Published online Aug 21, 2020. doi: 10.3748/wjg.v26.i31.4656
Peer-review started: March 4, 2020
First decision: April 25, 2020
Revised: June 7, 2020
Accepted: July 18, 2020
Article in press: July 18, 2020
Published online: August 21, 2020
Processing time: 170 Days and 5.3 Hours
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract. Surgical resection and tyrosine kinase inhibitors are defined as the main treatments but cannot cure patients with advanced GIST, which eventually develops into recurrence and acquired drug resistance. Therefore, it is necessary to identify prognostic biomarkers and new therapeutic targets for GISTs. CC chemokine receptor type 8 (CCR8) protein participates in regulation of immune responses. Recent studies on CCR8 in non-small cell lung cancer and colorectal cancer showed that it was highly expressed in tumor-infiltrating regulatory T cells and correlated with a poor prognosis.
To detect CCR8 expression in GIST tissues and analyze its relationships with clinicopathological features and prognosis in patients with GISTs.
Tissue samples were used for the tissue microarrays construction. The microarrays were then subjected to immunohistochemical analyses to detect CCR8 expression. Next, Kaplan–Meier analysis was utilized to calculate the survival rate of patients with complete follow-up data, and the potential prognostic value of CCR8 was evaluated by Cox regression analysis. Finally, a Gene Ontology/Kyoto Encyclopedia of Genes and Genomes single-gene enrichment chart of CCR8 was constructed using the STRING database.
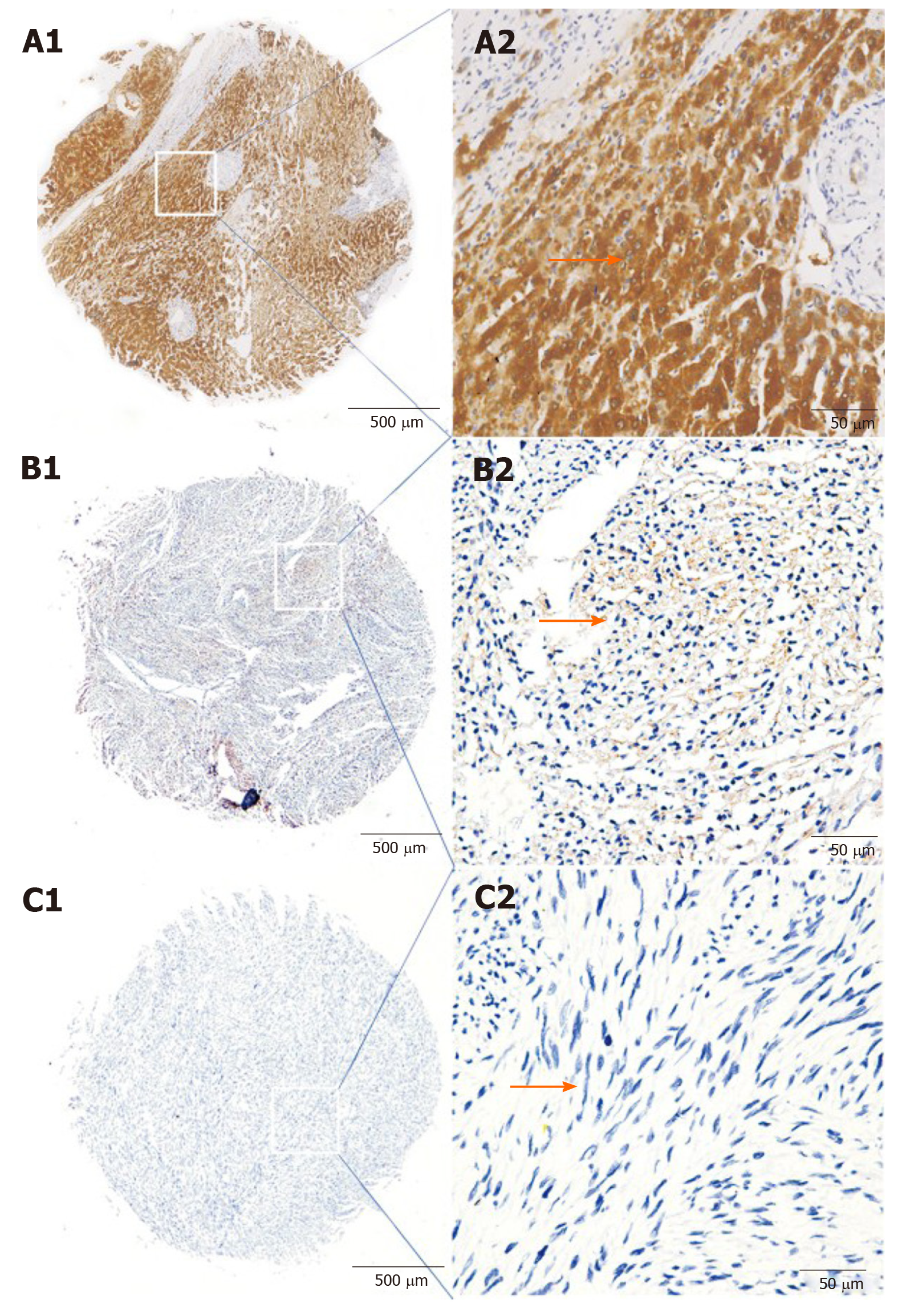
CCR8-positive signals were detected as brown or brown-yellow particles by immunohistochemistry located in the cytoplasm. Among 125 tissue samples, 74 had CCR8 high expression and 51 had low or negative expression. Statistical analyses suggested CCR8 was significantly correlated with tumor size, mitotic index, AFIP-Miettinen risk classification and tumor location. Kaplan–Meier and multivariate analyses showed that patients with low or negative CCR8 expression, mitotic index < 5/high-power fields (HPF) and tumor diameter < 5 cm had a better prognosis. Based on the STRING database, CCR8 was significantly enriched in biological processes such as tumor immunity, T lymphocyte chemotaxis, migration and pathways like the nuclear factor-κB and tumor necrosis factor pathways as well as intestinal immune regulation networks.
CCR8 is a prognostic biomarker for malignant potential of GISTs, with high expression correlated with malignancy and poor prognosis.
Core tip: Although the application of c-kit inhibitors prolonged survival for advanced gastrointestinal stromal tumor (GIST) patients, the prognosis was not optimistic. Because of the role in tumor immunity, chemokine receptors have been identified as independent prognostic factors in various cancers but have not been investigated in GISTs. We evaluated CC chemokine receptor type 8 expression in GISTs by immunohistochemistry and analyzed its relationships with clinicopathological features and prognosis. We also investigated its role in tumor immunity. These confirmed that CC chemokine receptor type 8 can be used as an independent prognostic factor for GISTs and even as a potential therapeutic target.
- Citation: Li HL, Wang LH, Hu YL, Feng Y, Li XH, Liu YF, Li P, Mao QS, Xue WJ. Clinical and prognostic significance of CC chemokine receptor type 8 protein expression in gastrointestinal stromal tumors. World J Gastroenterol 2020; 26(31): 4656-4668
- URL: https://www.wjgnet.com/1007-9327/full/v26/i31/4656.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i31.4656
Gastrointestinal stromal tumors (GISTs) are invasive tumors with malignant potential derived from the gastrointestinal mesenchymal tissue and account for 1%-3% of gastrointestinal malignant tumors[1]. Surgical resection remains the main treatment for GISTs. Unfortunately, more than 50% of patients eventually develop local recurrence or metastasis[2]. Although imatinib is the standard first-line therapy for patients with metastatic or advanced GISTs and can improve their median progression-free survival and median overall survival[3-6], it cannot cure the disease. The prognosis of the advanced cases is not optimistic.
Investigators have shown that the immune system was suppressed in the microenvironment of GISTs[7]. Chemokine receptors and their ligands played crucial roles in tumor immune responses. In the tumor microenvironment, chemokine receptors are beneficial for the recruitment of tumor immune cells, such as bone marrow-derived suppressor cells, tumor-associated macrophages and regulatory T cells (Tregs), which are associated with poor prognosis in various solid malignancies[8-11]. Recent studies suggested that the organ-selective patterns of tumor metastases were determined by the chemokine receptor/ligand axis[12,13].
CC chemokine receptor type 8 (CCR8) is one of the most important chemokine receptors and is mainly expressed in Tregs with small proportions of expression in T helper 2 cells and monocytes[14-17]. CCR8 has four known ligands: CCL1, CCL8, CCL16 and CCL18[18]. Inngjerdingen et al[19] confirmed that CCL1/CCR8 is the key axis for tumor immunity. Specifically, CCL1 enhances Treg immunosuppressive activity by recruiting CCR8, FOXp3 and IL-10 with a positive feedback mechanism. Recently, CCR8 was reported to be correlated with poor prognosis in non-small cell lung cancer and colorectal cancer[20]. However, the expression of CCR8 in GISTs, its relationship with clinical features and its impact on prognosis remain unclear.
In this study, we examined CCR8 expression in GISTs by immunohistochemistry and analyzed its clinical and prognostic importance. Using the STRING database, we mapped a Gene Ontology (GO)/Kyoto Encyclopedia of Genes and Genomes (KEGG) single-gene enrichment chart for CCR8. The present results may offer a basis for prognosis assessment and identify a new therapeutic target for GISTs.
The study included 125 patients with GISTs at the Department of Pathology of the Affiliated Hospital of Nantong University and Third Affiliated Hospital of Nanjing Medical University from 2002 to 2012 with relatively complete clinicopathological and follow-up data. The diagnostic criteria for GISTs were consistent with histo-pathological features and positive for CD117 immunohistochemistry, negative for CD117 but positive for DOG1 or negative for both, while immunohistochemistry for desmin, S-100 and smooth muscle actin were negative (to exclude neurogenic and smooth muscle tumors)[21]. The basic clinical data included tumor size, sex, age, mitotic index, total classification, tumor grade and tumor location. The AFIP-Miettinen risk classification was used to assess the potential risk of malignancy. All patients included in this study were treated with surgical resection, without preoperative radiotherapy or chemotherapy. Cases with distant metastasis were not enrolled in the research. In addition, patients were not treated with postoperative tyrosine kinase inhibitors, such as imatinib.
The tissue microarray was manufactured by Shanghai Outdo Biotech Co. Ltd. (Shanghai, China). Based on the results of hematoxylin and eosin staining, representative GIST regions were marked on specific paraffin blocks. The above 125 formalin-fixed and paraffin-embedded GIST tissues were collected, and 2 mm diameter samples were acquired by inserting tissue array needles. The obtained samples were aligned on blank paraffin blocks and cut into 4 µm sections.
The sections (4 µm) were conventionally dewaxed and rehydrated. The tissue microarray was incubated for 1 h with an anti-CCR8 primary antibody (1:200; Abcam, Cambridge, United Kingdom) in tris-buffered saline, washed and incubated with a horseradish peroxidase-conjugated anti-goat secondary antibody (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, United States). Immunostaining of CCR8 was scored by two pathologists based on the intensity and density of the CCR8-positive signals. Staining intensity was scored according to four grades: 0, 1, 2 or 3, ranging from negative and weak to strong intensity. Positive staining intensity: 0 for colorless, 1 for light yellow, 2 for brown and 3 for tan. The density of CCR8-positive cells was also scored at four levels: 0 for 0-25%, 1 for 26%-50%, 2 for 51%-75% and 3 for 76%-100%[21]. The product of the intensity and density scores was used as the CCR8 staining score. The degree of CCR8 staining was quantified using a two-level grading system, and staining scores were defined as follows: 0 or 1: Low expression; and 2-9: High expression.
The correlations between CCR8 and clinicopathological features were analyzed by the chi-square test. Survival rates were assessed by Kaplan–Meier analysis, and Cox regression was utilized to confirm the factors affecting the prognosis of GISTs. All statistical analyses were performed with SPSS version 18.0 software (SPSS, Chicago, IL, United States). Values of P < 0.05 were considered statistically significant.
Single-gene GO/KEGG enrichment was conducted with STRING Version 11.0 software (https://string-db.org), the settings include basic settings and advanced settings. The basic settings section mainly has several parts: Meaning of network edges: Evidence; active interaction sources: Text-mining; experiments; databases; coexpression; neighborhood; gene fusion; cooccurrence; minimum required interaction score: High confidence (0.700); max n of interactors to show: No more than 20 interactors. Advanced settings include two parts: Network display mode: Interactive svg; and display simplifications: Hide disconnected nodes in the network.
The study included a total of 125 patients comprised of 62 men and 63 women. It included 81 patients aged ≤ 60 years and 44 aged > 60 years. For tumor sizes, 25 had tumor diameters < 5 cm, 80 had tumor diameters 5-10 cm, and 20 had tumor diameters > 10 cm. Furthermore, 92 patients had single nodules, and 33 had multiple nodules. For tumor locations, 69 were located in the stomach, 42 in the intestine and 14 in other organs. Regarding the mitotic index, 70 patients were 0-5, 30 were 5-10, and 25 were > 10. According to the AFIP-Miettinen risk classification assessment, 85 patients were low to moderate risk, and 40 were high risk.
Immunohistochemical analysis showed that CCR8-positive signals as brown-yellow or brown particles were localized in the cytoplasm of GIST tissues (Figure 1). In tumor cells, 74 (59.20%) of the 125 GIST tissues exhibited high CCR8 protein expression with cytoplasm staining, and the remaining 51 showed low or no staining of the CCR8 protein.
We analyzed the correlations between CCR8 expression and clinicopathological features of the 125 patients with GISTs. The results indicated that CCR8 expression was associated with tumor size (P = 0.018), mitotic index (P = 0.017), AFIP-Miettinen risk classification (P = 0.038) and tumor location (P = 0.003) regardless of gender, age, gross classification and other features (Table 1).
| Characteristic | n | CCR8-, n (%) | CCR8+, n (%) | Pearson χ2 | P value |
| Total | 125 | 51 (40.80 ) | 74 (59.20) | ||
| Gender | 1.439 | 0.230 | |||
| Male | 62 | 22 (35.48) | 40 (64.52) | ||
| Female | 63 | 29 (46.03) | 34 (53.97) | ||
| Age | 2.268 | 0.132 | |||
| ≤ 60 yr | 81 | 37 (45.68) | 44 (54.32) | ||
| > 60 yr | 44 | 14 (31.82) | 30 (68.18) | ||
| Tumor size, cm | 8.999 | 0.01 | |||
| < 5 | 25 | 16 (64.00) | 9 (36.00) | ||
| 5-10 | 80 | 30 (37.50) | 50 (62.50) | ||
| > 10 | 20 | 5 (25.00) | 15 (75.00) | ||
| Mitotic index, per 50 HPFs | 8.196 | 0.017 | |||
| 0-5 | 70 | 34 (48.57) | 36 (51.43) | ||
| 6-10 | 30 | 13 (43.33) | 17 (56.67) | ||
| > 10 | 25 | 4 (16.00) | 21 (84.00) | ||
| Gross classification | 1.035 | 0.309 | |||
| Single nodule | 92 | 40 (43.48) | 52 (56.52) | ||
| Multiple nodules | 33 | 11 (33.33) | 22 (66.67) | ||
| Tumor location | 11.673 | 0.003 | |||
| Stomach | 69 | 34 (49.28) | 35 (50.72) | ||
| Intestine | 42 | 9 (21.42) | 33 (78.58) | ||
| Others | 14 | 9 (64.29) | 5 (35.71) | ||
| AFIP-Miettinen risk classification | 4.308 | 0.038 | |||
| Very low–Moderate risk | 85 | 40 (47.06) | 45 (52.94) | ||
| High risk | 40 | 11 (27.50) | 29 (72.50) |
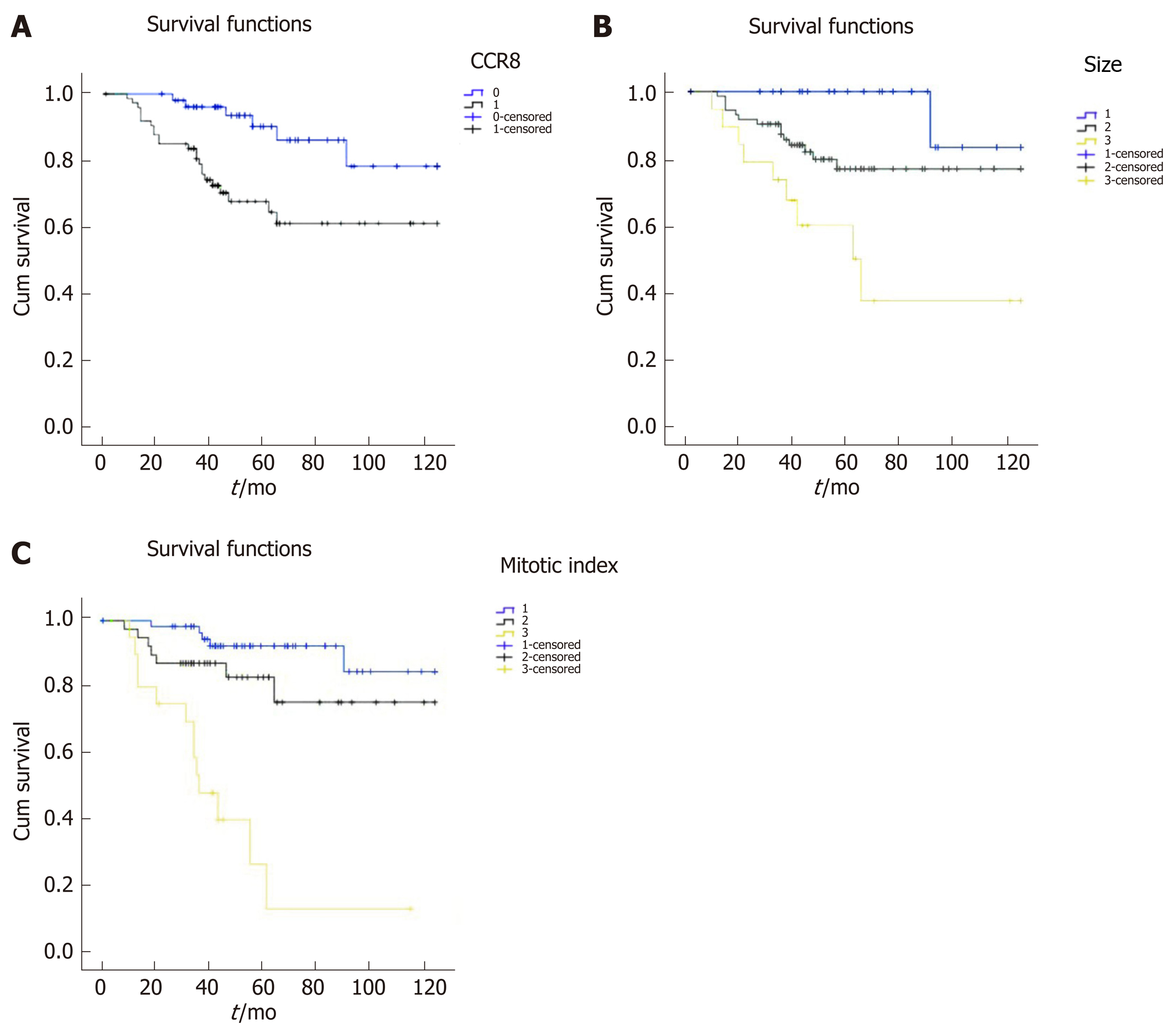
Kaplan–Meier survival curves showed that patients with low CCR8 expression (Figure 2A), mitotic index < 5/HPF (Figure 2B) and tumor diameter < 5 cm (Figure 2C) had a better prognosis. Multivariate analyses proved that CCR8 expression was significantly associated with poor prognosis, 5-year overall survival rate (P = 0.005) and 10-year overall survival rate (P = 0.011) in patients with GISTs (Table 2). As other important clinical factors, tumor size was significantly correlated with 10-year survival rate (univariate analysis P = 0.004, multivariate analysis P = 0.047). Mitotic index was correlated with poor prognosis, with 5-year overall survival rate (P < 0.001) and 10-year overall survival rate (univariate analysis P < 0.001, multivariate analysis kP = 0.002).
| Variable | Univariate analysis | Multivariate analysis | |||||||
| Yr | P > |z| | 95%CI | P > |z| | 95%CI | |||||
| Gender | |||||||||
| Male vs Female | 10/5 | 0.448/0.140 | 0.754/0.547 | 0.364/0.246 | 1.563/1.219 | ||||
| Age in yr | |||||||||
| ≤ 60 vs > 60 | 10/5 | 0.964/0.524 | 0.983/0.767 | 0.469/0.339 | 2.060/1.735 | ||||
| Tumor size in cm | |||||||||
| < 5 vs 5-10 vs > 10 | 10/5 | 0.004/0.019 | 2.417/2.160 | 1.336/1.137 | 4.371/4.102 | 0.0470 | 1.876 | 1.010 | 3.487 |
| Mitotic index/5 HPFs | |||||||||
| 0-5 vs 6-10 vs > 10 | 10/5 | < 0.001/< 0.001 | 2.696/2.727 | 1.687/1.645 | 4.310/4.522 | 0.002/0.001 | 2.177/2.335 | 1.345/1.401 | 3.523/3.889 |
| Gross classification | |||||||||
| Single vs Multiple | 10/5 | 0.300/ 0.315 | 1.569/1.601 | 0.670/ 0.639 | 3.677/4.009 | ||||
| Tumor location | |||||||||
| Stomach vs Intestine | 10/5 | 0.185/0.281 | 1.396/1.339 | 0.853/0.787 | 2.284/2.279 | ||||
| CCR8 expression | |||||||||
| High vs Low | 10/5 | 0.011/0.005 | 2.738/4.651 | 1.261/1.593 | 5.949/13.580 | 0.037/0.027 | 2.663/3.432 | 1.062/1.151 | 6.677/10.232 |
According to the built-in KEGG/GO module in the STRING database for single-gene enrichment analysis, 143 significantly up-regulated GO pathways and 19 significantly up-regulated KEGG pathways were found (P < 0.05). We choose 15 GO pathways and 8 KEGG pathways for further examination and found that they were mainly related to immune processes, T lymphocyte chemotaxis and lymphocyte migration in the GO pathways (Table 3) and enriched in the tumor necrosis factor and nuclear factor (NF)-κB pathways and intestinal immune regulation networks for the KEGG pathways (Table 4).
| Term | Count % | P value | |
| GO: 0070098~chemokine mediated signaling pathway | 20 | 26.67 | 1.25E-43 |
| GO: 0060326~cell chemotaxis | 20 | 10.93 | 3.99E-37 |
| GO:0006935~chemotaxis | 21 | 4.28 | 7,57E-32 |
| GO: 0030595~leukocyte chemotaxis | 17 | 13.08 | 2.39E-31 |
| GO: 0048247~lymphocyte chemotaxis | 14 | 31.11 | 1.33E-29 |
| GO: 0010469~regulation of signaling receptor activity | 20 | 3.47 | 6.99E-28 |
| GO: 0035821~modification of morphology or physiology of other organism | 16 | 8.79 | 1.02E-26 |
| GO: 0031640~killing of cells of other organism | 14 | 15.73 | 4.18E-26 |
| GO: 0061844~antimicrobial humoral immune response mediated by antimicrobial peptide | 14 | 13.08 | 3.62E-25 |
| GO: 0006954~inflammatory response | 18 | 3.73 | 9.59E-25 |
| GO: 0002685~regulation of leukocyte migration | 15 | 8.57 | 9.59E-25 |
| GO: 0002687~positive regulation of leukocyte migration | 14 | 11.02 | 2.56E-24 |
| GO: 0007186~G protein-coupled receptor signaling pathway | 21 | 1.68 | 3.62E-24 |
| GO: 0002690~positive regulation of leukocyte chemotaxis | 13 | 14.29 | 8.72E-24 |
| GO: 0006955~immune response | 21 | 1.34 | 3.10E-22 |
| GO: 0097529~myeloid leukocyte migration | 12 | 11.65 | 7.52E-21 |
| GO: 0002548~monocyte chemotaxis | 10 | 24.39 | 4.97E-20 |
| GO: 0071347~cellular response to interleukin-1 | 12 | 9.68 | 5.34E-20 |
| GO: 0006592~defense response | 19 | 1.54 | 8.02E-20 |
| GO: 0032103~positive regulation of response to external stimulus | 14 | 4.81 | 8.98E-20 |
| Term | Count | % | P value |
| Hsa04062: Chemokine signaling pathway | 2.10E+01 | 11.60 | 2.30E-41 |
| Hsa04060: Cytokine-cytokine receptor infiltration | 2.10E+01 | 7.98 | 2.03E-38 |
| Hsa04064: NF kappa B signaling pathway | 7.00E+00 | 7.53 | 9.35E-11 |
| Hsao04620: Toll-like receptor signaling pathway | 7.00E+00 | 6.86 | 1.30E-10 |
| Hsa05323: Rheumatoid arthritis | 6.00E+00 | 7.14 | 2.86E-09 |
| Hsa04668: TNF signaling pathway | 6.00E+00 | S.56 | 1.00E-08 |
| Hsa04657: lL-17 signaling pathway | 5.00E+00 | S.43 | 2.56E-07 |
| Hsa04623: Cytosolic DNA-sensing pathway | 4.00E+00 | 6.45 | 2.95E-06 |
| Hsa05132: Salmonella infection | 4.00E+00 | 4.76 | 1.90E-04 |
| Hsa05120: Epithelial cell signaling in Helicobacter pylori infection | 3.00E+00 | 4.55 | 2.40E-03 |
| Hsa05164: Influenza A | 3.00E+00 | 1.79 | 2.40E-03 |
| Hsa04621: N0D-like receptor signaling pathway | 3.00E+00 | 1.88 | 2.70E-03 |
| Hsa05167: Kaposi’s sarcoma-associated herpesvirus infection | 3.00E+00 | 1.81 | 3.62E-24 |
| Hsa04672: Intestinal immune network for IgA production | 2.00E+00 | 4.55 | 2.80E-03 |
| Hsa05134: Legionellosis | 2.00E+00 | 3.70 | 3.80E-03 |
GISTs are the most common digestive tract mesenchymal tumors with malignant potential. The National Comprehensive Cancer Network GIST guidelines describe that their malignant potential is associated with tumor size and mitotic index[22,23], both of which are considered in the National Institutes of Health risk classification of GISTs. Early studies showed that the malignant phenotype of GISTs was not only associated with these clinical pathological features but also related to molecular factors, such as long noncoding RNAs, microRNAs and other specific molecules that can accurately predict the risk of malignancy[24]. HOX antisense intergenic RNA was reported to be highly expressed in the high-risk group of GISTs and to facilitate proliferation, invasion and metastasis[25]. Besides, overexpression of miR-196a was reported to be associated with high-risk grade and poor survival in patients with GISTs.
In the present study, we first discovered that CCR8 was expressed in the cytoplasm, and the immunohistochemistry was brown or brown-yellow particles. The positive expression rates of CCR8 in the GIST tissues with tumor diameter < 5 cm, 5-10 cm and > 10 cm were 36.00%, 62.50% and 75.00% (P < 0.05), respectively. The positive rates of CCR8 in the GIST tissues with mitotic index 0-5/HPFs, 6-10/HPF and > 10/HPFs, were 51.43%, 56.67% and 84.00%, respectively (P < 0.05), indicating that the expression of CCR8 may be related to the uncontrolled increase of GIST. In addition, the expression of CCR8 is associated with tumor localization. In the stomach and small intestine, the positive expression rates of CCR8 were 50.72% and 79.07%, respectively (P < 0.05). Miettinen et al[25] found that the malignant potential of small intestinal stromal tumors was higher than that of gastric stromal tumors, which may be associated with higher exon 9 mutation rate in small intestinal stromal tumors, regardless of CCR8 expression. The prognosis of GIST patients in two sites was not statistically significant. In this study, high expression of CCR8 was correlated with tumor size and mitotic index and may be a biomarker for prediction of the malignant potential of GISTs.
The STRING database was used to map a GO single-gene enrichment chart of CCR8, which was enriched in biological processes, such as immune response, T lymphocyte chemotaxis and lymphocyte migration. Recent studies showed that the CCR4/CCL22 axis recruited Tregs to the tumor site, leading to tumor immune tolerance and was negatively correlated with tumor prognosis[26-29]. CCR8 recruited FOXp3+ Treg cells to exert an immunosuppressive function, ultimately resulting in a decreased proportion of CD8+ T cells/Tregs and leading to a poor prognosis of solid tumors[30-33]. Karin[22] even proposed the idea that chemokines and their receptors can act as immunological checkpoints for tumor therapy, pointing out that CCR8+ FOXp3+ regulatory T cells are the main driving factors of immune regulation and provided the possibility of using CCR8 as a therapeutic target.
Very recently, monoclonal antibodies targeting CCR8 were shown to significantly inhibit tumor growth and improve long-term survival in a colorectal cancer mouse model, which associated with increased infiltration of CD4+ CD8+ T cells and a significant decrease in the frequency of tumor-resident CD4+ CCR8+ Tregs. This resulted in an increased proportion of CD8+ T cells/Tregs[34]. Thus, blockage of CCR8 expression may improve the prognosis of patients with these tumors. In this study, survival curves suggested that patients with GISTs showing high CCR8 expression had a poor prognosis. Thus, we inferred that expression of CCR8 was correlated with progression of GISTs and may even be a potential therapeutic target for GISTs.
A KEGG single-gene enrichment chart of CCR8 was obtained using the STRING database and was enriched in the tumor necrosis factor and NF-κB pathways and intestinal immune regulation networks. Interestingly, upregulation of Notch1 by CCR7 promoted the invasion and metastasis of prostate cancer cells by activating the MAPK and NF-κB signaling pathways in prostate cancer[35]. In colorectal cancer, CCR4 in the downstream of the tumor necrosis factor-α pathway promoted metastasis via the ERK/NF-κB/MMP13 pathway[36]. Besides, CCR6 was reported to facilitate tumor angiogenesis by the NF-κB/VEGF pathway in colorectal cancer[37]. Moreover, the CCL20/CCR6 axis was proven to promote the invasion and migration of thyroid cancer cells through MMP3 induced by NF-κB signaling[38]. Estrogen promoted progression of hormone-dependent breast cancer through the CCL2/CCR2 axis by upregulating Twist via PI3K/AKT/NF-κB signaling[39]. CCR5 upregulated the expression of transforming growth factor β1, which ultimately induced epithelial-mesenchymal transition and migration via PI3K/AKT/GSK3β signaling in melanoma. Recently, CCL1 was shown to enhance migration, invasion and epithelial-mesenchymal transition by binding to CCR8 in bladder cancer[40-44]. Therefore, we speculated that expression of CCR8 can affect the progression of GISTs through the above mechanisms.
There are some limitations to this study. We lacked a large sample and multi-center study. Meanwhile, there are few advanced GIST cases. In addition, we also lacked relevant cytological studies. Our future studies will continue to solve those problems and examine the specific role of CCR8 in GIST immunization. In conclusion, high expression of CCR8 may be related to the malignant phenotype and negatively correlated with prognosis in GISTs. CCR8 can be used as an independent predictor of GIST prognosis and a potential therapeutic target.
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract. Surgical resection remains the main treatment for GISTs. Unfortunately, more than 50% of patients eventually develop local recurrence or metastasis. Although imatinib is the standard first-line therapy for patients with metastatic or advanced GISTs, it cannot cure the disease. The prognosis of the advanced cases is not optimistic. CC chemokine receptor type 8 (CCR8) protein participates in regulation of immune responses. Recent studies on CCR8 in non-small cell lung cancer and colorectal cancer showed that it was highly expressed in tumor-infiltrating regulatory T cells and correlated with a poor prognosis. However, there are only a few references on the expression of CCR8 protein in gastrointestinal stromal tumors.
The following problems, which we need to solve urgently, are also the research motivation of this article: (1) The function of the CCR8 in tumor immunity; (2) The relationship between the CCR8 expression and GIST clinical data; and (3) The relationship between the prognosis of GIST and CCR8.
In this study, we examined the expression of CCR8 in GISTs by immuno-histochemistry to explore the clinical significance of GIST expression and its prognosis. Based on the STRING database, we predicted the potential immunological function of CCR8. This research will provide evidence and support for the diagnosis of GIST, prognostic evaluation and new tumor treatment targets.
Tissue samples were used for the tissue microarray construction. The microarrays were then subjected to immunohistochemical analyses to detect CCR8 expression. Next, Kaplan–Meier analysis was utilized to calculate the survival rate of patients with complete follow-up data, and the potential prognostic value of CCR8 was evaluated by Cox regression analysis. Finally, a Gene Ontology/Kyoto Encyclopedia of Genes and Genomes single-gene enrichment chart of CCR8 was constructed using the STRING database.
CCR8-positive signals were detected as brown or brown-yellow particles by im-munohistochemistry located in the cytoplasm. Among 125 tissue samples, 74 had CCR8 high expression, and 51 had low or negative expression. Statistical analyses suggested CCR8 was significantly correlated with tumor size, mitotic index, AFIP-Miettinen risk classification and tumor location. Kaplan–Meier and multivariate analyses showed that patients with low or negative CCR8 expression, mitotic index < 5/high-power field and tumor diameter < 5 cm had a better prognosis. Based on the STRING database, CCR8 was significantly enriched in biological processes such as tumor immunity, T lymphocyte chemotaxis, migration and pathways like the nuclear factor-κB and tumor necrosis factor pathways as well as intestinal immune regulation networks.
The expression of CCR8 protein in GISTs correlated with tumor size, mitotic index, AFIP-Miettinen risk classification. Each index suggested that the higher the degree of malignancy was associated with higher CCR8 expression. When compared with other factors (age, sex, tumor location, etc), there was no relationship with CCR8. CCR8 low expression was positively correlated with the survival of patients with GISTs, which has some implications for the prognosis of patients. CCR8 can be used as an independent factor to evaluate the prognosis of GISTs. CCR8 was significantly enriched in biological processes such as tumor immunity, T lymphocyte chemotaxis, migration and pathways like the nuclear factor-κB and tumor necrosis factor pathways as well as intestinal immune regulation networks. These will provide ideas for future research about the specific mechanism of CCR8 in tumor immunity.
Tumor immunity is currently a hotspot in cancer research, and the chemokine ligand/receptor axis plays an important role in the tumor immune mechanism of which CCR8 is the most brilliant one. Meanwhile, more and more studies focus on the immunopathogenesis of gastrointestinal stromal tumors. However, data on the relationship between CCR8 and the pathogenesis and treatment of GIST still remains unclear. This article is only a preliminary exploration of the study of CCR8 in gastrointestinal stromal tumors. The specific immunological mechanism of CCR8 in gastrointestinal stromal tumors is worthy of further exploration. We believe CCR8 could be a potential therapeutic target of GISTs.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Muguruma N, Sezer S S-Editor: Zhang L L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Sorour MA, Kassem MI, Ghazal Ael-H, El-Riwini MT, Abu Nasr A. Gastrointestinal stromal tumors (GIST) related emergencies. Int J Surg. 2014;12:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg. 2006;244:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, Corless CL, Fletcher CD, Roberts PJ, Heinz D, Wehre E, Nikolova Z, Joensuu H. Long-term results from a randomized phase II trial of standard-vs higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 751] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 4. | Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, Raymond AK, Bramwell VH, Baker LH, Maki RG, Tanaka M, Hecht JR, Heinrich MC, Fletcher CD, Crowley JJ, Borden EC. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 768] [Article Influence: 45.2] [Reference Citation Analysis (1)] |
| 5. | Patrikidou A, Chabaud S, Ray-Coquard I, Bui BN, Adenis A, Rios M, Bertucci F, Duffaud F, Chevreau C, Cupissol D, Domont J, Pérol D, Blay JY, Le Cesne A; French Sarcoma Group. Influence of imatinib interruption and rechallenge on the residual disease in patients with advanced GIST: results of the BFR14 prospective French Sarcoma Group randomised, phase III trial. Ann Oncol. 2013;24:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M, Bertulli R, Judson I. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1270] [Cited by in RCA: 1205] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 7. | Tan Y, Garcia-Buitrago MT, Trent JC, Rosenberg AE. The immune system and gastrointestinal stromal tumor: a wealth of opportunities. Curr Opin Oncol. 2015;27:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | van Dongen M, Savage ND, Jordanova ES, Briaire-de Bruijn IH, Walburg KV, Ottenhoff TH, Hogendoorn PC, van der Burg SH, Gelderblom H, van Hall T. Anti-inflammatory M2 type macrophages characterize metastasized and tyrosine kinase inhibitor-treated gastrointestinal stromal tumors. Int J Cancer. 2010;127:899-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Rusakiewicz S, Semeraro M, Sarabi M, Desbois M, Locher C, Mendez R, Vimond N, Concha A, Garrido F, Isambert N, Chaigneau L, Le Brun-Ly V, Dubreuil P, Cremer I, Caignard A, Poirier-Colame V, Chaba K, Flament C, Halama N, Jäger D, Eggermont A, Bonvalot S, Commo F, Terrier P, Opolon P, Emile JF, Coindre JM, Kroemer G, Chaput N, Le Cesne A, Blay JY, Zitvogel L. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res. 2013;73:3499-3510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 10. | Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 677] [Cited by in RCA: 803] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 11. | Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2285] [Cited by in RCA: 2231] [Article Influence: 202.8] [Reference Citation Analysis (0)] |
| 12. | Zhang S, Ma X, Zhu C, Liu L, Wang G, Yuan X. The Role of Myeloid-Derived Suppressor Cells in Patients with Solid Tumors: A Meta-Analysis. PLoS One. 2016;11:e0164514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 13. | Varn FS, Wang Y, Mullins DW, Fiering S, Cheng C. Systematic Pan-Cancer Analysis Reveals Immune Cell Interactions in the Tumor Microenvironment. Cancer Res. 2017;77:1271-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 14. | Kondo J, Chijimatsu H, Kijima T, Nagashima Y, Hayashi H, Morita K, Sakata K, Setoguchi M. [A Case of Gastric GIST with Pathological Complete Response Achieved by Long-Term Chemotherapy with Imatinib Mesylate]. Gan To Kagaku Ryoho. 2018;45:515-517. [PubMed] |
| 15. | Klug LR, Bannon AE, Javidi-Sharifi N, Town A, Fleming WH, VanSlyke JK, Musil LS, Fletcher JA, Tyner JW, Heinrich MC. LMTK3 is essential for oncogenic KIT expression in KIT-mutant GIST and melanoma. Oncogene. 2019;38:1200-1210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3911] [Cited by in RCA: 3963] [Article Influence: 165.1] [Reference Citation Analysis (0)] |
| 17. | Wang T, Zhao H, Gao H, Zhu C, Xu Y, Bai L, Liu J, Yan F. Expression and phosphorylation of FOXO1 influences cell proliferation and apoptosis in the gastrointestinal stromal tumor cell line GIST-T1. Exp Ther Med. 2018;15:3197-3202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Watson-Fargie T, Grosset DG, White J, Cowie F. Possible modulation of concurrent Parkinson's disease in the management of metastatic GIST: a review of two cases. J R Coll Physicians Edinb. 2018;48:242-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Inngjerdingen M, Damaj B, Maghazachi AA. Human NK cells express CC chemokine receptors 4 and 8 and respond to thymus and activation-regulated chemokine, macrophage-derived chemokine, and I-309. J Immunol. 2000;164:4048-4054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | De Simone M, Arrigoni A, Rossetti G, Gruarin P, Ranzani V, Politano C, Bonnal RJP, Provasi E, Sarnicola ML, Panzeri I, Moro M, Crosti M, Mazzara S, Vaira V, Bosari S, Palleschi A, Santambrogio L, Bovo G, Zucchini N, Totis M, Gianotti L, Cesana G, Perego RA, Maroni N, Pisani Ceretti A, Opocher E, De Francesco R, Geginat J, Stunnenberg HG, Abrignani S, Pagani M. Transcriptional Landscape of Human Tissue Lymphocytes Unveils Uniqueness of Tumor-Infiltrating T Regulatory Cells. Immunity. 2016;45:1135-1147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 502] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 21. | Islam SA, Ling MF, Leung J, Shreffler WG, Luster AD. Identification of human CCR8 as a CCL18 receptor. J Exp Med. 2013;210:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Karin N. Chemokines and cancer: new immune checkpoints for cancer therapy. Curr Opin Immunol. 2018;51:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 23. | Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, Feirt N, Gordon R, Nagi CS, Wang Y, Entenberg D, Condeelis J, Skobe M. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J Exp Med. 2013;210:1509-1528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Chen JJ, Wang XF, Wang Q. Clinical and prognostic significance of Raf kinase inhibitory protein expression in gastrointestinal stromal tumors. World J Gastroenterol. 2018;24:2508-2517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1304] [Article Influence: 72.4] [Reference Citation Analysis (33)] |
| 26. | Rubin BP, Blanke CD, Demetri GD, Dematteo RP, Fletcher CD, Goldblum JR, Lasota J, Lazar A, Maki RG, Miettinen M, Noffsinger A, Washington MK, Krausz T; Cancer Committee, College of American Pathologists. Protocol for the examination of specimens from patients with gastrointestinal stromal tumor. Arch Pathol Lab Med. 2010;134:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Bure I, Haller F, Zaletaev DV. Coding and Non-coding: Molecular Portrait of GIST and its Clinical Implication. Curr Mol Med. 2018;18:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Niinuma T, Suzuki H, Nojima M, Nosho K, Yamamoto H, Takamaru H, Yamamoto E, Maruyama R, Nobuoka T, Miyazaki Y, Nishida T, Bamba T, Kanda T, Ajioka Y, Taguchi T, Okahara S, Takahashi H, Nishida Y, Hosokawa M, Hasegawa T, Tokino T, Hirata K, Imai K, Toyota M, Shinomura Y. Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res. 2012;72:1126-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 29. | Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y, Karbach J, Jäger E, Sakaguchi S. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A. 2013;110:17945-17950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 531] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 30. | Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1354] [Article Influence: 150.4] [Reference Citation Analysis (0)] |
| 31. | Kimpfler S, Sevko A, Ring S, Falk C, Osen W, Frank K, Kato M, Mahnke K, Schadendorf D, Umansky V. Skin melanoma development in ret transgenic mice despite the depletion of CD25+Foxp3+ regulatory T cells in lymphoid organs. J Immunol. 2009;183:6330-6337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3582] [Cited by in RCA: 3878] [Article Influence: 184.7] [Reference Citation Analysis (0)] |
| 33. | Soler D, Chapman TR, Poisson LR, Wang L, Cote-Sierra J, Ryan M, McDonald A, Badola S, Fedyk E, Coyle AJ, Hodge MR, Kolbeck R. CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes. J Immunol. 2006;177:6940-6951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 34. | Sjödahl G, Lövgren K, Lauss M, Chebil G, Patschan O, Gudjonsson S, Månsson W, Fernö M, Leandersson K, Lindgren D, Liedberg F, Höglund M. Infiltration of CD3+ and CD68+ cells in bladder cancer is subtype specific and affects the outcome of patients with muscle-invasive tumors. Urol Oncol. 2014;32:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 35. | Diana A, Wang LM, D'Costa Z, Allen P, Azad A, Silva MA, Soonawalla Z, Liu S, McKenna WG, Muschel RJ, Fokas E. Prognostic value, localization and correlation of PD-1/PD-L1, CD8 and FOXP3 with the desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget. 2016;7:40992-41004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Asano Y, Kashiwagi S, Goto W, Kurata K, Noda S, Takashima T, Onoda N, Tanaka S, Ohsawa M, Hirakawa K. Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br J Surg. 2016;103:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 37. | Villarreal DO, L'Huillier A, Armington S, Mottershead C, Filippova EV, Coder BD, Petit RG, Princiotta MF. Targeting CCR8 Induces Protective Antitumor Immunity and Enhances Vaccine-Induced Responses in Colon Cancer. Cancer Res. 2018;78:5340-5348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 38. | Du R, Tang G, Tang Z, Kuang Y. Ectopic expression of CC chemokine receptor 7 promotes prostate cancer cells metastasis via Notch1 signaling. J Cell Biochem. 2019;120:9639-9647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Ou B, Zhao J, Guan S, Feng H, Wangpu X, Zhu C, Zong Y, Ma J, Sun J, Shen X, Zheng M, Lu A. Correction: CCR4 promotes metastasis via ERK/NF-κB/MMP13 pathway and acts downstream of TNF-α in colorectal cancer. Oncotarget. 2017;8:41779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Zhu CC, Chen C, Xu ZQ, Zhao JK, Ou BC, Sun J, Zheng MH, Zong YP, Lu AG. CCR6 promotes tumor angiogenesis via the AKT/NF-κB/VEGF pathway in colorectal cancer. Biochim Biophys Acta Mol Basis Dis. 2018;1864:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Zeng W, Chang H, Ma M, Li Y. CCL20/CCR6 promotes the invasion and migration of thyroid cancer cells via NF-kappa B signaling-induced MMP-3 production. Exp Mol Pathol. 2014;97:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Han R, Gu S, Zhang Y, Luo A, Jing X, Zhao L, Zhao X, Zhang L. Estrogen promotes progression of hormone-dependent breast cancer through CCL2-CCR2 axis by upregulation of Twist via PI3K/AKT/NF-κB signaling. Sci Rep. 2018;8:9575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 43. | Liu J, Wang C, Ma X, Tian Y, Wang C, Fu Y, Luo Y. High expression of CCR5 in melanoma enhances epithelial-mesenchymal transition and metastasis via TGFβ1. J Pathol. 2019;247:481-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Liu X, Xu X, Deng W, Huang M, Wu Y, Zhou Z, Zhu K, Wang Y, Cheng X, Zhou X, Chen L, Li Y, Wang G, Fu B. CCL18 enhances migration, invasion and EMT by binding CCR8 in bladder cancer cells. Mol Med Rep. 2019;19:1678-1686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |