Published online Sep 28, 2020. doi: 10.3748/wjg.v26.i36.5520
Peer-review started: June 11, 2020
First decision: June 18, 2020
Revised: June 29, 2020
Accepted: September 5, 2020
Article in press: September 5, 2020
Published online: September 28, 2020
Processing time: 105 Days and 16.5 Hours
SMARCB1/INI1-deficient pancreatic undifferentiated rhabdoid carcinoma is a very aggressive tumor that is rarely reported in the literature. The tumor has a predominant rhabdoid cell component and different patterns of growth have been reported.
A 59-year-old woman presented with diffuse abdominal pain, increasing in severity and accompanied by weight loss, nausea, and vomiting. Imaging showed a pancreatic head mass. Fine needle aspiration demonstrated atypical epithelioid cells with a pseudopapillary growth pattern suggestive of solid pseudopapillary neoplasm. The excised neoplasm showed monotonous epithelioid and focally spindle cells with pseudopapillary structures, rhabdoid features, and loss of SMARCB1 protein expression with wild-type KRAS, consistent with a SMARCB1/INI1-deficient undifferentiated rhabdoid carcinoma. The patient’s condition deteriorated rapidly following surgery and she expired 3 mo post operation.
In this article, we report the first case of SMARCB1/INI1-deficient undifferentiated pancreatic rhabdoid carcinoma mimicking solid pseudopapillary neoplasm.
Core Tip: The presence of a pseudopapillary architectural pattern in pancreatic tumors sampled via fine needle aspiration commonly leads to solid pseudopapillary neoplasm as the primary diagnostic consideration, particularly in middle-aged women; however, an inconclusive immunohistochemistry profile may suggest an alternative diagnosis. Our case highlights that while SMARCB1/INI1-deficient pancreatic undifferentiated carcinomas may mimic solid pseudopapillary neoplasm, recognition of this aggressive malignancy is important as these rare tumors impart a dismal prognosis.
- Citation: Hua Y, Soni P, Larsen D, Zreik R, Leng B, Rampisela D. SMARCB1/INI1-deficient pancreatic undifferentiated rhabdoid carcinoma mimicking solid pseudopapillary neoplasm: A case report and review of the literature. World J Gastroenterol 2020; 26(36): 5520-5526
- URL: https://www.wjgnet.com/1007-9327/full/v26/i36/5520.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i36.5520
Pancreatic undifferentiated carcinoma is a group of neoplasms comprised of pleomorphic giant cell carcinoma, sarcomatoid carcinoma, rhabdoid carcinoma, and round cell carcinomas. Pancreatic undifferentiated rhabdoid carcinoma, characterized by a predominant rhabdoid cell component, is a very rare tumor with high lethality. Patients often die within weeks or months of diagnosis. The first case of pancreatic rhabdoid carcinoma was reported in 1997[1]. A recent study by Agaimy et al[2] reported the phenotypic and molecular heterogeneity of pancreatic undifferentiated rhabdoid carcinoma. Two subtypes of the tumor were described based on the tumor’s KRAS status and SMARCB1 expression. The pleomorphic giant cell subtype displays KRAS alterations with preservation of SMARCB1 expression; in contrast, the anaplastic monomorphic subtype shows absence of KRAS alterations with loss of SMARCB1 expression. To our best knowledge, eight SMARCB1/INI1-deficient pancreatic undifferentiated rhabdoid carcinomas have been reported in the literature[2-6]. Our case is the first reported case with a solid and pseudopapillary growth pattern mimicking solid pseudopapillary neoplasm (SPN).
A 59-year-old woman presented to the clinic with diffuse abdominal pain.
The patient complained of diffuse abdominal pain with increased intensity for approximately 4 wk, accompanied by weight loss, nausea, and vomiting.
The patient had a past medical history of back pain, gastroesophageal reflux disease, allergic rhinitis and hepatitis A; and a past surgical history of appendectomy, cholecystectomy, Cesarean section, and ovarian cyst removal.
The abdomen was soft, nondistended, but tender to palpation in the right upper quadrant with no rebound or guarding. A well-healed scar was noted below the umbilicus midline, intersecting with a Cesarean section scar.
The patient had elevated laboratory values of alkaline phosphatase and aspartate aminotransferase.
The patient underwent a computed topography (CT) scan of abdomen and pelvis, and an abdominal magnetic resonance imaging (MRI) scan. CT scan revealed a hypodense region in the head of the pancreas, and the follow-up MRI revealed a 3.1 cm pancreatic head mass. Endoscopic ultrasound-guided fine needle aspiration (FNA) of the pancreatic mass was performed and a diagnosis of low-grade neoplasm with papillary features most suggestive of a SPN was rendered.
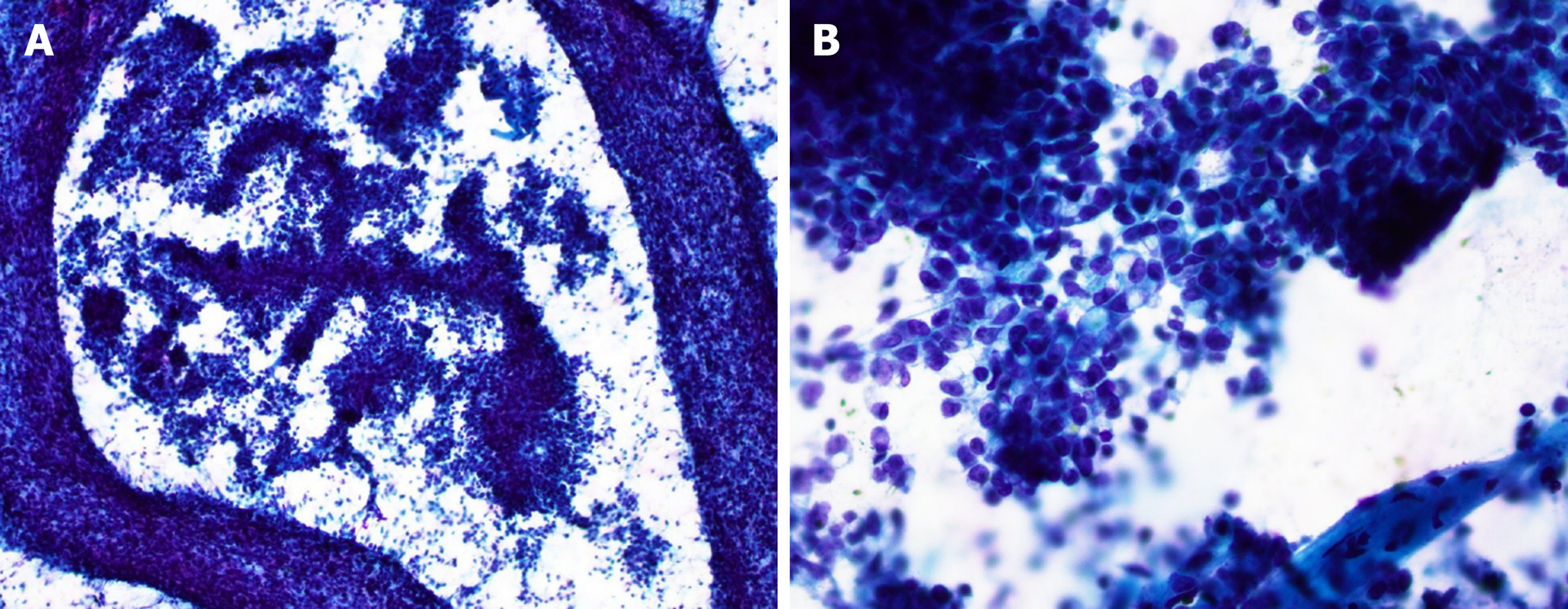
The FNA specimen was hypercellular with a prominent pseudopapillary growth pattern (Figure 1A). Abundant discohesive tumor cells were also present. The tumor cells were small to intermediate-sized, predominantly epithelioid with round to oval nuclei, inconspicuous to prominent nucleoli, and focal nuclear grooves. Some tumor cells exhibited eccentric nuclei, prominent nucleoli and large cytoplasmic vacuoles (Figure 1B). The tumor cells on the cell block section showed eosinophilic cytoplasm with no obvious rhabdoid features. Immunohistochemically, the tumor cells were diffusely positive for vimentin, very focally positive for synaptophysin and pankeratin (AE1/AE3), and negative for CD10, progesterone receptor, alpha 1-antitrypsin, CD56 and chromogranin. No nuclear beta-catenin stain was noted. Although the immunohistochemical profile was not conclusive, a diagnosis of a low grade neoplasm with papillary features most suggestive of SPN was made.
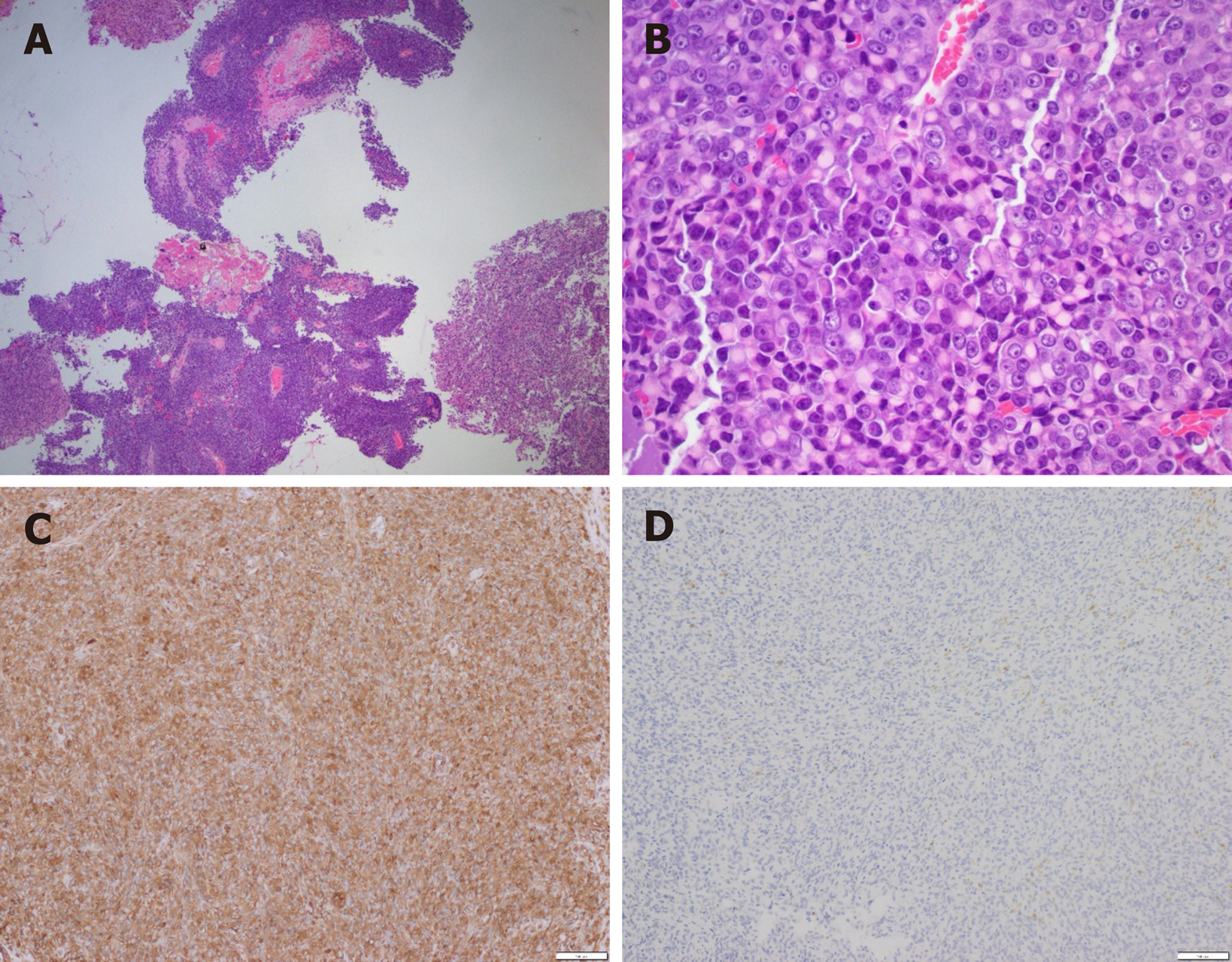
The enucleated pancreatic mass was received in two parts, measuring 3.4 cm × 2.4 cm × 1.1 cm and 2.0 cm × 1.0 cm × 0.4 cm. Histological sections showed a heter-ogeneous tumor with areas of solid and pseudopapillary growth and focal hemorrhage (Figure 2A). A subpopulation of the neoplastic cells was intermediate-sized, epithelioid and focally spindled with mild to moderate nuclear pleomorphism and indistinct to prominent nucleoli. Nuclear grooves were focally appreciated. However, a second subpopulation (approximately 60% of the neoplastic cells) showed rhabdoid features with eccentrically located nuclei, prominent nucleoli, abundant eosinophilic cytoplasm and eosinophilic cytoplasmic inclusions (Figure 2B). By immunohistochemistry, the neoplastic cells were diffusely positive for vimentin and focally positive for pankeratin (AE1/AE3), OSCAR keratin, CD34, Cyclin D1, synaptophysin and CD56. The tumor cells were negative for DOG1, CD117/KIT, desmin, STAT6, progesterone receptor, neuron-specific enolase, trypsin, chromogranin, CD99, CD10, SOX10, and S100. Beta-catenin immunostaining showed focal loss of membranous expression without nuclear expression. E-cadherin immunostaining showed patchy loss of membranous expression. The proliferation index Ki-67 demonstrated variable expression, ranging from less than 10% (non-rhabdoid cells) to over 50% (rhabdoid cells). PD-L1 highlighted less than 1% tumor cells, which was interpreted as negative for PD-L1 expression. SMARCB1/INI1 immunostaining showed complete loss of nuclear staining in all tumor cells. Next generation sequencing using the Ion AmpliSeqTM Cancer Hotspot Panel v2 confirmed loss of SMARCB1 gene with wild type KRAS gene.
The final diagnosis was SMARCB1/INI1-deficient pancreatic undifferentiated rhabdoid carcinoma.
The patient was treated with pancreatic mass enucleation approximately 4 mo after the initial presentation of abdominal pain.
The patient’s post operation course was complicated by small mesenteric artery (SMA) thrombosis, small bowel ischemia, perforated small bowel and intra-abdominal abscess. She underwent a small bowel resection, an open thrombectomy of the SMA and a placement of a drainage for the abdominal abscess. She was readmitted twice to the hospital following episodes of upper gastrointestinal bleeding due to multiple ulcers, which were treated with epinephrine injections and cauteries. Follow up CT of the abdomen and pelvis 1 mo and 2 mo post-pancreatic mass enucleation showed multiple intrahepatic cystic masses that increased in size, suspicious for malignancy. Subsequent core biopsy of the liver mass was consistent with a metastatic pancreatic undifferentiated rhabdoid carcinoma. The patient’s condition deteriorated rapidly and she expired 3 mo after pancreatic mass enucleation.
SMARCB1/INI1, ubiquitously expressed in the nuclei of all normal cells, is an important player in the SWI/SNF ATP-dependent chromatin-remodeling complex[7]. Cell biology studies have revealed a wide spectrum of signaling cascades regulated by SMARCB1, including p16-RB pathway, canonical WNT pathway, sonic hedgehog pathway, and polycomb pathway[8]. More importantly, aberrant expression of SMARCB1 has been found in a variety of tumors[7,9]. In particular, malignant rhabdoid tumor, epithelioid sarcoma, sinonasal carcinoma, renal medullary carcinoma, and pancreatic undifferentiated rhabdoid carcinoma can exhibit complete loss of SMARCB1 expression. SMARCB1 immunohistochemistry has been well established as a highly sensitive and specific tool for detecting malignant rhabdoid neoplasms[9].
SMARCB1/INI1-deficient pancreatic undifferentiated rhabdoid carcinoma is an aggressive and rare primary malignancy of the pancreas. According to the current World Health Organization classification of pancreatic tumors, undifferentiated pancreatic carcinoma is defined as a malignant epithelial neoplasm in which a substantial component of the neoplasm does not show a definitive line of differentiation[10]. The incidence of undifferentiated pancreatic carcinoma is only about 2% to 7% of all pancreatic cancers. A spectrum of histologic variants, including anaplastic rhabdoid carcinoma, sarcomatoid spindle cell carcinoma, carcinosarcoma with recognizable adenocarcinoma and high-grade spindle cell areas, as well as undifferentiated carcinoma with osteoclast-like giant cells, have been re-cognized[1-6,11-17]. Agaimy et al[2] pointed out that pancreatic undifferentiated rhabdoid carcinoma (undifferentiated carcinoma with rhabdoid features) showed heterogeneous morphology but could be separated into two subtypes: Pleomorphic giant cell subtype (positive for KRAS alterations and intact SMARCB1 expression) and anaplastic monomorphic cell subtype (loss of SMARCB1 expression and wild type KRAS expression). The prognosis of both subtypes of the undifferentiated rhabdoid carcinoma is poor (no reported cases with 5-year survival). In contrast, the undifferentiated carcinoma with osteoclastic giant cells appears to confer a more favorable prognosis with 5-year survival of approximately 50%[18,19].
To our best knowledge, eight SMARCB1/INI1-deficient pancreatic undifferentiated rhabdoid carcinomas have been reported in the literature (Table 1). Eight of the nine cases (including our case) had clinical data. The patients consisted of three males and five females, ranging from 35 to 76 years. Tumor sizes ranged from 1.9 cm to 10 cm. Five cases originated in the pancreatic head, two in the tail, and one in the body. Six cases had distant metastases and three were not specified. Six cases had follow up and all of the patients died of disease either post-operatively or within 2 wk to 7 mo following the initial diagnosis (mean: 3.6 mo, median: 3.5 mo). The histologic patterns of the tumors were heterogeneous with pseudopapillary acantholytic gland-like spaces, angiosarcoma-like, proximal-type epithelioid sarcoma-like, solid/diffuse, mucoid/myxoid stromal and solid patterns. Our case had solid and pseudopapillary features mimicking SPN. All nine cases had complete loss of SMARCB1 by immunohistochemistry. Six cases had wild-type KRAS, one was positive for KRAS mutation, and two cases were not tested. PD-L1 immunostaining were performed on two cases including our case, and both cases were negative for PD-L1 expression. Based on the limited number of the cases, the prognosis of SMARCB1/INI1-deficient pancreatic undifferentiated rhabdoid carcinoma is dismal.
| Ref. | Age/gender | Size/pancreatic location | Histology | Treatment | Metastasis | Outcome | KRAS mutation | SMARCB1 (IHC) |
| Agaimy et al[2] | 76/M | 5 cm/head | Rhabdoid | Surgery | NS | DOD in 1 mo | Wild type | Complete loss |
| Agaimy et al[2] | 44/F | 6 cm/head | Rhabdoid, angiosarcoma-like | Surgery | NS | NS | Positive (pGly12Asp) | Complete loss |
| Agaimy et al[2] | 72/M | 4 cm/head | Rhabdoid, pseudopapillary acantholytic gland-like spaces | Surgery | Liver, lymph nodes | DOD post-operatively | Wild type | Complete loss |
| Agaimy et al[2] | 61/M | 5 cm/tail | Rhabdoid, prominent neutrophils and focal glandular formation | Surgery | Intra-abdominal, stomach | NS | Wild type | Complete loss |
| Sano et al[3] | 68/F | 10 cm/body, tail | Rhabdoid | Palliative | Liver, kidneys, lungs, right adrenal gland, omentum, peritoneum | DOD in 2 wk | NS | Complete loss |
| Ohike et al[4] | 35/F | 6 cm/head | Rhabdoid, solid/diffuse | Chemo | Liver | DOD in 7 mo | Wild type | Complete loss |
| Tahara et al[5] | 67/F | 1.9 cm/body | Rhabdoid, solid/diffuse | Chemo | Liver, lung, bone, tongue, right thigh | DOD in half year | Wild type | Complete loss |
| Lehrke et al[6] | NS | NS | Rhabdoid | NS | NS | NS | NS | Complete loss |
| Our case | 59/F | 3.1 cm/head | Rhabdoid, solid pseudopapillary-like | Surgery | Liver | DOD in 7 mo | Wild type | Complete loss |
SPN is a rare low-grade malignant pancreatic neoplasm composed of poorly cohesive epithelial cells forming solid and pseudopapillary structures that lack of a specific line of pancreatic epithelial differentiation. The histology shows solid nests of poorly cohesive cells forming a cuff surrounding blood vessels, resulting in a pseudopapillary architecture. The stroma usually shows various degrees of hyalinization or evidence of degeneration such as hemorrhage, foamy macrophages, calcification and cholesterol clefts. The tumor cells usually have a moderate amount of eosinophilic cytoplasm with perinuclear vacuoles, relatively uniform nuclei with finely textured chromatin, inconspicuous nucleoli and longitudinal grooves, and often containing intracytoplasmic hyaline globules. High-grade malignant transformation of SPN with an aggressive clinical course has been reported[20-23]. The morphological features predicting high-grade transformation include diffuse growth pattern, extensive tumor necrosis, significant nuclear atypia, high mitotic rate and presence of an undifferentiated component[20], while typical histologic architecture and cytological features of a SPN are always present. The tumor cells of SPN express vimentin, CD56, alpha 1-antichymotrypsin, alpha 1-antitrypsin, cyclin D1, CD10 and progesterone receptor. The tumor cells may also show focal immunoreactivity for synaptophysin and cytokeratins. The tumor always shows beta-catenin mutation with nuclear and cytoplasmic staining for beta-catenin immunostaining, including areas of high-grade transformation[20,24]. On the other hand, majority of the pancreatic undifferentiated carcinoma shows only diffuse positivity for vimentin and focal for cytokeratin[10] with no nuclear immunoreactivity for beta-catenin. Although our case showed solid/pseudopapillary architecture and cytological features mimicking SPN, especially on the FNA specimen, the immunostaining pattern and molecular findings on the enucleation specimen did not support the diagnosis of SPN. The findings were consistent with a SMARCB1/INI1-deficient undifferentiated rhabdoid carcinoma.
In conclusion, here we report a rare case of SMARCB1/INI1-deficient pancreatic undifferentiated rhabdoid carcinoma with cytological and architectural features mimicking SPN. Recognition of this entity is very important due to the poor prognosis of undifferentiated rhabdoid carcinoma compared to SPN, which has much better prognosis. More studies are warranted for the better understanding of pancreatic undifferentiated rhabdoid carcinoma, particularly the SMARCB1/INI1-deficient subtype.
We thank Dr. Rondell Graham at Mayo Clinic for his expertise.
Manuscript source: Unsolicited manuscript
Specialty type: Pathology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carloni R S-Editor: Wang JL L-Editor: A P-Editor: Zhang YL
| 1. | Nishihara K, Katsumoto F, Kurokawa Y, Toyoshima S, Takeda S, Abe R. Anaplastic carcinoma showing rhabdoid features combined with mucinous cystadenocarcinoma of the pancreas. Arch Pathol Lab Med. 1997;121:1104-1107. [PubMed] |
| 2. | Agaimy A, Haller F, Frohnauer J, Schaefer IM, Ströbel P, Hartmann A, Stoehr R, Klöppel G. Pancreatic undifferentiated rhabdoid carcinoma: KRAS alterations and SMARCB1 expression status define two subtypes. Mod Pathol. 2015;28:248-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 3. | Sano M, Homma T, Hayashi E, Noda H, Amano Y, Tsujimura R, Yamada T, Quattrochi B, Nemoto N. Clinicopathological characteristics of anaplastic carcinoma of the pancreas with rhabdoid features. Virchows Arch. 2014;465:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Ohike N, Suzuki R, Isobe T, Norose T, Shiokawa A, Kunimura T, Kohashi K, Oda Y. A case of pancreatic undifferentiated carcinoma mimicking proximal-type epithelioid sarcoma. J Pancreas. 2016;17:75-80. |
| 5. | Tahara S, Takeyari M, Morii E. Usefulness of cytological analysis in the diagnosis of pancreatic undifferentiated rhabdoid carcinoma. Pathol Int. 2018;68:56-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Lehrke HD, Graham RP, McWilliams RR, Lam-Himlin DM, Smyrk TC, Jenkins S, Dong H, Zhang L. Undifferentiated Pancreatic Carcinomas Display Enrichment for Frequency and Extent of PD-L1 Expression by Tumor Cells. Am J Clin Pathol. 2017;148:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Hollmann TJ, Hornick JL. INI1-deficient tumors: diagnostic features and molecular genetics. Am J Surg Pathol. 2011;35:e47-e63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 307] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 8. | Kohashi K, Oda Y. Oncogenic roles of SMARCB1/INI1 and its deficient tumors. Cancer Sci. 2017;108:547-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 9. | Agaimy A. The expanding family of SMARCB1(INI1)-deficient neoplasia: implications of phenotypic, biological, and molecular heterogeneity. Adv Anat Pathol. 2014;21:394-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 10. | Hruban RH, Adsay NV, Esposito I. Pancreatic ductal adenocarcinoma. World Health Organization Classification of Tumor. Digestive System Tumours. 5th Edition. Lyon: IARC Press, 2019; 322-332. |
| 11. | Kuroda N, Sawada T, Miyazaki E, Hayashi Y, Toi M, Naruse K, Fukui T, Nakayama H, Hiroi M, Taguchi H, Enzan H. Anaplastic carcinoma of the pancreas with rhabdoid features. Pathol Int. 2000;50:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Chadha MK, LeVea C, Javle M, Kuvshinoff B, Vijaykumar R, Iyer R. Anaplastic pancreatic carcinoma. A case report and review of literature. JOP. 2004;5:512-515. [PubMed] |
| 13. | Cho YM, Choi J, Lee OJ, Lee HI, Han DJ, Ro JY. SMARCB1/INI1 missense mutation in mucinous carcinoma with rhabdoid features. Pathol Int. 2006;56:702-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Jamali M, Serra S, Chetty R. Adenosquamous carcinoma of the pancreas with clear cell and rhabdoid components. A case report. JOP. 2007;8:330-334. [PubMed] |
| 15. | Kuroda N, Iwamura S, Fujishima N, Ohara M, Hirouchi T, Mizuno K, Hayashi Y, Lee GH. Anaplastic carcinoma of the pancreas with rhabdoid features and hyaline globule-like structures. Med Mol Morphol. 2007;40:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Al-Nafussi A, O'Donnell M. Poorly differentiated adenocarcinoma with extensive rhabdoid differentiation: clinicopathological features of two cases arising in the gastrointestinal tract. Pathol Int. 1999;49:160-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Layfield LJ, Bentz J. Giant-cell containing neoplasms of the pancreas: an aspiration cytology study. Diagn Cytopathol. 2008;36:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Paniccia A, Hosokawa PW, Schulick RD, Henderson W, Kaplan J, Gajdos C. A matched-cohort analysis of 192 pancreatic anaplastic carcinomas and 960 pancreatic adenocarcinomas: A 13-year North American experience using the National Cancer Data Base (NCDB). Surgery. 2016;160:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Muraki T, Reid MD, Basturk O, Jang KT, Bedolla G, Bagci P, Mittal P, Memis B, Katabi N, Bandyopadhyay S, Sarmiento JM, Krasinskas A, Klimstra DS, Adsay V. Undifferentiated Carcinoma With Osteoclastic Giant Cells of the Pancreas: Clinicopathologic Analysis of 38 Cases Highlights a More Protracted Clinical Course Than Currently Appreciated. Am J Surg Pathol. 2016;40:1203-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol. 2005;29:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 21. | Matsuo H, Ikoma H, Morimura R, Arita T, Kosuga T, Konishi H, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Nakanishi M, Ichikawa D, Fujiwara H, Okamoto K, Otsuji E. [A Case Report of an Undifferentiated Pancreatic Carcinoma Diagnosed as a Solid Pseudopapillary Neoplasm before Surgery]. Gan To Kagaku Ryoho. 2016;43:2350-2352. [PubMed] |
| 22. | Kim JH, Lee JM. Clinicopathologic Review of 31 Cases of Solid Pseudopapillary Pancreatic Tumors: Can We Use the Scoring System of Microscopic Features for Suggesting Clinically Malignant Potential? Am Surg. 2016;82:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Tomioka K, Ohike N, Aoki T, Enami Y, Fujimori A, Koizumi T, Kusano T, Nogaki K, Tashiro Y, Wada Y, Hakozaki T, Shibata H, Hirai T, Yamazaki T, Fujimasa K, Norose T, Isobe T, Murakami M. Solid Pseudopapillary Neoplasm of the Pancreas with High-Grade Malignant Transformation Involving p16-RB Pathway Alterations. Case Rep Surg. 2020;2020:5980382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Tanaka Y, Kato K, Notohara K, Hojo H, Ijiri R, Miyake T, Nagahara N, Sasaki F, Kitagawa N, Nakatani Y, Kobayashi Y. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001;61:8401-8404. [PubMed] |