source of these enzymes may be, in addition to the neoplastic tissue, neutrophils infiltrating the tumor. It is believed that CB determination may be helpful in the diagnosis and monitoring of colon and rectal cancer therapy[38-40].
In our study, the average CB activity determined in the blood serum of patients with colorectal cancers using a synthetic Z-Arg-Arg-N-MC substrate was about 1.5 times higher than that of healthy individuals (P < 0.05). Statistically significant results were obtained in the group of patients with different degrees of clinical advancement (ABCD: P < 0.05) (Table 2 and Figure 1A). On the other hand, CB did not statistically significantly differentiate patients according to Dukes’ staging of colorectal cancer (A, B, C, D), although the average values in patients exceeded the average values in the control group (Table 3). Among other parameters, CB correlated only with ATA (P < 0.001) (Table 5).
The activity of CB in serums of patients with colon and rectal cancers was also studied by other authors. Dufek et al[41] observed a fivefold increase in this parameter. It was found that the CB activity in patients with colon and rectal cancers was significantly higher than in patients with polyps and in healthy individuals. This difference may be due to the use of the other substrate (Z-Ala-Arg-Arg-N-MC), which may also have been hydrolyzed by other proteases. These studies have also shown a high level of alkali-stable form of CB. In people with mild lesions (polyps) and in healthy people, the level of this form did not reach the threshold of determination. After the effective treatment, CB levels decreased significantly and increased again in case of metastases or resistant chemotherapy.
Kos et al[40] also observed the fivefold increase in CB concentration using immune-enzymatic method. The CB level correlated well with stage C and D of Dukes’ staging system. As in our research, no correlation between CB and age or gender was observed. However, it should be stressed that the antibodies used in this method detected both the active and non-active precursor form of this enzyme and their complexes with inhibitors, such as cystatins.
Similar studies on the proteolytic activity of blood serum with colon and rectal cancer were performed by Amiguet et al[42]. Similar to our research, the proteolytic activities of CB and elastase, were determined. Patients in Dukes’ B and D stages were examined. The activities of the examined proteases were increased in relation to the control group of healthy individuals. In Dukes’ stage D, the increase in CB levels was directly proportional to the weight of the tumor. In metastatic carcinomas, the increase in CB was accompanied by an increase in AAT concentration.
Padilla et al[43] showed, with the use of the immunoreactive method, that CB levels in colorectal cancer patients were different from the control group.
Clinical and pathological evaluation of patients with the use of serum CB and cathepsin D, based on the TNM system before and after the operation, was performed by Skrzydlewska et al[44]. The CB activity before the cancer tumor resection was significantly higher. However, in relation to the control group, both before and after the procedure, the CB activity was approximately 8.4 times lower. The authors concluded that the postoperative level of CB was associated with the involvement of the surrounding lymph nodes and higher when not accompanied by lymph node involvement. However, a relatively non-specific Z-Arg-pNA substrate was used to test the CB activity, and thus the observations might have been biased by the activity of other proteases.
Zore et al[45] examined CB levels in the complex with cystatin C using the ELISA method. Their observations show that CB in the Dukes’ CD stage was significantly lower than in the AB stage (P = 0.02). Our study did not observe any significant differences between these groups (the results are not presented). The inhibitory capacity of cystatin C does not compensate for the increase in CB levels in patients suffering from colorectal cancers. This supports the hypothesis that inhibitory capacity might have been impaired during colorectal cancer progression.
Cysteine proteases — CB and cathepsin L levels in blood serum in patients with colorectal cancer were studied by Herszényi et al[46], who used the ELISA method for this purpose. CB correlated with the progressive Dukes’ scale, reaching a 2.3 times higher level in patients compared to the control group. Analysis of the ROC curve confirmed the diagnostic importance of the examined factors, including CB. The sensitivity and specificity in the ROC analysis of CB were similar to the results obtained in our study (72 and 89% respectively vs 82 and 88%, and the areas under the ROC curve 0.85 vs 0.87), thus confirming the high diagnostic value of the studied parameter. Comparing CB with other biochemical parameters such as TSA and ATA in the ROC analysis results in an even higher level of sensitivity and specificity. Also, other researchers noticed that proteolytic enzymes are excellent indicators for colorectal cancers, often better than the commonly used tumor markers[47].
The imbalance of protease/inhibitors ratio is particularly relevant for LE and AAT involvement in the pathogenesis of cancer. The level of AAT, an acute-phase protein, increases in cancer patients in response to the increased levels of proteolytic enzymes released from leukocytes into circulation. In patients, despite an increase in their levels, the functional activity of the inhibitor decreases, thus disturbing the LE/AAT balance. This imbalance is further exacerbated by the fact that membrane forms of proteases, such as CB or elastase are more resistant to these inhibitors. Moreover, LE has itself ability to degrade those inhibitors[48]. A disturbed balance between LE and AAT may be associated with an increased risk of liver, cholecystitis, bladder, lymphoma, or lung cancer[49-55]. Apart from AAT, other protein inhibitors present in the blood serum are also responsible for the physiological regulation of LE: α2MG, SLPI, or elaphin. SLPI, as it is clear from the work by Sugino et al[54], performed in various types of cancer, including colorectal cancer, has a dual effect. On the one hand, it suppresses the invasion of neoplastic cells, and on the other hand, it promotes metastasis that transmits through blood circulation.
The inclusion of serum ATA and LE, as indicated by the results of our research, may be a factor informing about the balance of serine proteases.
LE in all listed patient groups according to the Dukes’ classification: Combined ABCD stages and A, B, C, D stages separately (Table 2 and Figure 1D), shows a clear statistically significant difference when compared to the control group. Its activity reached the highest values in groups B and D. It is possible that it is associated with subsequent stages of cancer spread and the presence of metastatic foci rich in granulocytic intumescence, increased elastase-induced adhesion of cancer cells to the endothelium of vessels, or through mitogenic activity[56,57].
The different activity of serum elastase, in successive stages of the disease according to the Dukes’ classification eliminates the possibility of using this parameter for early diagnosis in colorectal cancers. In addition, serum elastase is characterized by relatively low sensitivity in the ROC analysis (49%) (Figure 1D). On the other hand, some authors have found it as a putative diagnostic biomarker and also a potential therapeutic target[58].
AAT is a blood plasma protein belonging to α1-globulin fraction, one of the strongest inhibitors of circulating serine proteases (serpins). It is also an acute-phase protein, synthesized mainly in the liver but also by macrophages. AAT has the ability to inactivate many proteolytic enzymes, but its most important effect is the inactivation of LE released by neutrophils as a result of an inflammatory reaction.
In our study, statistically significant (P < 0.001) elevated ATA levels were observed in the cumulative group of patients, regardless of the Dukes’ classification stage (ABCD) in relation to the control group. In the B, C, and D stages, in relation to the control, these levels were highly statistically significant (P < 0.001; P < 0.004; P < 0.001). Also, ATA differentiated very well patients with Dukes’ stages B against C and C against D (P < 0.05 and P < 0.002, respectively). In our ROC analysis, with the cut-off value > 2400 U/mL, the ATA value reached the sensitivity of 63% and specificity of 84%, respectively. The listed sensitivity and specificity parameters are lower than in the ROC analysis for CB (72% and 90%, respectively).
There is little clinical work on the contribution of AAT to the diagnosis or monitoring of the treatment of colorectal cancer[59-64]. Yüceyar et al[65] and Gallardo-Valverde et al[66] did not find relationship between the AAT and the severity of colorectal cancer and showed a statistically significant correlation between AAT and other biochemical factors such as acute-phase protein, carcinoembryonic antigen (CEA), or tumor-associated trypsin inhibitor (TATI).
Bernacka et al[67] studied plasma levels of AAT in patients with gastrointestinal cancers: Stomach and colorectal cancers of adenocarcinoma type. This marker did not differentiate colorectal cancer patients in terms of local and metastatic lesions. However, it had the highest level in stage C and differentiated patients in terms of the histological degree of tumor stage (G). The author postulates that AAT levels are associated with increased production by liver cells in response to the increased release of lysosomal proteases of tumor cells or from mononuclear inflammatory tumor-infiltrating cells. It seems that tumor cells may be the third source of antiproteases.
Ward et al[68], using proteomic profiling, identified AAT as having the potential to classify the colorectal patients with 95% sensitivity and 91% specificity.
Interesting conclusions were drawn from the work of Bujanda et al[64], who studied a group of 42 colorectal cancer patients using combined AAT, matrix metalloproteinase 7 (MMP-7), urokinase-type plasmin activator receptor (uPAR), and cyclooxygenase-2 (COX-2). Compared to the control group, AAT levels were about 1.4 times higher at stages B and C, and the AAT level was high and reached its value under the ROC curve (0.88). The above results are similar to those obtained in our study. The level under the ROC curve was 0.77. In patients in stages B and C, ATA levels were 1.5 and 1.6 times higher, respectively, than in the control group. AAT has a promising diagnostic profile and, most importantly, at the early stages of colorectal cancer.
The neoplastic process is associated not only with the activation of the cascade system of proteolytic enzymes, the activation of which mainly takes place with the participation of CB and LE, but also induces the activation of acute-phase proteins.
The interest in the sialic acids (TSA, LASA) as markers useful in diagnosing and monitoring the course of many diseases, including colorectal cancer, is reflected in the publications briefly reviewed in[69-72]. Increased levels of sialic acid expression are associated with changes in biosynthesis and posttranslational processes of acute-phase proteins glycosylation in the liver. This phenomenon is associated with increased expression of sialyltransferases by cancer cells[73]. The mechanism of increased TSA levels in serum takes the following into account: (1) Spontaneous release of the compounds from the surface of cancer cells; (2) Increase in concentration and/or glycosylation of serum glycoproteins; and (3) Secondary inflammatory reaction associated with the increase in acute-phase proteins[32,74]. Increased sialyltransferases activities, observed in cancer cells, results in increased glycoprotein secretion as well as the secretion of cell membrane components into the culture medium. The cancer cell hypoxia may also contribute to the above[75]. Increased sialisation of glycosphingolipids leads to abnormal adhesion and a disturbed premembranous signal exchange[75]. Determination of sialic acids is a laboratory marker of many pathological lesions. A significant increase in serum levels of sialic acids was observed in many malignant diseases[76]. Elevated levels of TSA or LASA were observed in malignant melanoma, lung, breast, ovary, and laryngeal cancers[28,32,77], as well as in colorectal cancer[26,31,70,78-82].
In our study, TSA in blood serum was elevated and statistically significantly different from the control group. The above observation concerns the cumulative group of patients (ABCD: P < 0.001) as well as individual groups (B, C, D in relation to the control: P < 0.001; P < 0.003; P < 0.001 respectively). The lowest level among patients was found in group A, and the highest in group D. The above clearly shows differences between particular patient groups. TSA was only less effective than ATA. The TSA level moderately but statistically significantly correlates with LE and ATA. The results of the ROC analysis are interesting. With a cut-off value for TSA > 75.34 mg%, the sensitivity of the method was 66%, and its specificity was 77%. In ROC analysis TSA clearly benefits when combined CB with the same cut-off values) sensitivity 74% and specificity 100%were obtained. The sensitivity further increases if we also take the ATA into account, which is also studied by the authors and reflects the AAT level. In the TSA test, the main component determined is the sialic acid associated with proteins. The level of sialic acids increases in the sera of cancer patients as a result of increased concentration of acute-phase proteins rather than gangliosides from decaying cancer cells[65,74,75,83].
We have found a statistically significant increase in the CB activity in the blood serum of the examined individuals suffering from colorectal cancers. The highest CB level was observed in Dukes’ stage A patients, and in stages B, C and D, it was lower and comparable to each other.
Concordantly, the following increased concentrations in blood serum of investigated markers were observed: LE, TSA, and ATA. According to Dukes’ classification, these values were statistically significantly increased with respect to the control group, gradually rising from the A to D stage.
The ROC analysis showed the high diagnostic value of CB, TSA, ATA determinations in blood serum, both in the single and combined analysis (biomarker panels) with two biochemical parameters: CB and TSA, and with three parameters: CB, TSA, and ATA. The above results suggest high diagnostic usefulness in determining these 2 or 3 combined parameters in relation to single determinations, obtaining sensitivity and specificity of 88.2% and 100% for three parameters.
Recognition of the mechanisms involved in neoplastic cell spreading is indispensable for the early diagnosis and detection of colorectal cancer.
Colorectal cancer is the third most common type of cancer, making up about 10% of all cases. In 2018, there were 1.09 million new cases and 551000 deaths from the disease. Consequently, early diagnosis of colorectal cancer remains a significant medical and economic problem.
Using several biomarkers involved in cancer progression, we have tried to identify a panel that could be used for effective early diagnosis.
Before surgery, we analyzed the blood serum of 185 patients with colorectal cancer and determined: Cathepsin B (CB), leukocytic elastase (LE), total sialic acid (TSA), lipid-bound sialic acid (LASA), and antitrypsin activity (ATA).
The receiver operating characteristic analysis revealed the potential diagnostic value of CB, TSA, and ATA. The sensitivity and specificity for the three-parameter panel CB-TSA-ATA were equal to 88.2% and 100%, respectively.
The increased value of CB, TSA, and ATA parameters are associated with tumor biology, invasion, and metastasis of colorectal cancer.
The presented evidence suggests the potential diagnostic and prognostic value of the CB-TSA-ATA biochemical marker panel.
We thank Dr. Filipowski H, PhD, for help with the statistical analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Batyrbekov K S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li JH
| 1. | Guinec N, Dalet-Fumeron V, Pagano M. "In vitro" study of basement membrane degradation by the cysteine proteinases, cathepsins B, B-like and L. Digestion of collagen IV, laminin, fibronectin, and release of gelatinase activities from basement membrane fibronectin. Biol Chem Hoppe Seyler. 1993;374:1135-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Korzus G, Turyna B. [Cysteine proteinases and their endogenous inhibitors]. Postepy Biochem. 1988;34:23-46. [PubMed] |
| 3. | Calkins CC, Sameni M, Koblinski J, Sloane BF, Moin K. Differential localization of cysteine protease inhibitors and a target cysteine protease, cathepsin B, by immuno-confocal microscopy. J Histochem Cytochem. 1998;46:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Olszewska D, Drewa T, Makarewicz R, Drewa J, Woźniak A, Maciak R. [Significance of cathepsin B and D in physiologic and pathologic processes]. Pol Merkur Lekarski. 2001;10:65-70. [PubMed] |
| 5. | Qian F, Chan SJ, Gong QM, Bajkowski AS, Steiner DF, Frankfater A. The expression of cathepsin B and other lysosomal proteinases in normal tissues and in tumors. Biomed Biochim Acta. 1991;50:531-540. [PubMed] |
| 6. | Howie AJ, Burnett D, Crocker J. The distribution of cathepsin B in human tissues. J Pathol. 1985;145:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Nomura T, Katunuma N. Involvement of cathepsins in the invasion, metastasis and proliferation of cancer cells. J Med Invest. 2005;52:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 169] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Talieri M, Papadopoulou S, Scorilas A, Xynopoulos D, Arnogianaki N, Plataniotis G, Yotis J, Agnanti N. Cathepsin B and cathepsin D expression in the progression of colorectal adenoma to carcinoma. Cancer Lett. 2004;205:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Sheahan K, Shuja S, Murnane MJ. Cysteine protease activities and tumor development in human colorectal carcinoma. Cancer Res. 1989;49:3809-3814. [PubMed] |
| 10. | Troy AM, Sheahan K, Mulcahy HE, Duffy MJ, Hyland JM, O'Donoghue DP. Expression of Cathepsin B and L antigen and activity is associated with early colorectal cancer progression. Eur J Cancer. 2004;40:1610-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Shuja S, Sheahan K, Murnane MJ. Cysteine endopeptidase activity levels in normal human tissues, colorectal adenomas and carcinomas. Int J Cancer. 1991;49:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Kruszewski WJ, Rzepko R, Wojtacki J, Skokowski J, Kopacz A, Jaśkiewicz K, Drucis K. Overexpression of cathepsin B correlates with angiogenesis in colon adenocarcinoma. Neoplasma. 2004;51:38-43. [PubMed] |
| 13. | Campo E, Muñoz J, Miquel R, Palacín A, Cardesa A, Sloane BF, Emmert-Buck MR. Cathepsin B expression in colorectal carcinomas correlates with tumor progression and shortened patient survival. Am J Pathol. 1994;145:301-309. [PubMed] |
| 14. | Berquin IM, Sloane BF. Cathepsin B expression in human tumors. Adv Exp Med Biol. 1996;389:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Hirai K, Yokoyama M, Asano G, Tanaka S. Expression of cathepsin B and cystatin C in human colorectal cancer. Hum Pathol. 1999;30:680-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Adenis A, Huet G, Zerimech F, Hecquet B, Balduyck M, Peyrat JP. Cathepsin B, L, and D activities in colorectal carcinomas: relationship with clinico-pathological parameters. Cancer Lett. 1995;96:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Travis J, Dubin A, Potempa J, Watorek W, Kurdowska A. Neutrophil proteinases. Caution signs in designing inhibitors against enzymes with possible multiple functions. Ann N Y Acad Sci. 1991;624:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Watorek W, Farley D, Salvesen G, Travis J. Neutrophil elastase and cathepsin G: structure, function, and biological control. Adv Exp Med Biol. 1988;240:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Imamura T, Maeda H. [Alpha1-proteinase inhibitor: structure and functions]. Nihon Rinsho. 2005;63 Suppl 4:81-87. [PubMed] |
| 20. | Okada Y, Watanabe S, Nakanishi I, Kishi J, Hayakawa T, Watorek W, Travis J, Nagase H. Inactivation of tissue inhibitor of metalloproteinases by neutrophil elastase and other serine proteinases. FEBS Lett. 1988;229:157-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 143] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Potempa J, Dubin A, Watorek W, Travis J. An elastase inhibitor from equine leukocyte cytosol belongs to the serpin superfamily. Further characterization and amino acid sequence of the reactive center. J Biol Chem. 1988;263:7364-7369. [PubMed] |
| 22. | Varki NM, Varki A. Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab Invest. 2007;87:851-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 405] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 23. | Sillanaukee P, Pönniö M, Jääskeläinen IP. Occurrence of sialic acids in healthy humans and different disorders. Eur J Clin Invest. 1999;29:413-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 170] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Akcay F, Taysi S, Uslu C, Doğru Y, Gümüştekin K. Levels of soluble intercellular adhesion molecule-1 and total sialic acid in serum of patients with laryngeal cancer. Jpn J Clin Oncol. 2001;31:584-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | de Albuquerque Garcia Redondo P, Nakamura CV, de Souza W, Morgado-Díaz JA. Differential expression of sialic acid and N-acetylgalactosamine residues on the cell surface of intestinal epithelial cells according to normal or metastatic potential. J Histochem Cytochem. 2004;52:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Feijoo-Carnero C, Rodríguez-Berrocal FJ, Páez de la Cadena M, Ayude D, de Carlos A, Martínez-Zorzano VS. Clinical significance of preoperative serum sialic acid levels in colorectal cancer: utility in the detection of patients at high risk of tumor recurrence. Int J Biol Markers. 2004;19:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Katopodis N, Hirshaut Y, Geller NL, Stock CC. Lipid-associated sialic acid test for the detection of human cancer. Cancer Res. 1982;42:5270-5275. [PubMed] |
| 28. | López Sáez JJ, Senra-Varela A. Evaluation of lipid-bound sialic acid (LSA) as a tumor marker. Int J Biol Markers. 1995;10:174-179. [PubMed] |
| 29. | Miyazaki K, Ohmori K, Izawa M, Koike T, Kumamoto K, Furukawa K, Ando T, Kiso M, Yamaji T, Hashimoto Y, Suzuki A, Yoshida A, Takeuchi M, Kannagi R. Loss of disialyl Lewis(a), the ligand for lymphocyte inhibitory receptor sialic acid-binding immunoglobulin-like lectin-7 (Siglec-7) associated with increased sialyl Lewis(a) expression on human colon cancers. Cancer Res. 2004;64:4498-4505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Shen Y, Tiralongo J, Kohla G, Schauer R. Regulation of sialic acid O-acetylation in human colon mucosa. Biol Chem. 2004;385:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Shen Y, Kohla G, Lrhorfi AL, Sipos B, Kalthoff H, Gerwig GJ, Kamerling JP, Schauer R, Tiralongo J. O-acetylation and de-O-acetylation of sialic acids in human colorectal carcinoma. Eur J Biochem. 2004;271:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Uslu C, Taysi S, Akcay F, Sutbeyaz MY, Bakan N. Serum free and bound sialic acid and alpha-1-acid glycoprotein in patients with laryngeal cancer. Ann Clin Lab Sci. 2003;33:156-159. [PubMed] |
| 33. | Barrett AJ. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem J. 1980;187:909-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 313] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Jourdian GW, Dean L, Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971;246:430-435. [PubMed] |
| 35. | Tautu C, Verazin G, Prorok JJ, Alhadeff JA. Improved procedure for determination of serum lipid-associated sialic acid: application for early diagnosis of colorectal cancer. J Natl Cancer Inst. 1988;80:1333-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Warwas M, Knapik-Kordecka M, Kowal-Gierczak B. Stężenie i aktywność alfa1-inhibitora proteaz oraz alfa-2 makroglobuliny w osoczu krwi chorych na cukrzycę typu II. Diagn Lab. 1991;44:552-556. |
| 37. | Dietz AA, Rubinstein HM, Hodges LK. Use of alpha-N-benzoyl-L-arginine-p-nitroanilide as trypsin substrate in estimation of alpha 1-antitrypsin. Clin Chem. 1976;22:1754-1755. [PubMed] |
| 38. | Herszényi L, Farinati F, Plebani M, Carraro P, Roveroni G, De Paoli M, Cardin R, Naccarato R, Tulassay Z. [Prognostic role of cisteine and serin proteases in gastriC cancer]. Orv Hetil. 1996;137:1637-1641. [PubMed] |
| 39. | Herszényi L, Farinati F, Plebani M, István G, Sápi Z, Carraro P, De Paoli M, Naccarato R, Tulassay Z. [The role of cathepsins and the plasminogen activator/inhibitor system in colorectal cancer]. Orv Hetil. 1999;140:1833-1836. [PubMed] |
| 40. | Kos J, Nielsen HJ, Krasovec M, Christensen IJ, Cimerman N, Stephens RW, Brünner N. Prognostic values of cathepsin B and carcinoembryonic antigen in sera of patients with colorectal cancer. Clin Cancer Res. 1998;4:1511-1516. [PubMed] |
| 41. | Dufek V, Jirásek V, Král V, Matous B, Drazná E. Changes in serum cathepsin B-like activity in patients with colorectal cancer. Neoplasma. 1985;32:51-54. [PubMed] |
| 42. | Amiguet JA, Jiménez J, Monreal JI, Hernández MJ, López-Vivanco G, Vidán JR, Conchillo F, Liso P. Serum proteolytic activities and antiproteases in human colorectal carcinoma. J Physiol Biochem. 1998;54:9-13. [PubMed] |
| 43. | Padilla D, Cubo T, Molina JM, García M, De la Osa G, Palomino T, Pardo R, Martín J, Arévalo E, Hernández Calvo J. [Prognostic significance and clinic utility of serum and immunohistochemical cathepsin B levels in colorectal cancer]. An Med Interna. 2003;20:521-525. [PubMed] |
| 44. | Skrzydlewska E, Sulkowska M, Wincewicz A, Koda M, Sulkowski S. Evaluation of serum cathepsin B and D in relation to clinicopathological staging of colorectal cancer. World J Gastroenterol. 2005;11:4225-4229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Zore I, Krasovec M, Cimerman N, Kuhelj R, Werle B, Nielsen HJ, Brünner N, Kos J. Cathepsin B/cystatin C complex levels in sera from patients with lung and colorectal cancer. Biol Chem. 2001;382:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Herszényi L, Plebani M, Carraro P, De Paoli M, Roveroni G, Cardin R, Foschia F, Tulassay Z, Naccarato R, Farinati F. Proteases in gastrointestinal neoplastic diseases. Clin Chim Acta. 2000;291:171-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Herszényi L, Farinati F, Cardin R, István G, Molnár LD, Hritz I, De Paoli M, Plebani M, Tulassay Z. Tumor marker utility and prognostic relevance of cathepsin B, cathepsin L, urokinase-type plasminogen activator, plasminogen activator inhibitor type-1, CEA and CA 19-9 in colorectal cancer. BMC Cancer. 2008;8:194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Geraghty P, Rogan MP, Greene CM, Boxio RM, Poiriert T, O'Mahony M, Belaaouaj A, O'Neill SJ, Taggart CC, McElvaney NG. Neutrophil elastase up-regulates cathepsin B and matrix metalloprotease-2 expression. J Immunol. 2007;178:5871-5878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Sun Z, Yang P. Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. Lancet Oncol. 2004;5:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 50. | Xu Y, Zhang J, Han J, Pan X, Cao Y, Guo H, Pan Y, An Y, Li X. Curcumin inhibits tumor proliferation induced by neutrophil elastase through the upregulation of α1-antitrypsin in lung cancer. Mol Oncol. 2012;6:405-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 51. | Sato T, Takahashi S, Mizumoto T, Harao M, Akizuki M, Takasugi M, Fukutomi T, Yamashita J. Neutrophil elastase and cancer. Surg Oncol. 2006;15:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 52. | Kataoka H, Nabeshima K, Komada N, Koono M. New human colorectal carcinoma cell lines that secrete proteinase inhibitors in vitro. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Keppler D, Markert M, Carnal B, Berdoz J, Bamat J, Sordat B. Human colon carcinoma cells synthesize and secrete alpha 1-proteinase inhibitor. Biol Chem Hoppe Seyler. 1996;377:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Sugino T, Yamaguchi T, Ogura G, Kusakabe T, Goodison S, Homma Y, Suzuki T. The secretory leukocyte protease inhibitor (SLPI) suppresses cancer cell invasion but promotes blood-borne metastasis via an invasion-independent pathway. J Pathol. 2007;212:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Korkmaz B, Attucci S, Jourdan ML, Juliano L, Gauthier F. Inhibition of neutrophil elastase by alpha1-protease inhibitor at the surface of human polymorphonuclear neutrophils. J Immunol. 2005;175:3329-3338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Meyer-Hoffert U, Wiedow O. Neutrophil serine proteases: mediators of innate immune responses. Curr Opin Hematol. 2011;18:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 57. | Nozawa F, Hirota M, Okabe A, Shibata M, Iwamura T, Haga Y, Ogawa M. Elastase activity enhances the adhesion of neutrophil and cancer cells to vascular endothelial cells. J Surg Res. 2000;94:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Ho AS, Chen CH, Cheng CC, Wang CC, Lin HC, Luo TY, Lien GS, Chang J. Neutrophil elastase as a diagnostic marker and therapeutic target in colorectal cancers. Oncotarget. 2014;5:473-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 59. | Kovcić V, Jelić S, Filipović I, Tomasević Z. [Serum haptoglobin and alpha-1 antitrysin levels as biological evolution markers in patients with gastric and colorectal cancer]. Srp Arh Celok Lek. 1994;122:311-313. [PubMed] |
| 60. | Millán Núñez-Cortés J. [Biological and clinical aspects of alpha 1-antitrypsin with special reference to malignant tumor processes]. Rev Esp Oncol. 1981;28:591-641 contd. [PubMed] |
| 61. | Simpson WG, Heys SD, Whiting PH, Eremin O, Broom J. Acute phase proteins and recombinant IL-2 therapy: prediction of response and survival in patients with colorectal cancer. Clin Exp Immunol. 1995;99:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Stamatiadis AP, St Toumanidou M, Vyssoulis GP, Manouras AJ, Apostolidis NS. Value of serum acute-phase reactant proteins and carcinoembryonic antigen in the preoperative staging of colorectal cancer. A multivariate analysis. Cancer. 1990;65:2055-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Jaberie H, Hosseini SV, Naghibalhossaini F. Evaluation of Alpha 1-Antitrypsin for the Early Diagnosis of Colorectal Cancer. Pathol Oncol Res. 2020;26:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Bujanda L, Sarasqueta C, Cosme A, Hijona E, Enríquez-Navascués JM, Placer C, Villarreal E, Herreros-Villanueva M, Giraldez MD, Gironella M, Balaguer F, Castells A. Evaluation of alpha 1-antitrypsin and the levels of mRNA expression of matrix metalloproteinase 7, urokinase type plasminogen activator receptor and COX-2 for the diagnosis of colorectal cancer. PLoS One. 2013;8:e51810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Yüceyar S, Ertürk S, Dirican A, Cengiz A, Saner H. The role of acute-phase reactant proteins, carcinoembryonic antigen and CA 19-9 as a marker in the preoperative staging of colorectal cancer: a prospective clinical study. Int Surg. 1996;81:136-139. [PubMed] |
| 66. | Gallardo-Valverde JM, Calañas-Continente A, Baena-Delgado E, Zurera-Tendero L, Vázquez-Martínez C, Membrives-Obrero A, Muntané J, Arévalo-Jiménez E. Obstruction in patients with colorectal cancer increases morbidity and mortality in association with altered nutritional status. Nutr Cancer. 2005;53:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Bernacka K, Kuryliszyn-Moskal A, Sierakowski S. The levels of alpha 1-antitrypsin and alpha 1-antichymotrypsin in the sera of patients with gastrointestinal cancers during diagnosis. Cancer. 1988;62:1188-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 68. | Ward DG, Suggett N, Cheng Y, Wei W, Johnson H, Billingham LJ, Ismail T, Wakelam MJ, Johnson PJ, Martin A. Identification of serum biomarkers for colon cancer by proteomic analysis. Br J Cancer. 2006;94:1898-1905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 69. | Kątnik-Prastowska I. Struktura i biologia kwasów sjalowych. Adv Clin Exp Med. 2003;5:653-663. |
| 70. | Painbeni T, Gamelin E, Cailleux A, Le Bouil A, Boisdron-Celle M, Daver A, Larra F, Allain P. Plasma sialic acid as a marker of the effect of the treatment on metastatic colorectal cancer. Eur J Cancer. 1997;33:2216-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 71. | Romppanen J, Eskelinen M, Tikanoja S, Mononen I. Total and lipid-bound serum sialic acid in benign and malignant breast disease. Anticancer Res. 1997;17:1249-1253. [PubMed] |
| 72. | Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14:351-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 830] [Cited by in RCA: 764] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 73. | Brockhausen I, Yang JM, Burchell J, Whitehouse C, Taylor-Papadimitriou J. Mechanisms underlying aberrant glycosylation of MUC1 mucin in breast cancer cells. Eur J Biochem. 1995;233:607-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 253] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 74. | Inagaki Y, Tang W, Guo Q, Kokudo N, Sugawara Y, Karako H, Konishi T, Nakata M, Nagawa H, Makuuchi M. Sialoglycoconjugate expression in primary colorectal cancer and metastatic lymph node tissues. Hepatogastroenterology. 2007;54:53-57. [PubMed] |
| 75. | Miyagi T, Wada T, Yamaguchi K. Roles of plasma membrane-associated sialidase NEU3 in human cancers. Biochim Biophys Acta. 2008;1780:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 76. | Schutter EM, Visser JJ, van Kamp GJ, Mensdorff-Pouilly S, van Dijk W, Hilgers J, Kenemans P. The utility of lipid-associated sialic acid (LASA or LSA) as a serum marker for malignancy. A review of the literature. Tumour Biol. 1992;13:121-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Cylwik B, Chrostek L, Szmitkowski M. [Diagnostic value of total and lipid-bound sialic acid in malignancies]. Pol Merkur Lekarski. 2005;19:237-241. [PubMed] |
| 78. | Feijoo C, Páez de la Cadena M, Rodríguez-Berrocal FJ, Martínez-Zorzano VS. Sialic acid levels in serum and tissue from colorectal cancer patients. Cancer Lett. 1997;112:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Sebzda T, Saleh Y, Gburek J, Warwas M, Andrzejak R, Siewinski M, Rudnicki J. Total and lipid-bound plasma sialic acid as diagnostic markers in colorectal cancer patients: correlation with cathepsin B expression in progression to Dukes stage. J Exp Ther Oncol. 2006;5:223-229. [PubMed] |
| 80. | İliklerden ÜH, Peksen C, Kalayci T, Kemik O. Evaluation of preoperative and postoperative total serum sialic acid levels in patients with colon cancer. Ann Ital Chir. 2020;91:649-647. [PubMed] |
| 81. | Zhang Z, Wuhrer M, Holst S. Serum sialylation changes in cancer. Glycoconj J. 2018;35:139-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 82. | Herszényi L, Barabás L, Hritz I, István G, Tulassay Z. Impact of proteolytic enzymes in colorectal cancer development and progression. World J Gastroenterol. 2014;20:13246-13257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Yin J, Hashimoto A, Izawa M, Miyazaki K, Chen GY, Takematsu H, Kozutsumi Y, Suzuki A, Furuhata K, Cheng FL, Lin CH, Sato C, Kitajima K, Kannagi R. Hypoxic culture induces expression of sialin, a sialic acid transporter, and cancer-associated gangliosides containing non-human sialic acid on human cancer cells. Cancer Res. 2006;66:2937-2945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |