Published online Sep 28, 2020. doi: 10.3748/wjg.v26.i36.5463
Peer-review started: June 9, 2020
First decision: July 29, 2020
Revised: July 30, 2020
Accepted: August 29, 2020
Article in press: August 29, 2020
Published online: September 28, 2020
Processing time: 106 Days and 20.6 Hours
There are few reports on major gastrointestinal (GI) bleeding among patients receiving an antithrombotic.
To describe clinical characteristics, bleeding locations, management and in-hospital mortality related to these events.
Over a three-year period, we prospectively identified 1080 consecutive adult patients admitted in two tertiary care hospitals between January 1, 2013 and December 31, 2015 for major GI bleeding while receiving an antithrombotic. The bleeding events were medically validated. Clinical characteristics, causative lesions, management and fatalities were described. The distribution of antithrombotics prescribed was compared across the bleeding lesions identified.
Of 576 patients had symptoms of upper GI bleeding and 504 symptoms of lower GI bleeding. No cause was identified for 383 (35.5%) patients. Gastro-duodenal ulcer was the first causative lesion in the upper tract (209 out of 408) and colonic diverticulum the first causative lesion in the lower tract (120 out of 289). There was a larger proportion of direct oral anticoagulant use among patients with lower GI than among those with upper GI lesion locations (P = 0.03). There was an independent association between gastro-duodenal ulcer and antithrombotic use (P = 0.03), taking account of confounders and proton pump inhibitor co-prescription. Pair wise comparisons pointed to a difference between vitamin K antagonist, direct oral anticoagulants, and antiplatelet agents in monotherapy vs dual antiplatelet agents.
We showed a higher rate of bleeding lesion identification and suggested a different pattern of antithrombotic exposure between upper and lower GI lesion locations and between gastro-duodenal ulcer and other identified upper GI causes of bleeding. Management was similar across antithrombotics and in-hospital mortality was low (5.95%).
Core tip: A large population requires long-term treatment with antithrombotics and gastrointestinal (GI) bleeding is the commonest bleeding manifestation. However, there are few reports on major GI bleeding among patients receiving an antithrombotic. We prospectively identified 1080 adult patients consecutively referred for major GI bleeding to emergency departments in two tertiary care hospitals between January 2013 and December 2015 while receiving an antithrombotic. Based on these data, we described clinical characteristics, bleeding locations, management and in-hospital mortality related to these events.
- Citation: Bouget J, Viglino D, Yvetot Q, Oger E. Major gastrointestinal bleeding and antithrombotics: Characteristics and management. World J Gastroenterol 2020; 26(36): 5463-5473
- URL: https://www.wjgnet.com/1007-9327/full/v26/i36/5463.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i36.5463
The prevalence of vascular diseases is increasing, resulting in a large proportion of patients requiring long-term treatment with antithrombotics-antiplatelet agents or anticoagulants-particularly among the elderly. Consequently, the risk of hemorrhage related to antithrombotic use will increase, including gastrointestinal (GI) bleeding, which is the commonest manifestation[1,2].
There are few reports on the clinical and pathological characteristics of major GI bleeding in a large population, and reports are often limited to oral anticoagulants (vitamin K antagonists and direct oral anticoagulants) or antiplatelet agents[3,4], only exceptionally including parenteral anticoagulants[5]. Information on the location of the causative bleeding lesion, on management, and on resource consumption for patients with GI bleeding and their associations with different antithrombotics is scarce, and we thought the issue was relevant and of clinical importance. Differences in GI bleeding locations according to the presence of antiplatelet agents (AP) drugs, vitamin K antagonists (VKA) or direct oral anticoagulants (DOAC), and the relative distribution between upper and lower GI bleeding locations have been reported[6-10]. Varying methodologies, retrospective or prospective designs, different definitions of GI bleeding and patient selection according to antithrombotic indication could explain these conflicting results[6-10]. In addition, little is known about the severity of GI bleeding, the causative lesions or fatalities among patients admitted to emergency department for acute major GI bleeding while receiving an antithrombotic.
Our primary objective was to describe the clinical characteristics, bleeding locations, management and fatalities related to upper and lower major GI bleeding events among patients receiving an antithrombotic, whatever the indication. Our second objective was to compare the distribution of antithrombotics between patients with upper and lower bleeding lesions, and between patients with gastro-duodenal ulcer and patients with other identified causes of upper GI bleeding.
The SACHA study is a French prospective population-based cohort on the incidence and outcome of major bleeding among patients treated with antithrombotics (parenteral or oral anticoagulant, or antiplatelet agent). The detailed methods have already been published[11].
For the current analysis, we studied all consecutive adult patients admitted in two tertiary care hospitals between January 1, 2013 and December 31, 2015 for major GI bleeding. Briefly, patients were first identified at emergency admission from computerised requests on electronic health records on the basis of several GI haemorrhage diagnostic codes (Supplementary Table 1, ICD-10 code list), and on the basis of specific emergency therapies suggesting the patient might have been prescribed an antithrombotic. In each emergency department, the referent medical doctor validated the final inclusion of all screened records for major bleeding. Major bleeding was defined from at least one of the following criteria[12]: Unstable hemodynamic (systolic arterial pressure < 90 mmHg or mean arterial pressure < 65 mmHg) or haemorrhagic shock, uncontrollable bleeding, need for transfusion or haemostatic procedure (endoscopic procedure, embolization, surgery). Of note, we excluded (1) patients who had major GI bleeding during hospitalization whereas they were referred to emergency for another reason; and (2) patients referred for intentional overdoses of antithrombotics.
| Variable | Total (n = 1080) | Upper GI bleeding (n = 576) | Lower GI bleeding (n = 504) | P value |
| Female | 454 | 40.6 (234) | 43.7 (220) | 0.3149 |
| Age (yr) | 78.5 ± 11.7 | 80.6 ± 11.0 | 0.0028 | |
| Arterial hypertension | 735 | 66.7 (384) | 69.6 (351) | 0.2953 |
| CAD | 439 | 41.5 (239) | 39.7 (200) | 0.5456 |
| Heart failure | 166 | 16.8 (97) | 13.7 (69) | 0.1522 |
| Diabetes mellitus | 302 | 29.7 (171) | 26 (131) | 0.1770 |
| Cancer | 231 | 22.2 (128) | 20.4 (103) | 0.4752 |
| PVD | 190 | 20 (115) | 14.9 (75) | 0.0286 |
| Chronic renal insufficiency | 217 | 20.8 (120) | 19.2 (97) | 0.5160 |
| Liver cirrhosis | 58 | 7.1 (41) | 3.4 (17) | 0.0065 |
| Alcohol consumption | 117 | 13.2 (76) | 8.1 (41) | 0.0076 |
| Tobacco use | 93 | 11.6 (67) | 5.2 (26) | 0.0002 |
| History of bleeding | ||||
| GI | 257 | 20.8 (120) | 27.2 (137) | 0.0275 |
| ICH | 20 | 2.60 (15) | 1.00 (5) | - |
| Other | 80 | 7.60 (44) | 7.10 (36) | - |
| Gastro-duodenal ulcer | 195 | 25.0 (144) | 10.1 (51) | < 0.0001 |
| With PPI treatment | 85 | 37.5 (54) | 60.8 (31) | 0.0040 |
| Antithrombotic treatment | ||||
| VKA alone | 321 | 30.6 (176) | 28.8 (145) | 0.3735 |
| DOAC alone | 66 | 4.86 (28) | 7.54 (38) | - |
| Parenteral alone | 53 | 4.17 (24) | 5.75 (29) | - |
| AP mono alone | 389 | 36.5 (210) | 35.5 (179) | - |
| Dual AP alone | 72 | 6.60 (38) | 6.75 (34) | - |
| Other | 179 | 17.4 (100) | 15.7 (79) | - |
| MAP (mmHg) on admission | 93 ± 20 | 93 ± 20 | 0.8971 | |
| Creatinine (µmol/L) on admission | 104 ± 72 | 104 ± 67 | 0.9005 | |
| Hemoglobin (g/dL) on admission | 11 ± 3 | 11 ± 3 | 0.9495 |
Clinical and biological data were collected from emergency department clinical records: Demographics (age, gender), medical history, co-morbid conditions, antithrombotic class, concomitant medical treatment (in particular proton pump inhibitor), type of bleeding/outcome, vital signs at admission (mean blood pressure), contributory procedures that led to a diagnosis of major GI bleeding, biological data at admission (haemoglobin and creatinine levels), therapeutic management of the haemorrhagic event in the emergency unit. From hospital medical records, we extracted the length of stay in hospital, intensive care unit stay and fatalities, defined as in-hospital deaths. In addition, medical records were carefully analyzed for a detailed description of endoscopic and abdominal computed tomography scan findings. Lastly, specific endoscopic procedures (haemostatic treatment, sclerotherapy with epinephrine injection, electro-cautery therapy, mucosal resection, ablation) were specifically collected. If GI diagnostic procedures were not performed, the reasons were sought in the medical records.
Firstly, the clinical characteristics were described according to gastrointestinal symptoms: Hematemesis or melena indicating upper GI bleeding and hematochezia indicating lower GI bleeding.
Secondly, we described the causative lesions, clinical characteristics across causative lesions summarized as a four-class variable (gastro-duodenal ulcer, other upper GI lesion, lower GI lesion, and unknown cause), and the distribution of five or six mutually exclusive antithrombotic classes (VKA alone, DOAC alone, parenteral anticoagulants alone, AP alone mono or dual, and any combination). We compared the distribution of antithrombotic classes between patients with upper and lower causative bleeding lesions and between gastro-duodenal ulcer (vs other upper GI causes) and antithrombotic classes, stratifying for proton pump inhibitor co-prescription.
Thirdly, case management and fatalities were compared across antithrombotic classes, excluding patients with a limitation of care decision, and stratifying for bleeding symptoms.
For the stratified statistical analysis we used the general association statistic which tests the alternative hypothesis that, for at least one stratum, there is some kind of association. We then took potential confounders into account in a multivariate logistic regression model.
All statistical tests were two-tailed and P values < 0.05 were considered significant. Statistical analyses were performed using SAS software 9.4 (SAS Institute, Cary, NC, United States).
Over a 3-year period, we identified 1080 eligible patients: 576 (53.3%) patients with symptoms of upper GI bleeding (hematemesis or melena) and 504 (46.7%) patients with symptoms of lower GI bleeding (hematochezia). The characteristics of the patients are reported in Table 1. Of note, 257 patients out of 1080 (23.8%) had a history of gastrointestinal bleeding, either major or not; 20 patients out of 1080 (1.85%) had a history of intracranial hemorrhage and 80 patients out of 1080 (7.41%) had a history of bleeding in other location.
The distribution of antithrombotic regimens was as follows (Supplementary Table 2): 461 patients were prescribed AP alone, 321 VKA alone, 53 parenteral anticoagulant alone, and 177 various combinations. For 2 patients, the type of antithrombotic remained unknown. Coagulation parameters according to antithrombotic regimen are shown in Supplementary Table 2.
Twenty-one patients (1.9%) were subject to limitation of care at admission, 14 with upper GI symptoms and 7 with lower GI symptoms.
The cause of GI bleeding was identified for 697 patients (64.5%), 408 with upper GI symptoms, and 289 with lower GI symptoms. No cause of bleeding was identified for 383 patients (35.5%), because investigations yielded negative results (174 patients) or because of no investigations were performed (209 patients). Those patients had upper GI symptoms (191 patients) or with lower GI symptoms (192 patients). Gastrointestinal investigations were performed on 862 patients without limitation of care decision, 479 with upper GI symptoms and 383 with lower GI symptoms. Details are shown in Supplementary Table 3.
Gastro-duodenal ulcer was the first causative lesion of the upper tract (209 out of 408) followed by erosive gastric lesion (75 out of 408) and angiodysplasia (51 out of 408). In the lower GI tract, colonic diverticulum was the principal causative lesion (120 out of 288) followed by colon cancer (51 out 288).
Among 504 patients with symptoms of lower GI bleeding (hematochezia) 55 (11%) were diagnosed to have upper GI bleeding.
Clinical characteristics that significantly differed across causative lesions were age, gender, a history of liver cirrhosis or gastro-duodenal ulcer, and tobacco use
The matrix crossing detailed causative lesions and antithrombotic classes is provided in Supplementary Tables 5 and 6.
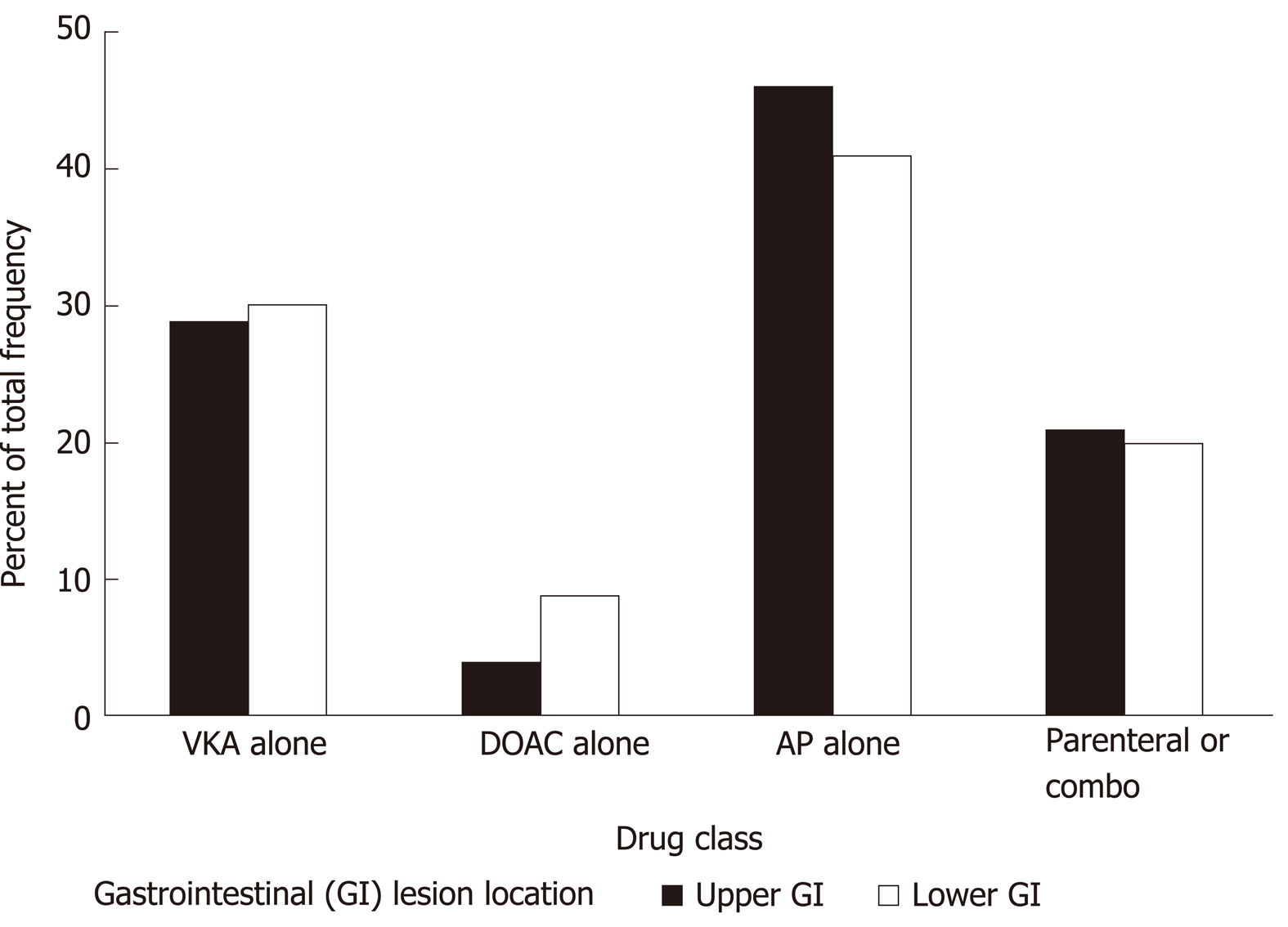
When crossing GI lesion location (upper vs lower) and antithrombotic classes, the proportions were fairly similar (Supplementary Table 7 and Figure 1) except for DOAC for which there was a larger proportion of lower GI than upper GI lesion locations, and for antiplatelet drugs with a larger proportion of upper GI than lower GI lesion locations (overall P value = 0.03). Indeed pair wise comparison with Bonferroni correction pointed to a difference between DOAC and antiplatelet drugs (P value = 0.02).
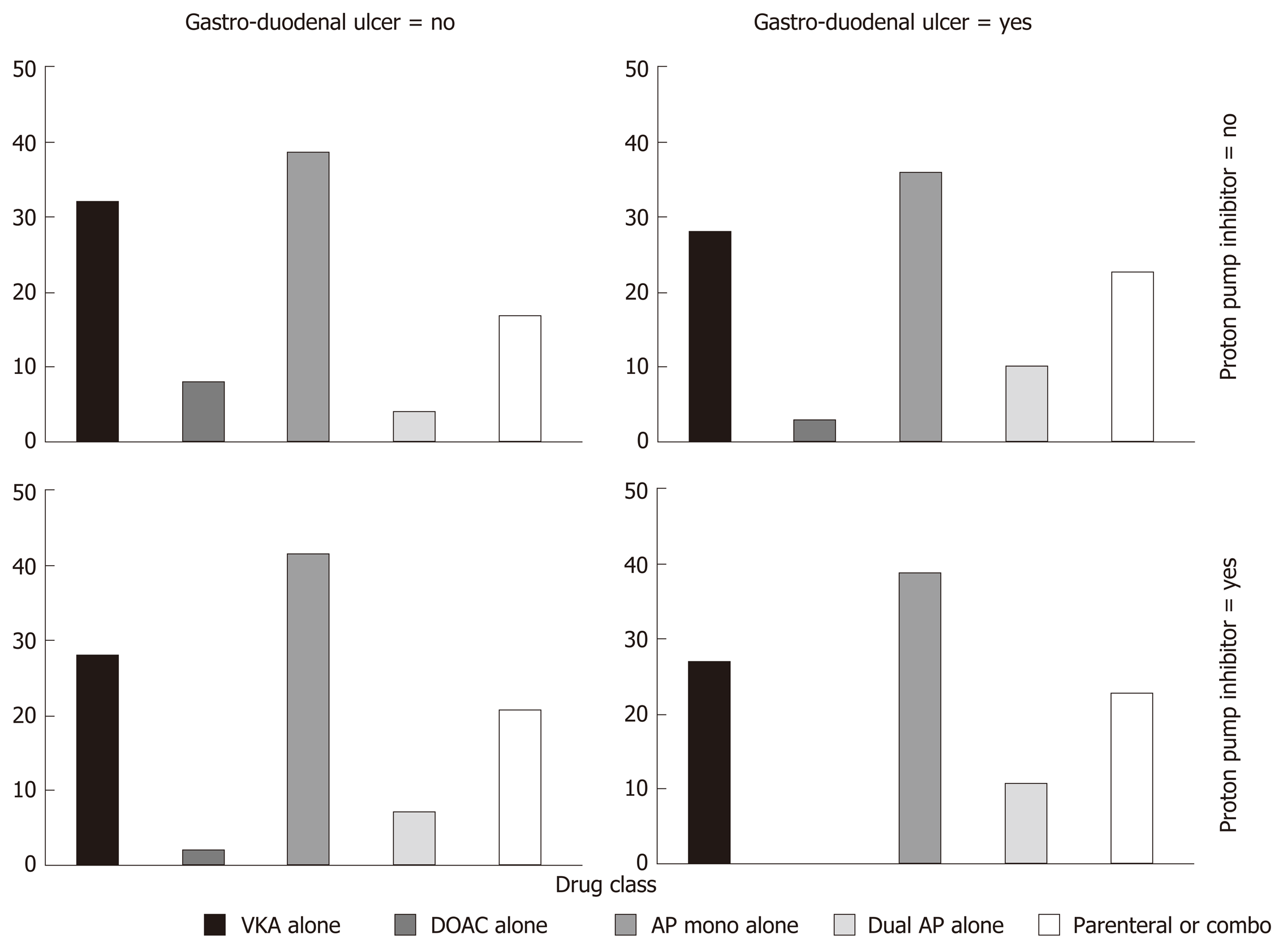
In a stratified statistical analysis of the relationship between gastro-duodenal ulcer as a causative lesion (vs other upper GI causes) and antithrombotic drug type, controlling for proton pump inhibitor (PPI) co-prescription, the general association statistic rejected the null hypothesis (P = 0.05, Figure 2). The multivariate logistic regression model adjusting for gender, a history of cancer, liver cirrhosis or gastro-duodenal ulcer showed that the antithrombotic class (P = 0.03) and PPI co-prescription [adjusted odds ratio (OR) = 0.55, 95%CI: 0.35-0.88] were independently associated with gastro-duodenal ulcer. Bonferroni adjusted pair wise comparisons evidenced differences between dual AP vs VKA (adjusted OR = 3.1, 95%CI: 1.2-7.7), dual vs mono AP (adjusted OR = 2.7, 95%CI: 1.1-6.7), dual AP vs DOAC (adjusted OR = 9.0, 95%CI: 2.0-39) and parenteral antithrombotic drug vs DOAC (adjusted OR = 4.4, 95%CI: 1.2-16).
Our results showed lower resource consumption for the management of lower GI bleeding compared to upper GI bleeding, whatever the antithrombotic type.
Upper GI bleeding management: PPI injection was prescribed to about 80% of patients and red cell transfusions were required for more than 80%, whatever the antithrombotic. Thirty patients required surgery and 2 an embolization. About one-fifth of the patients required endoscopy with haemostatic procedures. Only 50.6% and 31.5% of patients under VKA received reversal therapy with vitamin K and prothrombin complex concentrate (PCC) respectively. PCC was prescribed to only 23% of the patients under DOACs (Supplementary Table 8, panel A).
Lower GI bleeding management: PPI injection was also the most frequent treatment used, whatever the antithrombotic (28.4% overall). Red cell transfusions were needed for about 60% of the patients. Reversal therapy with vitamin K and PCC was required for 51.7% and 27.3% of patients under VKA respectively. PCC was prescribed to 7.9% of the patients under DOACs. Forty-one patients required surgery and fourteen an embolization (Supplementary Table 8, panel B).
Most patients needed hospitalization, 87.5% for upper GI bleeding, and 81.7% for lower GI bleeding (Supplementary Table 9). Length of stay and the need for critical care were similar whatever the antithrombotic and type of GI bleeding.
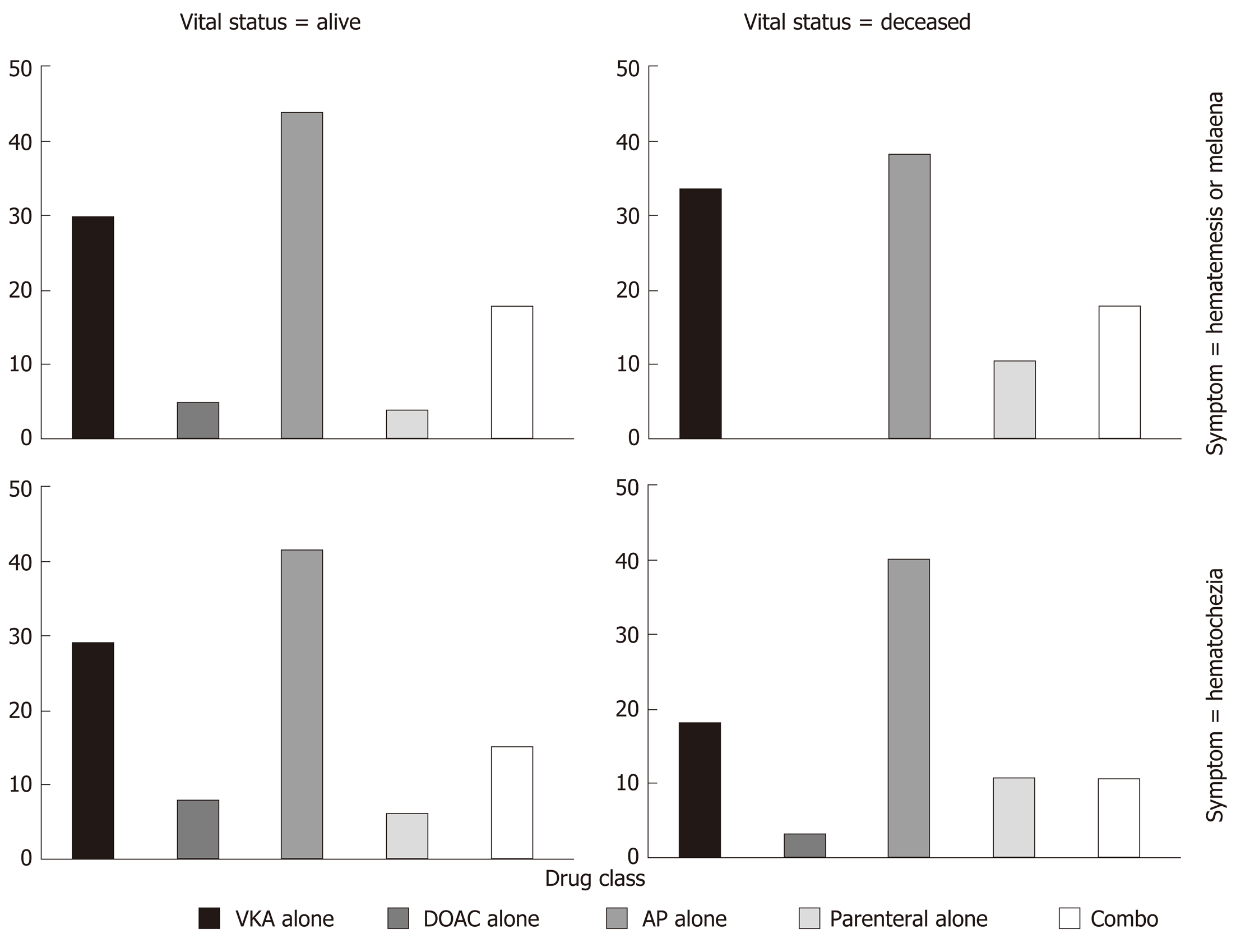
Among the 1059 patients without a limitation of care decision, 63 patients (5.95%) died, 39 with upper GI bleeding (out of 523, 6.94%) and 24 with lower GI bleeding (out of 437, 4.83%). In-hospital mortality, whatever the GI bleeding type, was not statistically different across antithrombotics (P = 0.09, Figure 3).
Our large, multicentre, prospective, comprehensive cohort of patients who had been prescribed an antithrombotic and who were referred for major GI bleeding made it possible to report on GI investigations, causative GI lesions, management, and fatalities.
Among patients undergoing GI investigations, a bleeding lesion was identified for 64.5%, which is higher than in other reports: 42%-44% in the prospective study by Pannach et al[7], 58.4% in the post-hoc study by Kolb et al[13] within the RELY study.
There was a larger proportion of DOAC prescription among patients with a lower GI location than among those with an upper GI lesion location. A similar distribution was reported by Pannach et al[7] and by post-hoc analyses in pivotal trials[13,14]. Several reasons are given: Incomplete absorption of DOAC across the GI mucosa and a potential for topical drug activity leading to relevant concentrations of active drug in the lower GI tract[15], non-absorbed active DOAC being excreted into the feces[16]. In addition, more active drug in the lumen could exacerbate bleeding from existing lesions[17]. All these reasons contrast with the high absorption and excretion for VKA and AP[7]. No patient with gastro-duodenal ulcer received dabigatran, but a few with gastric erosive lesion did: The low oral bioavailability of the dabigatran pro-drug etexilate (6%) and the causticity of tartric acid associated with dabigatran could explain these findings[8]. Few patients with lower GI lesions were receiving DOAC, which contrasts with results from the study by Sherwood et al[15]. This could be explained by our strict definition of major bleeding.
There was a larger proportion of antiplatelet drug use among patients with upper GI locations than among those with lower GI lesion locations. Our results are in line with previous reports that showed gastro-duodenal ulcer as the most frequent bleeding lesion with acetylsalicylic acid and P2Y12 inhibitors[18]. Acetylsalicylic acid inhibits cyclo-oxygenase 1 in the GI mucosa, leading to a reduction in the synthesis of cyto-protective prostaglandin in the GI tract, allowing GI lesions to develop[19]. P2Y12 inhibitors inhibit adenosine diphosphate-induced platelet aggregation without inhibiting cyclo-oxygenase 1 function and prostaglandin formation[20]. Adenosine diphosphate receptor antagonists can cause GI lesions through an impairment of ulcer healing[21]. Nevertheless, P2Y12 inhibitors induce upper GI bleeding with the same frequency as acetylsalicylic acid[18,20,22]. Taking account of the protective role of PPI[18,23,24] on the incidence of gastro-duodenal ulcer, our results showed an over-representation of dual AP use among patients with ulcers.
All drugs that prolong bleeding time induce lower GI bleeding from preexisting lesions, which explains the increased risk of diverticulum bleeding with acetylsalicylic acid whatever the dose, and with P2Y12 inhibitors[25,26].
Percentages of patients with specific therapies, reversal therapy and transfusions were similar irrespective of antithrombotic used and GI bleeding location. Patients on antiplatelet drugs can require platelet transfusions[17], prescribed here to a few patients. For patients under VKA, reversal therapy with cryopoor plasma and vitamin K was used in accordance with guidelines[12,27]. There were no differences in the rates of hospitalization nor in length of stay across antithrombotics nor according to GI bleeding location.
Our results differ from other studies: Pannach et al[7] showed low resource consumption, shorter hospitalization and lower rates of transfusion with DOAC than with VKA among patients hospitalized for GI bleeding. Cangemi et al[9] reported a significantly lower incidence of transfusions and shorter length of stay for patients under DOAC compared to warfarin. Nagatas et al[28] reported a significantly higher transfusion needs among warfarin users than among DOAC users, with no differences in the levels of use of endoscopy therapy. In this study, few patients required surgery, embolization or endoscopy with haemostatic procedures, without any differences across antithrombotics[28]. Fewer hospitalizations and fewer transfusions in the DOAC group than in the warfarin group, irrespective of GI bleeding type and anticoagulant indication, were reported by Brodie et al[29]. Diamantopoulos et al[30] showed more frequent endoscopic hemostasis for patients under DOAC, fewer hospitalization days with no difference for blood transfusion needs or embolization/surgery. In these studies, different inclusion criteria and bleeding definitions could explain these conflicting results. We think that our strict definition of major bleeding and its medical validation are relevant, and led to greater population homogeneity. This could explain the absence of any difference with regard to management and outcomes across antithrombotics.
Overall in-hospital mortality was 5.95% in the present study. We were not able to reject the homogeneity hypothesis across antithrombotics. There is clearly a lack of power. Our results were nevertheless in line with the results reported by Pannach et
Our population-based multicenter cohort can be thought to be representative of a real-world population. Like others[3], we hypothesized that bleeding risk related to antithrombotics was mostly related to patient characteristics, not to the antithrombotic used. We used strict criteria for major bleeding, based on the French guidelines[12] and criteria close to the ISTH criteria[31]. In addition, the medical validation minimized bias.
We cannot exclude a risk of misclassification related to coding errors at the time of hospital admissions, although this may not be very likely for a serious condition like bleeding. Our study was restricted to two tertiary care hospitals. We required extensive clinical data, and a trade-off had to be made between the number of participating centers and feasibility. We focused on major bleeding, and lastly we provided here only descriptive statistics.
In conclusion, our study showed a high rate of bleeding lesion identification and suggested a different pattern of antithrombotic exposure between upper GI and lower GI lesion locations, and between gastro-duodenal ulcer and other identified causes of upper GI bleeding. We did not detect any difference in management or outcomes across a range of antithrombotics. In-hospital mortality was low.
There are few reports on the characteristics of major gastrointestinal (GI) bleeding in patients exposed to different antithrombotics.
There are conflicting results when reporting GI bleeding causative lesions across different antithrombotics. In addition, severity and case fatality are poorly known.
The main objective was to describe the characteristics, causative lesions, management and fatalities related to major GI bleeding events for patients receiving an antithrombotic. A secondary objective was to compare the distribution of antithrombotics between upper and lower GI bleeding, and finally to compare the distribution of antithrombotics between patients with gastro-duodenal ulcer and patients with other identified causes of upper GI bleeding.
Over a three-year period (2013-2015), in two tertiary care hospitals in France, we prospectively identified adult patients admitted for major GI bleeding while receiving an antithrombotic. Patients were screened at emergency admission by computerised requests on electronic health records. All screened records were medically validated. Major bleeding was defined on pre-specified criteria. Data were collected from emergency department clinical records and hospital medical records.
We observed a high rate of identification of causative bleeding lesions. There was a higher proportion of direct oral anticoagulant use among patients with lower GI locations than among those with upper GI lesion locations. Dual antiplatelet regimen was more frequently encountered among patients with gastro-duodenal ulcers. Our data did not support differences in management and outcomes across the various antithrombotics. In-hospital mortality was low.
Our results suggest a different pattern of antithrombotic exposure between GI lesion locations.
Future research could assess potential difference between direct oral anticoagulants.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Spiliopoulos S S-Editor: Gao CC L-Editor: A P-Editor: Zhang YL
| 1. | Sørensen R, Hansen ML, Abildstrom SZ, Hvelplund A, Andersson C, Jørgensen C, Madsen JK, Hansen PR, Køber L, Torp-Pedersen C, Gislason GH. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet. 2009;374:1967-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 429] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 2. | Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3181] [Cited by in RCA: 3670] [Article Influence: 333.6] [Reference Citation Analysis (0)] |
| 3. | Di Minno A, Spadarella G, Prisco D, Scalera A, Ricciardi E, Di Minno G. Antithrombotic drugs, patient characteristics, and gastrointestinal bleeding: Clinical translation and areas of research. Blood Rev. 2015;29:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Lanas Á, Carrera-Lasfuentes P, Arguedas Y, García S, Bujanda L, Calvet X, Ponce J, Perez-Aísa Á, Castro M, Muñoz M, Sostres C, García-Rodríguez LA. Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants. Clin Gastroenterol Hepatol. 2015;13:906-12.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 192] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 5. | Bouget J, Oger E, Nicolas N. Emergency admissions for major haemorrhage associated with antithrombotics: a cohort study. Thromb Res. 2015;135:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Di Minno MN, Ambrosino P, Di Minno A, Tremoli E, Di Minno G. The risk of gastrointestinal bleeding in patients receiving dabigatran etexilate: a systematic review and meta-analysis of the literature. Ann Med. 2017;49:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Pannach S, Goetze J, Marten S, Schreier T, Tittl L, Beyer-Westendorf J. Management and outcome of gastrointestinal bleeding in patients taking oral anticoagulants or antiplatelet drugs. J Gastroenterol. 2017;52:1211-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Desai J, Kolb JM, Weitz JI, Aisenberg J. Gastrointestinal bleeding with the new oral anticoagulants--defining the issues and the management strategies. Thromb Haemost. 2013;110:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Cangemi DJ, Krill T, Weideman R, Cipher DJ, Spechler SJ, Feagins LA. A Comparison of the Rate of Gastrointestinal Bleeding in Patients Taking Non-Vitamin K Antagonist Oral Anticoagulants or Warfarin. Am J Gastroenterol. 2017;112:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Lanas A, Wu P, Medin J, Mills EJ. Low doses of acetylsalicylic acid increase risk of gastrointestinal bleeding in a meta-analysis. Clin Gastroenterol Hepatol. 2011;9:762-768.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Bouget J, Balusson F, Scailteux LM, Maignan M, Roy PM, L'her E, Pavageau L, Nowak E. Major bleeding with antithrombotic agents: a 2012-2015 study using the French nationwide Health Insurance database linked to emergency department records within five areas - rationale and design of SACHA study. Fundam Clin Pharmacol. 2019;33:443-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Pernot G, Godiér A, Gozalo C, Tremey B, Sié P; French National Authority for Health. French clinical practice guidelines on the management of patients on vitamin K antagonists in at-risk situations (overdose, risk of bleeding, and active bleeding). Thromb Res. 2010;126:e167-e174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Kolb JM, Flack KF, Chatterjee-Murphy P, Desai J, Wallentin LC, Ezekowitz M, Connolly S, Reilly P, Brueckmann M, Ilgenfritz J, Aisenberg J. Locations and Mucosal Lesions Responsible for Major Gastrointestinal Bleeding in Patients on Warfarin or Dabigatran. Dig Dis Sci. 2018;63:1878-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Sherwood MW, Nessel CC, Hellkamp AS, Mahaffey KW, Piccini JP, Suh EY, Becker RC, Singer DE, Halperin JL, Hankey GJ, Berkowitz SD, Fox KAA, Patel MR. Gastrointestinal Bleeding in Patients With Atrial Fibrillation Treated With Rivaroxaban or Warfarin: ROCKET AF Trial. J Am Coll Cardiol. 2015;66:2271-2281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 15. | Di Minno A, Spadarella G, Spadarella E, Tremoli E, Di Minno G. Gastrointestinal bleeding in patients receiving oral anticoagulation: Current treatment and pharmacological perspectives. Thromb Res. 2015;136:1074-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Feagins LA, Weideman RA. GI Bleeding Risk of DOACs Versus Warfarin: Is Newer Better? Dig Dis Sci. 2018;63:1675-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Abraham NS, Horsley-Silva JL. Gastrointestinal bleeding secondary to the new anticoagulants. Curr Opin Gastroenterol. 2016;32:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 432] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 19. | Eikelboom JW, Hirsh J, Spencer FA, Baglin TP, Weitz JI. Antiplatelet drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e89S-e119S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 271] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 20. | Lin CC, Hu HY, Luo JC, Peng YL, Hou MC, Lin HC, Lee FY. Risk factors of gastrointestinal bleeding in clopidogrel users: a nationwide population-based study. Aliment Pharmacol Ther. 2013;38:1119-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Tsai TJ, Lai KH, Hsu PI, Lin CK, Chan HH, Yu HC, Wang HM, Lin KH, Wang KM, Chang SN, Liu CP, Hsiao SH, Huang HR, Lin CH, Tsay FW. Upper gastrointestinal lesions in patients receiving clopidogrel anti-platelet therapy. J Formos Med Assoc. 2012;111:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel vs aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4584] [Cited by in RCA: 4180] [Article Influence: 144.1] [Reference Citation Analysis (0)] |
| 23. | Sehested TSG, Carlson N, Hansen PW, Gerds TA, Charlot MG, Torp-Pedersen C, Køber L, Gislason GH, Hlatky MA, Fosbøl EL. Reduced risk of gastrointestinal bleeding associated with proton pump inhibitor therapy in patients treated with dual antiplatelet therapy after myocardial infarction. Eur Heart J. 2019;40:1963-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 24. | Ray WA, Chung CP, Murray KT, Smalley WE, Daugherty JR, Dupont WD, Stein CM. Association of Proton Pump Inhibitors With Reduced Risk of Warfarin-Related Serious Upper Gastrointestinal Bleeding. Gastroenterology. 2016;151:1105-1112.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Yuhara H, Corley DA, Nakahara F, Nakajima T, Koike J, Igarashi M, Suauki T, Mine T. Aspirin and non-aspirin NSAIDs increase risk of colonic diverticular bleeding: a systematic review and meta-analysis. J Gastroenterol. 2014;49:992-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Chen WC, Lin KH, Huang YT, Tsai TJ, Sun WC, Chuah SK, Wu DC, Hsu PI. The risk of lower gastrointestinal bleeding in low-dose aspirin users. Aliment Pharmacol Ther. 2017;45:1542-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | ASGE Standards of Practice Committee. Acosta RD, Abraham NS, Chandrasekhara V, Chathadi KV, Early DS, Eloubeidi MA, Evans JA, Faulx AL, Fisher DA, Fonkalsrud L, Hwang JH, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Shergill AK, Wang A, Cash BD, DeWitt JM. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016;83:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 458] [Article Influence: 50.9] [Reference Citation Analysis (1)] |
| 28. | Nagata N, Sakurai T, Moriyasu S, Shimbo T, Okubo H, Watanabe K, Yokoi C, Yanase M, Akiyama J, Uemura N. Impact of INR monitoring, reversal agent use, heparin bridging, and anticoagulant interruption on rebleeding and thromboembolism in acute gastrointestinal bleeding. PLoS One. 2017;12:e0183423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Brodie MM, Newman JC, Smith T, Rockey DC. Severity of Gastrointestinal Bleeding in Patients Treated with Direct-Acting Oral Anticoagulants. Am J Med. 2018;131:573.e9-573.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Diamantopoulou G, Konstantakis C, Skroubis G, Theocharis G, Theopistos V, Triantos C, Thomopoulos K. Acute Lower Gastrointestinal Bleeding in Patients Treated With Non-Vitamin K Antagonist Oral Anticoagulants Compared With Warfarin in Clinical Practice: Characteristics and Clinical Outcome. Gastroenterology Res. 2019;12:21-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3234] [Cited by in RCA: 3918] [Article Influence: 195.9] [Reference Citation Analysis (0)] |