Published online Mar 28, 2020. doi: 10.3748/wjg.v26.i12.1317
Peer-review started: December 6, 2019
First decision: January 16, 2020
Revised: February 21, 2020
Accepted: March 10, 2020
Article in press: March 10, 2020
Published online: March 28, 2020
Processing time: 112 Days and 15.4 Hours
We studied the protective effects of Qingyi decoction (QYD) (a Traditional Chinese Medicine) against severe acute pancreatitis (SAP)-induced myocardial infarction (MI).
To study the function and mechanism of QYD in the treatment of myocardial injuries induced by SAP.
Ultrasonic cardiography, hematoxylin and eosin staining, immunohistochemistry, qRT-PCR, western blot, enzyme-linked immunosorbent assays, and apoptosis staining techniques were used to determine the effects of QYD following SAP-induced MI in Sprague-Dawley rats.
Our SAP model showed severe myocardial histological abnormalities and marked differences in the symptoms, mortality rate, and ultrasonic cardiography outputs among the different groups compared to the control. The expression of serum cytokines [interleukin (IL)-1ß, IL-6, IL-8, IL-12, amyloid β, and tumor necrosis factor-α] were significantly higher in the SAP versus QYD treated group (P < 0.05 for all). STIM1 and Orai1 expression in myocardial tissue extracts were significantly decreased post QYD gavage (P < 0.001). There was no significant histological difference between the 2-aminoethyl diphenylborinate inhibitor and QYD groups. The SAP group had a significantly higher apoptosis index score compared to the QYD group (P < 0.001).
QYD conferred cardio-protection against SAP-induced MI by regulating myocardial-associated protein expression (STIM1 and Orai1).
Core tip: This manuscript illustrates the protective role of the Traditional Chinese Medicine, Qingyi decoction (QYD), on severe acute pancreatitis-induced myocardial injuries. QYD treatment was able to play a double role as a preventive and curative agent for both myocardial injuries and acute severe pancreatitis via the STIM1/Orai1-SOCE pathway. QYD treatment reduced myocardial injuries and the mortality rate by 30% in Sprague-Dawley (SD) rats within 24 h. Our findings reveal the success of QYD in improving the overall appearance of SD rats, together with decreasing the myocardial histological abnormalities and inflammatory cells infiltration caused by severe acute pancreatitis and myocardial injuries.
- Citation: Li L, Li YQ, Sun ZW, Xu CM, Wu J, Liu GL, Bakheet AM, Chen HL. Qingyi decoction protects against myocardial injuries induced by severe acute pancreatitis. World J Gastroenterol 2020; 26(12): 1317-1328
- URL: https://www.wjgnet.com/1007-9327/full/v26/i12/1317.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i12.1317
Severe acute pancreatitis (SAP) is one of the most common types of acute abdominal inflammatory diseases, with approximately 20% of new cases reported annually. SAP can lead to multiple organ dysfunction syndromes (MODS), such as SAP-induced myocardial injury (MI), which is associated with an approximate 10%-30% mortality rate among SAP patients[1-3]. Globally, the SAP mortality rate ranges between 15%-30%, while in China the rate is 11.8%-16.6%[4]. SAP is characterized by sudden onset of severe persistent upper abdominal pain, which is epigastric and radiates towards the back. SAP results from pathological dysfunction of the pancreatic microcirculation, which leads to pancreatic vascular thrombosis and reduced pancreatic blood flow[5]. Without prompt intervention, SAP progresses rapidly, leading to irreversible multiple organ damage and early death[6-8].
MI is a predominant complication of SAP-induced MODS[9,10]. Synchronized manifestations of myocardial dysfunction[11], such as abnormal electrocardiography, troponin imbalance, myocardium abnormalities, altered cardiac input/output ratio, arrhythmia, cardiac shock, myocarditis, pericarditis, and other types of MIs, have been reported in SAP patients[6,7,12]. Chinese herbs and Traditional Chinese Medicine (TCM) have long been used in China to treat various diseases[8,13-20]. Qingyi decoction (QYD), a TCM that includes several medicinal plants and minerals (Supplementary Table 1), has been used to treat acute pancreatitis in animal models.
| No | Primer | Sequence |
| 1 | GAPDH-F | 5’-TGTGTCCGTCGTGGATCTGA-3’ |
| 2 | GAPDH-R | 5’-TTGCTGTTGAAGTCGCAGGAG-3 |
| 3 | STIM1-F | 5’-TCCACCTGGAGAAGAAGCTG-3 |
| 4 | STIM1-R | 5’-CCTCAGGAGCATACCAGGAG-3’ |
| 5 | Orai1-SOCE-F | 5’-CGCCCTCATGATCAGTACCT-3’ |
| 6 | Orai1-SOCE-R | 5’-AGAACTTCACCCAGCAGAGC-3 |
QYD can cause purgation, promote hemostasis, and stimulate anti-inflammatory responses. Moreover, the role of QYD was illustrated in our previous study using an acute pancreatitis model, in which we reported that QYD reduced lung injury and protected pulmonary function[7,8,16]. Previous studies have also shown that QYD regulates the immune response in the gut. Specifically, QYD controls intestinal cell electrical activity by maintaining smooth muscle physiological function and directly neutralizing endotoxins that protect the intestinal barrier[5,9,10,12,13,21,22].
Our previous studies demonstrated that QYD protects against acute pancreatitis by suppressing G protein-coupled bile acid receptor 1 expression, subsequently inhibiting caspase-9 activation. Furthermore, QYD blocked the NF-kb/p-RIP inflammatory signaling pathway in an in vitro acute pancreatitis model, de-monstrating a beneficial effect by decreasing apoptosis of alveolar type II epithelial cells in a SAP rat model[11,23]. Moreover, Su et al[24] and Zhang et al[6] showed that QYD can ameliorate intestinal barrier damage by inhibiting overexpression of secretory phospholipase A (sPLA2) and Toll-like receptor 4, and increasing occludin-1 expression in a SAP rat model. Recent studies demonstrated that QYD can attenuate SAP-induced MI[12,21,22].
Microvascular endothelial cell apoptosis is controlled by the store-operated calcium entry (SOCE) associated proteins STIM1 and Orai1, by regulating intracellular calcium ion concentration; however, calcium flux is closely associated with cardiac cell damage[1-3,14]. Based on these findings, we hypothesized that QYD protects against SAP-induced MI via the STMI1/Orai1-SOCE pathway, resulting in regulation of cell apoptosis and pathogenesis.
Forty adult healthy male Sprague-Dawley (SD) rats, weighing between 180–220 g, were obtained from the Specific Pathogen Free Animal Department of Dalian Medical University (Dalian, China), and the study protocol followed the ethical guidelines approved by the Dalian Medical University Animal Care and Use Committee. The rats were randomly divided into four groups (n = 10 per group): (1) Healthy control (Sham); (2) SAP; (3) 2-aminoethyl diphenyl borinate (2-APB), which is a STIM1/Orai1-SOCE pathway inhibitor; and (4) QYD treatment.
The SAP model was induced by injecting 5% sodium taurocholate. Briefly, animals were fasted for 12 h with free access to water before surgery. Rats were anesthetized with 1% pentobarbital sodium anesthesia (intraperitoneal injection, 40 mg/100 g body weight) prior to surgery. A midline incision was made in the rat abdomen, and the pancreatic bile duct was identified. Sodium taurocholate 5% (1 mL/kg, cat no: T4009; Sigma-Aldrich, St. Louis, MO, United States) was retrogradely injected into the distal end of the pancreatic bile duct at a rate of 0.2 mL/min using a 1 mL syringe and 28-gauge needle. The injector needle was opened in the bile duct through the bile duct-duodenal connection, avoiding damage to the bile duct. The artery was clamped to prevent injecting the drug into the liver[13].
Following the establishment of SAP, rats in the QYD group received three doses of QYD orally (1 mL/100 g body mass): One dose immediately after SAP induction (0 h), then one after 12 h and 24 h, respectively[13]. The Sham group received no intervention, while the SOCE inhibitor group received 2-APB (2 mg/kg body mass) orally immediately after SAP induction. The Sham group only received laparotomy and subsequent exposure of the duodenum and was given the same treatment as the SAP group but with phosphate buffered saline (PBS). The rats were then placed in suitable living conditions with only free access to water. After 24 h, mortality and morbidity were monitored. The rats were anesthetized with 1% pentobarbital sodium anesthesia (intraperitoneal injection, 40 mg/100 g body weight) before sacrifice by ex-sanguination, and heart tissues and blood specimens were harvested under sterile conditions for analyses.
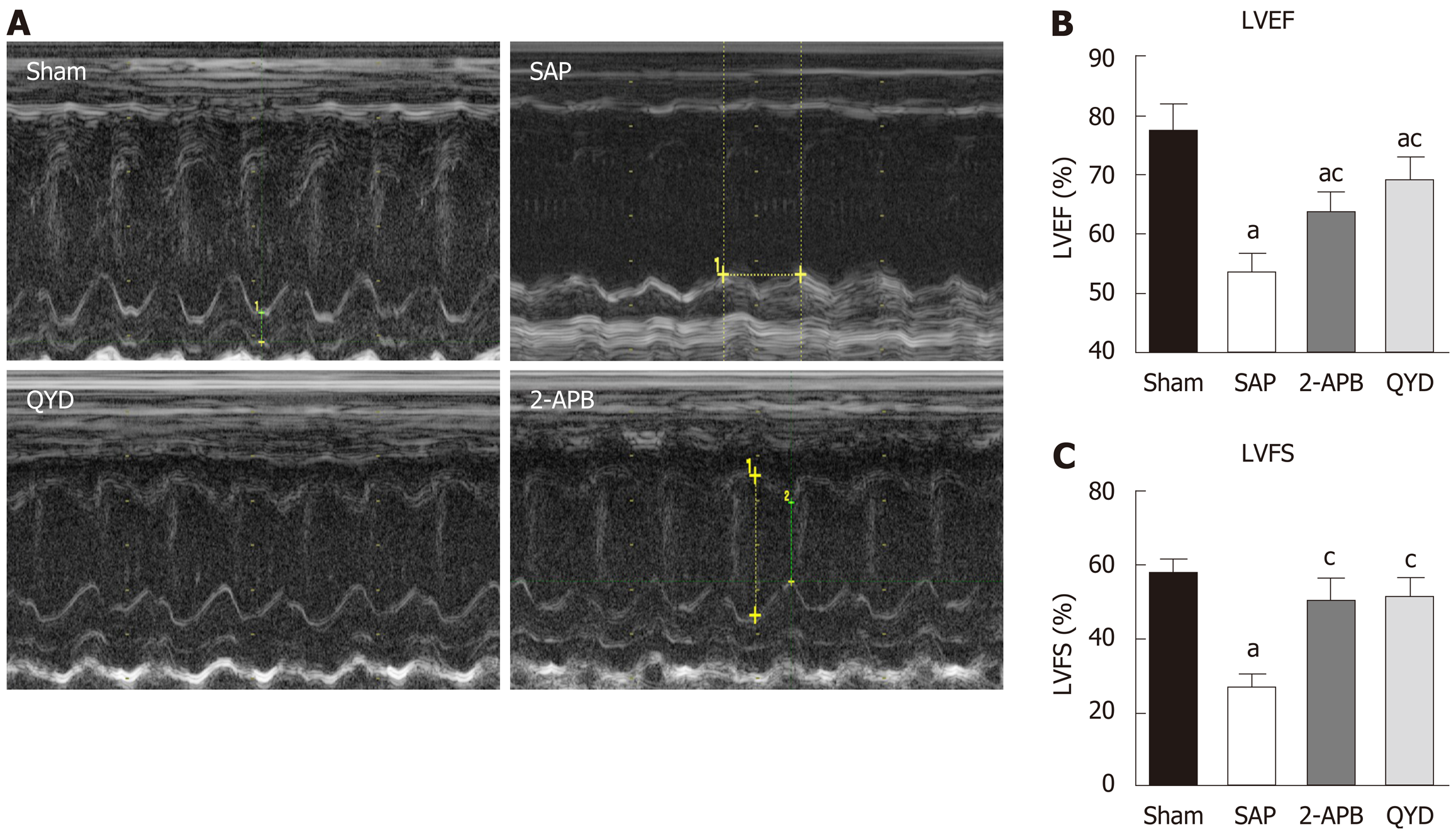
Transthoracic echocardiography assessment of left ventricular (LV) function was conducted 24 h after SAP induction and prior to euthanizing the rats. Heart function measurements were performed using the GE Logiq E9 ultrasound system with a probe (ML6–15-D). Cardiac function was assessed by a sonographer to determine changes in cardiac muscle function under 10% CH anesthesia. M-mode images of LV dimensions were obtained from the LV ejection fraction (LVEF %) and LV fractional shortening (LVFS %), as previously described, to distinguish cardiac function from myocardial infarction. Data were analyzed by a cardiologist blinded to the study.
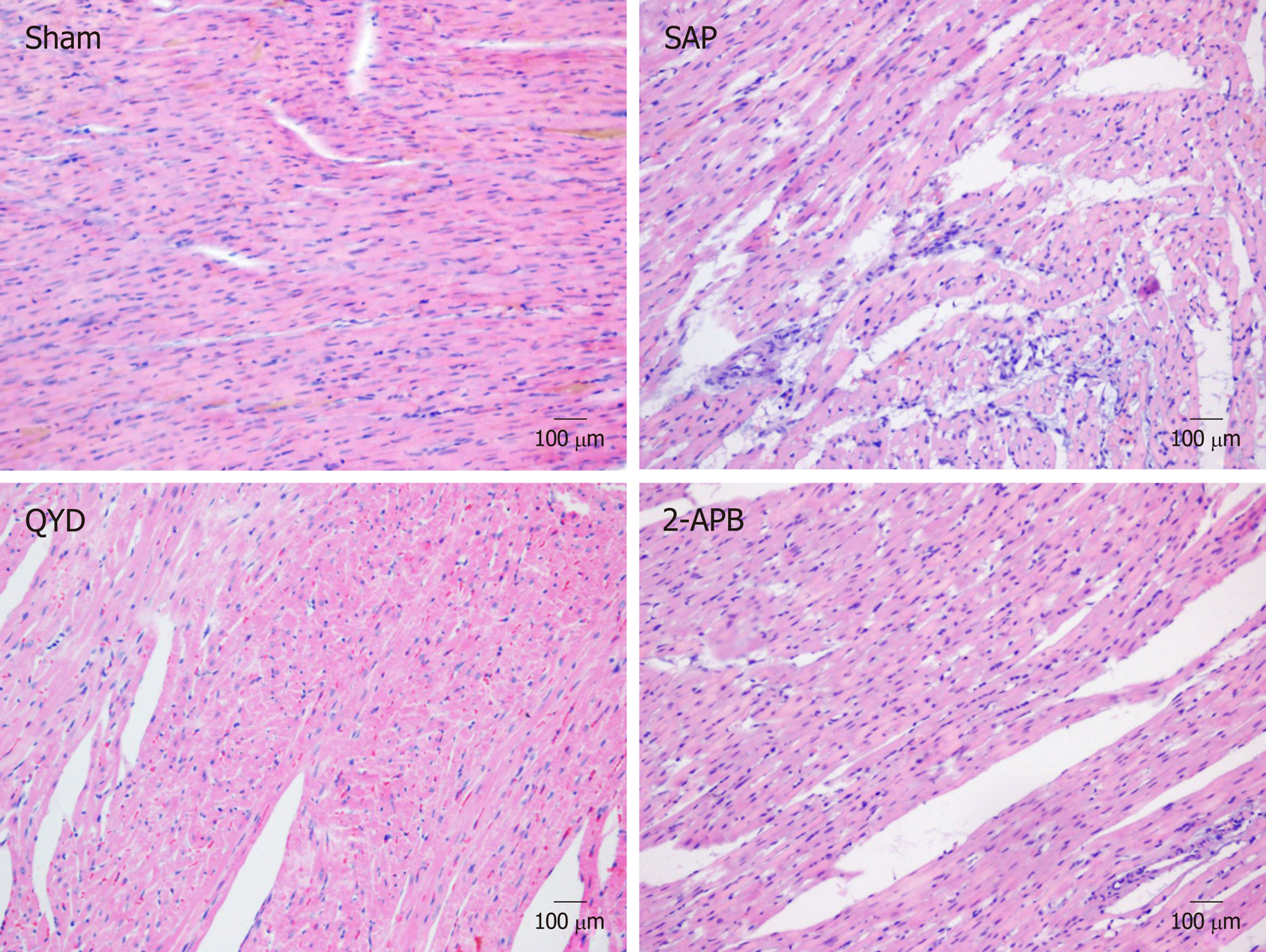
To evaluate morphological alterations in the myocardial tissue, rats were euthanized 24 h post-treatment and the LV myocardial tissues were collected and fixed in 10% formalin, processed, and embedded in paraffin wax. Thin sections (3-5 μm) were cut and mounted on microscopy slides. The sections were deparaffinized in xylene solution, rehydrated in decreasing serial dilutions of ethanol, and stained with hematoxylin for 10 min, washed with running tap water, decolorized for 3 s with 1% acid, stained with eosin for 1 min, washed with water, and left to air dry. The sections were covered with coverslips and a professional pathologist, who was blinded to the experimental design, examined the slides under a light microscope at a magnification of 40 ×.
Enzyme-linked immunosorbent assay (ELISA) was used to estimate the concentrations of inflammatory cytokines, including interleukin (IL) IL-1ß, IL-6, IL-8, IL-12, tumor necrosis factor alpha (TNF-α), and amyloid β (AMY), in rat serum. Serum samples were incubated at room temperature for a maximum of one hour before assaying. Protein concentrations were measured twice using rat IL-1ß, IL-6, IL-8, IL-12, TNF-α, and AMY ELISA kits (Cusabio Biotech, China) according to the manufacturer’s instructions. Measurements were recorded using a spectrophotometer (Thermo Scientific, United States) at 450 nm.
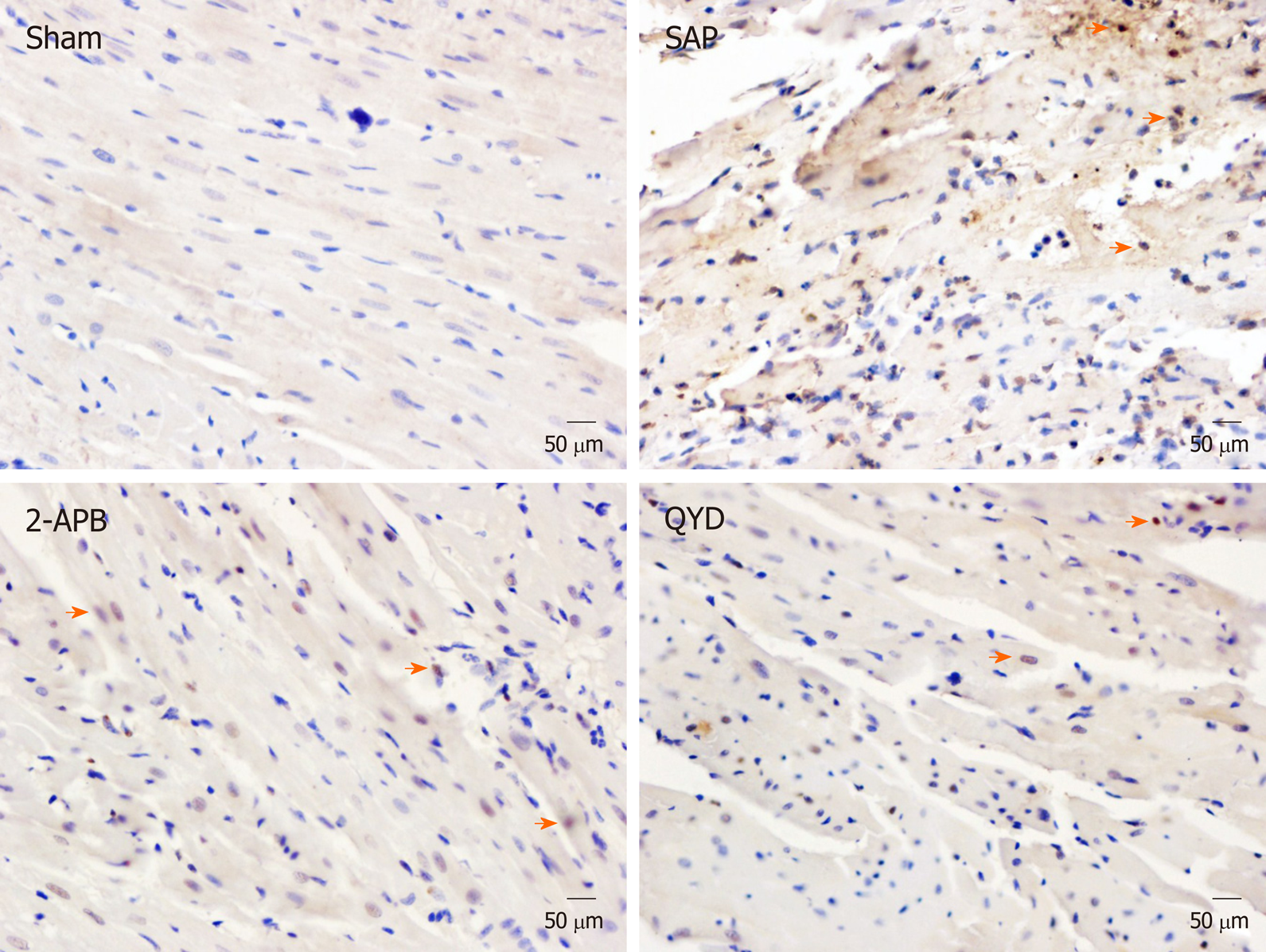
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) was performed on the tissue sections to assess apoptosis. Tissue sections were treated with 50 µL of the TUNEL reaction mixture and incubated in a humidified atmosphere for 60 min at 37 °C in the dark, and then rinsed with PBS (pH 7.4) three times, for 5 min each time, followed by 5 min staining with DAPI at room temperature, three 5 min PBS washes, and observed using fluorescence microscopy. The TUNEL-positive cells were marked by yellow/brown nuclei, whereas negative cells stained blue. The apoptotic index was calculated as the percent of TUNEL-positive nuclei relative to the total number of nuclei.
To determine STIM1 and Orai1 gene expression, total mRNA was extracted from the LV myocardial tissues from all groups using the TRIzol reagent (Invitrogen, United States) according to the manufacturer’s protocol. mRNA (500 ng) from each group was added to the super mix SYPR green II 5× reagents and DNA remover for cDNA synthesis (Transgene Biotech, China). A total volume of 20 μL was placed in the thermocycler (Bio-Rad, Singapore) and PCR was conducted at 42 °C for 15 min and then 85 °C for 5 s. Gene amplification conditions were: 94 °C for 30 s, 94 °C for 5 s, 55 °C for 15 s, and 72 °C for 10 s for 45 cycles. MXP/CXP software (Agilent Technologies, Germany) was used to analyze the PCR output, and GAPDH served as the internal control. The Ct2ΔΔ-CT method was used to calculate the relative mRNA expression of the different genes[25]. The primer sequences are listed in Table 1.
LV tissue was frozen in liquid nitrogen and then crushed for total protein extraction. Radio-Immune Precipitation Assay lysis buffer containing Dithiothreitol, Phenyl Methane Sulfonyl Fluoride and 100 × antiprotease (Roche, China) was used for total protein extraction, according to the manufacturer’s protocol. Protein concentrations were estimated using the Bicinchoninic acid assay kit (Thermo Scientific, United States) and standard bovine serum albumin. Protein concentration was quantified using a spectrophotometer (Thermo Scientific, United States) at 594 nm.
Western blot analysis was used to measure STIM1 and Orai1 protein expression in the LV myocardial tissues. Equal concentrations of proteins were separated according to their molecular weight using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were transferred onto 0.02 mm polyvinylidene fluoride membranes (Millipore, United States), and blocked with 5% low-fat silk milk (Claix, France) for 3 h at room temperature. The membranes were subsequently incubated with STIM1 monoclonal rabbit anti-rat primary antibody (1:2500; Abcam, United States), Orai1 monoclonal rabbit anti-rat primary antibody (1:2500; Abcam, United States), and GAPDH polyclonal antibody (1:1000; Proteintech, United States) overnight at 4°C. Polyvinylidene fluoride membranes were washed thrice in Tris-buffered saline with Tween 20 and incubated with goat anti-rabbit secondary antibodies (1:1000; LI-COR, United States). Protein bands were detected using a LI-COR scanner (LI-COR, United States). Tris-buffered saline with Tween 20 was used for all antibody dilutions. Quantification of protein expression was achieved using Image J software (International Institute of Health, United States).
Immunohistochemistry (IHC) was performed to determine the expression and location of STIM1 and Orai1 in the LV myocardial tissues. Tissue sections were prepared as described above for HE staining. The sections were hydrolyzed by placing them in three changes of xylene for 3 min each time, followed by washes in decreasing ethanol concentrations for 2 min per wash. Proteins were retrieved by heating the slides in citrate buffer (pH 6.0) in a microwave for 20 min. The tissues were then incubated in 3% H2O2 for 10 min at room temperature, followed by incubation in the blocking reagent (A) in the IHC kit (ZSGB-BIO, China) for 25 min at room temperature. The tissues were then incubated with STIM1 and Orai1 monoclonal rabbit anti-rat primary antibodies (1:2500; Abcam, United States) overnight at 4 °C, and then washed three times with cold PBS, for 5 min each time. The secondary antibody (B) in the IHC kit (ZSGB-BIO, China) was applied to the tissues for 30 min at room temperature, followed by washing with cold PBS three times, for 5 min each time. Subsequently, the tissues were treated with labeling reagent (C) in the IHC kit (ZSGB-BIO, China) for 30 min at 37 °C. Lastly, diaminobenzidine (ZSGB-BIO, China) was applied at room temperature for 1 min for protein visualization. The tissues were serially dehydrated in diluted alcohol and xylene and air-dried. Coverslips were mounted on the tissue sections and examined under a light microscope at a magnification of 40 ×.
SPSS version 17 (IBM, United States) and Graph Pad Prism version 6 (Graph Pad Software Inc., United States) statistical analysis software were used to analyze the data. Survival rates were calculated using an unpaired t-test. ANOVA and the t-test were used to compare differences between groups. Data are presented as mean ± SE. Statistical significance was set at P < 0.05.
The general condition of the rats was monitored 24 h after SAP induction and treatment. The Sham group appeared healthy, with no mortalities or abnormalities observed. The SAP group demonstrated several signs of abnormalities, including paralysis, laziness, depression, lack of responsiveness, and shortness of breath. We observed 40% (n = 4) mortality in the SAP group (Table 2). The QYD group showed significantly fewer signs of morbidity compared to the SAP group, and the survival rate was 90% (Table 2). Similarly, the 2-APB group exhibited less morbidity and significantly higher survival rates (Table 2). These observations suggest that QYD treatment reduces SAP-induced morbidity and mortality.
| Group | Number | Morbidity | % | Mortality | % | P value |
| Sham | 10 | 0 | 0 | 0 | 0 | - |
| SAP | 10 | 10 | 100 | 4 | 40 | < 0.0001 (Sham vs SAP) |
| QYD | 10 | 10 | 100 | 1 | 10 | 0.0047 (SAP vs QYD) |
| 2-APB | 10 | 10 | 100 | 1 | 10 | NS (QYD vs 2-APBSAP) |
Rats in all groups were subjected to echocardiography assessment 24 h after SAP induction and before euthanasia. LV circumferential strain measurements were assessed to determine physiological myocardial functions (Figure 1). As expected, cardiac function in the SAP group was lower compared to the Sham, QYD, and 2-APB groups (Figure 1A). QYD treatment significantly improved LVEF and LV fractional shortening compared to the Sham and the 2-APB groups, suggesting that QYD mitigated SAP-induced MI (Figure 1B and C).
To evaluate tissue damage caused by SAP, LV myocardial sections were stained with HE and examined by a blinded professional pathologist. The Sham group showed intact heart muscles without any signs of inflammation or necrosis. In contrast, the SAP group showed increased tissue necrosis, with an increased number of inflammatory cells infiltrating the cardiac muscle, leading to reduced myocardial fiber structures and damage to the myocardial membrane (Figure 2). QYD treatment markedly reduced the alterations in histological morphology in the SAP group (Figure 2).
We measured protein expression of selected inflammatory cytokines to determine the effect of QYD on the immune response following SAP induction. As shown in Supplementary Figure 1 and Table 3, ELISA assays revealed that the serum levels of IL-1ß, IL-6, IL-8, IL-12, AMY, and TNF-α were markedly elevated in the SAP group compared to the Sham, QYD, and 2-APB groups. There were no significant differences in protein concentrations between the QYD and 2-APB groups. Thus, QYD treatment can inhibit the SAP-induced inflammatory response.
| Group | Case (n) | IL-1β | IL-6 | IL-8 | IL-12 | AMY | TNF-α |
| Sham | 10 | 09.2 ± 1.8 | 61.7 ± 4.0 | 109.8 ± 6.0 | 062 ± 6.0 | 076 ± 3.0 | 121 ± 4.0 |
| SAP | 10 | 17.3 ± 2.2 | 93.0 ± 5.0 | 116.8 ± 4.0 | 099 ± 5.0 | 154 ± 6.0 | 354 ± 6.0 |
| 2-APB | 10 | 11.0 ± 09 | 76.0 ± 8.0 | 128.0 ± 9.0 | 076 ± 7.0 | 102 ± 2.0 | 163 ± 9.0 |
| QYD | 10 | 11.9 ± 1.7 | 69.0 ± 6.0 | 132.0 ± 2.0 | 073 ± 4.0 | 114 ± 4.0 | 161 ± 7.0 |
The TUNEL assay was performed to investigate the effect of QYD treatment on myocardial cell apoptosis following SAP (Figure 3). We counted the number of apoptotic cells (brown/yellow) and total cells (blue) and then calculated the apoptotic index [(apoptotic cell number/total cell number) × 100%] in all groups. The majority of myocardial cells in the Sham group stained blue, and only a few exhibited brown/yellow staining (Figure 3). However, a significant number of myocardial cells in the SAP group exhibited brown/yellow staining (P < 0.0001) (Figure 3, Table 4), indicating increased apoptosis. Although the number of cells with brown/yellow staining in the QYD group was higher compared to the Sham group, the numbers of apoptotic cells in the QYD and 2-APB groups were significantly lower compared to the SAP group (Figure 3, Table 4). As shown in Table 4, the apoptosis index in the SAP group was significantly higher compared to the Sham group (P < 0.0001). These results suggest that QYD treatment reduces SAP-induced myocardial cell apoptosis.
| Group | Cases (n) | Apoptotic % | P value |
| Sham | 10 | 1.5 ± 0.3 | - |
| SAP | 6 | 38.8 ± 2.7 | < 0.0001 (Sham vs SAP) |
| 2-APB | 9 | 15.4 ± 1.9 | < 0.0001 (SAP vs 2-APB) |
| QYD | 9 | 13.7 ± 1.4 | < 0.0001 (SAP vs QYD) |
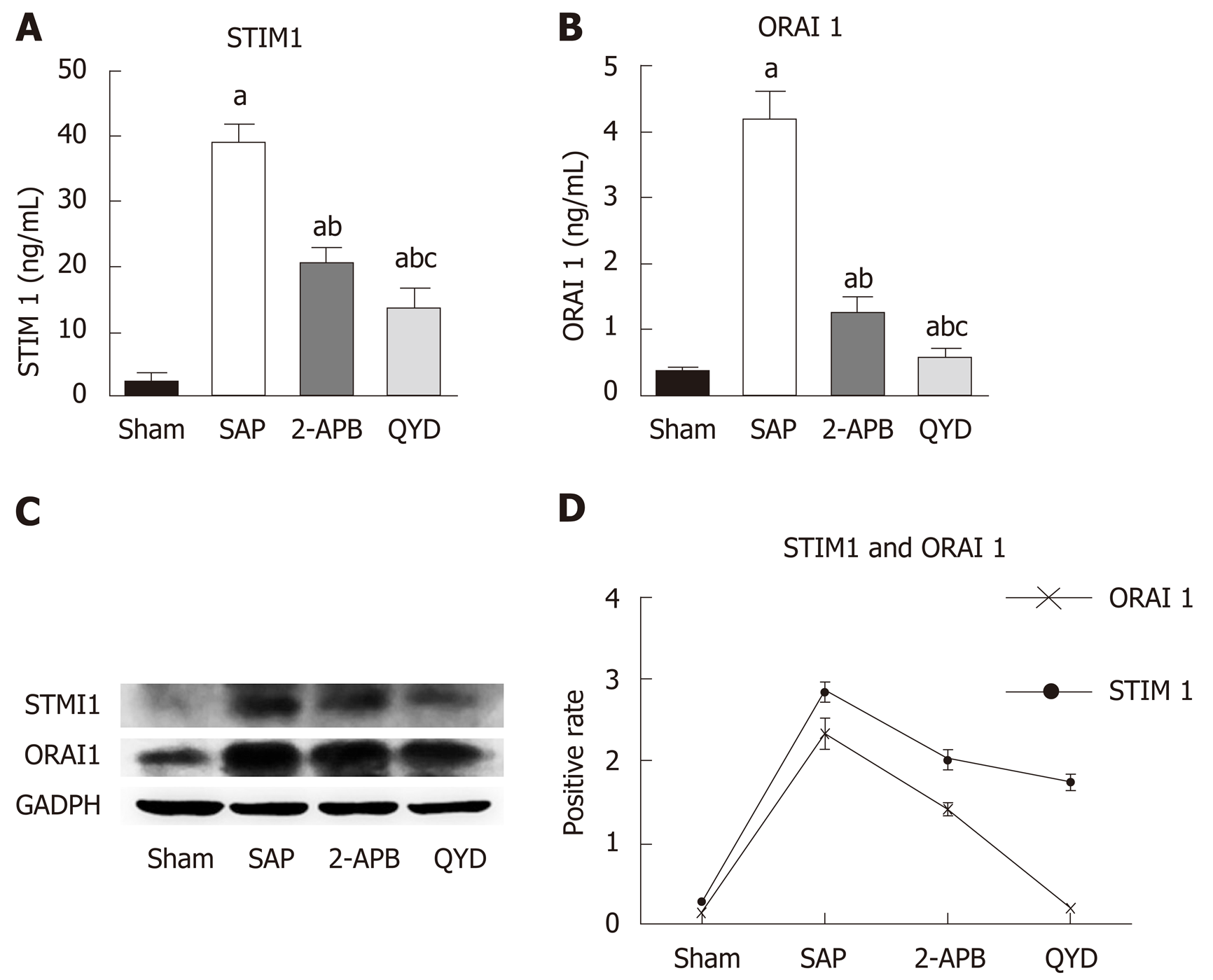
The STIM1/Orai1-SOCE pathway plays a significant role in regulating cell Ca+2 concentrations (Graph 1) and modulating cardiac function. We therefore examined the cardio-protective effects of QYD by assaying its impact on the STIM1/Orai1-SOCE pathway. As 2-APB is a known inhibitor of the STIM1/Orai1-SOCE pathway, we used this inhibitor as a comparative control. Following treatment, expression of STIM1 and Orai1 was measured using RT-PCR, western blot, and IHC. Both QYD and 2APB significantly decreased the SAP-induced increase in myocardial cell STIM1 and Orai1 gene and protein expression (Figure 4 and Supplementary Figure 2). These observations indicate that the STIM1/Orai1-SOCE pathway is activated following SAP, and that QYD treatment can mitigate SAP-induced activation of the STIM1/Orai1-SOCE pathway, conferring cardio-protection.
The goal of this study was to investigate whether the TCM QYD can protect against SAP-induced MI in a rat model. We found that QYD was able to protect against tissue damage, as well as reduce the inflammatory response and cell apoptosis. We further determined that QYD confers cardio-protection by regulating the STIM1/Orai1-SOCE pathway. These findings indicate that QYD could be used clinically to protect again SAP-induced MI and subsequent MODS.
Our results further support the literature showing that SAP can induce MI and mortality. We reported only 40% survival following SAP induction in our rat model. However, QYD treatment reduced the SAP-induced MI and lowered the mortality rate by 30%. This finding is similar to that reported for other TCM decoction subtypes (Lai Fu Cheng Qi decoction), which have also demonstrated roles in protecting against lung and intestinal injuries[13,16,19,26]. Collectively, these findings support the development of QYD and similar TCM decoctions as therapeutic approaches to prevent SAP-induced MI.
Our findings also revealed that QYD improves the overall morphological appearance of myocardial tissue following SAP. QYD decreased the myocardial histological abnormalities and infiltration of inflammatory cells caused by SAP-induced MI. QYD also attenuated myocardial cell apoptosis, enhanced cardiac functions, and partially restored the heart muscle. These results support our recently published work[27-30].
In this study, we showed that QYD treatment decreased the SAP-induced inflammatory response. Specifically, we found that both QYD and the SOCS inhibitor 2-APB significantly decreased the SAP-induced increase in an array of cytokines (IL-1ß, IL-6, IL-8, IL-12, AMY, and TNF-α). These results parallel previous findings for 2-APB[5-7,31]. Our data indicate that QYD can boost the immune response to SAP, and may therefore have preventive and therapeutic value for treating MI consistent with previous reports[17,20,32,33].
We further investigated the underlying mechanism by which QYD confers cardio-protection. Specifically, we determined the effect of QYD on the STIM1/Orai1-SOCE pathway (Graph 1). Our gene and protein expression analyses revealed that SAP increased STIM1 and Orai1 expression. These findings reflect the role of the STIM1/Orai1-SOCE pathway in SAP-induced MI. However, QYD treatment reduced the expression of STIM1 and Orai1, suggesting that QYD can stabilize Ca2+ ion flux and promote myocardial recovery, decrease cell inflammation and necrosis, and preserve cardiac function, consistent with previous studies that targeted the STIM1/Orai1-SOCE pathway[3,9,10,34]. Thus, QYD treatment could play a double role in preventing and preserving myocardial function by targeting the STIM1/Orai1-SOCE pathway.
One challenge in this study was our SAP experimental model; only a few rats survived SAP, limiting the number of rats that could be used for overall analyses.
In summary, QYD confers cardio-protection in response to SAP by controlling immune cytokine levels and cardiac tissue apoptosis by regulating the STIM1/Orai1-SOCE pathway. Further studies are needed to determine the specific mechanism by which QYD modulates Ca+2 flux in myocardial cells.
Severe acute pancreatitis (SAP) is a common acute abdominal inflammatory disease which can lead to multiple organ dysfunction syndromes including myocardial dysfunction. Qingyi decoction (QYD), a Traditional Chinese Medicine, has been used to treat acute pancreatitis in animal models. Previously we reported that QYD reduced lung injury and protected pulmonary function in patients with SAP.
The protective functions of Qingyi decoction against myocardial injuries induced by SAP will provide new therapeutic information on SAP.
To study the function and mechanism of QYD in the treatment of myocardial injuries induced by SAP.
We established a SAP model with Sprague-Dawley rats. Echocardiography was performed to assess cardiac function before the rats were euthanized. After euthanizing, hematoxylin and eosin staining of myocardial tissue was performed. Enzyme-linked immunosorbent assay was used to estimate the concentrations of inflammatory cytokines. Terminal deoxynucleotidyl transferase dUTP nick-end labeling was carried out to assess apoptosis. Real-time PCR was used to determine STIM1 and Orai1 gene expression. STIM1 and Orai1 protein expression was analyzed by Western blot. Immunohistochemistry was used to determine the location of STIM1 and Orai1 in myocardial tissue.
In the present study, our SAP model shows that QYD treatment effectively reduces SAP-induced myocardial injury (MI) associated morbidity and mortality. QYD treatment restores physiological heart function and reverses myocardial tissue damage and improves immunity in the SAP rat model. Our study shows that the STIM1/Orai1-SOCE pathway is mitigated by QYD treatment.
QYD treatment is able to play a double role as a preventive and curative agent for myocardial injuries induced by acute severe pancreatitis via the STIM1/Orai1-SOCE pathway.
This study provides insight into the function and mechanism of QYD in the treatment of SAP-induced MI in a rat model, suggesting that QYD might also be used in patients with SAP-induced MI.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Inal V, Yildiz K S-Editor: Zhang L L-Editor: Webster JR E-Editor: Zhang YL
| 1. | Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1347] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 2. | Vengadakrishnan K, Koushik AK. A study of the clinical profile of acute pancreatitis and its correlation with severity indices. Int J Health Sci (Qassim). 2015;9:410-417. [PubMed] |
| 3. | Hsu PC, Lin TH, Su HM, Lin ZY, Lai WT, Sheu SH. Acute necrotizing pancreatitis complicated with ST elevation acute myocardial infarction: a case report and literature review. Kaohsiung J Med Sci. 2010;26:200-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Chen W, Yang X, Huang L, Xue P, Wan M, Guo J, Zhu L, Jin T, Huang Z, Chen G, Tang W, Xia Q. Qing-Yi decoction in participants with severe acute pancreatitis: a randomized controlled trial. Chin Med. 2015;10:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Gruszczynska-Biegala J, Pomorski P, Wisniewska MB, Kuznicki J. Differential roles for STIM1 and STIM2 in store-operated calcium entry in rat neurons. PLoS One. 2011;6:e19285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Zhang JW, Zhang GX, Chen HL, Liu GL, Owusu L, Wang YX, Wang GY, Xu CM. Therapeutic effect of Qingyi decoction in severe acute pancreatitis-induced intestinal barrier injury. World J Gastroenterol. 2015;21:3537-3546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Zhang XM, Chen HL, Wang ZH. [Expression of secretory type II phospholipase A₂ in acute lung injury following acute pancreatitis and interventional effect of Qingyi decoction on it]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue Zazhi. 2010;22:518-521. [PubMed] |
| 8. | Li J, Zhang S, Zhou R, Zhang J, Li ZF. Perspectives of traditional Chinese medicine in pancreas protection for acute pancreatitis. World J Gastroenterol. 2017;23:3615-3623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (3)] |
| 9. | Tian G, Tepikin AV, Tengholm A, Gylfe E. cAMP induces stromal interaction molecule 1 (STIM1) puncta but neither Orai1 protein clustering nor store-operated Ca2+ entry (SOCE) in islet cells. J Biol Chem. 2012;287:9862-9872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Kurosaki T, Baba Y. Ca2+ signaling and STIM1. Prog Biophys Mol Biol. 2010;103:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Zhang GX, Zhan C, Wang K, Han J, Shang D, Chen HI. Qingyi Decoction amerliorates acute biliary pancreatitis by targeting Gpbar1/NF-kb pathway. Front Biosci (Landmark Ed). 2019;24:833-848. [PubMed] |
| 12. | Collins HE, Zhu-Mauldin X, Marchase RB, Chatham JC. STIM1/Orai1-mediated SOCE: current perspectives and potential roles in cardiac function and pathology. Am J Physiol Heart Circ Physiol. 2013;305:H446-H458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Li N, Tian Y, Wang C, Zhang P, You S. Protective effect of Lai Fu Cheng Qi decoction on severe acute pancreatitis-induced myocardial injury in a rat model. Exp Ther Med. 2015;9:1133-1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Dare AJ, Phillips AR, Hickey AJ, Mittal A, Loveday B, Thompson N, Windsor JA. A systematic review of experimental treatments for mitochondrial dysfunction in sepsis and multiple organ dysfunction syndrome. Free Radic Biol Med. 2009;47:1517-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Zhang CL, Lin ZQ, Luo RJ, Zhang XX, Guo J, Wu W, Shi N, Deng LH, Chen WW, Zhang XY, Bharucha S, Huang W, Sutton R, Windsor JA, Xue P, Xia Q. Chai-Qin-Cheng-Qi Decoction and Carbachol Improve Intestinal Motility by Regulating Protein Kinase C-Mediated Ca2+ Release in Colonic Smooth Muscle Cells in Rats with Acute Necrotising Pancreatitis. Evid Based Complement Alternat Med. 2017;2017:5864945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Zhou J, Li X, Deng P, Wei Y, Liu J, Chen M, Xu Y, Zhang D, Zhu L, Lou L, Dong B, Jin Q, Chai L. Chinese Herbal Formula, Modified Danggui Buxue Tang, Attenuates Apoptosis of Hematopoietic Stem Cells in Immune-Mediated Aplastic Anemia Mouse Model. J Immunol Res. 2017;2017:9786972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Li Y, Zhao Y, Feng L, Guo R. Comparison of the prognostic values of inflammation markers in patients with acute pancreatitis: a retrospective cohort study. BMJ Open. 2017;7:e013206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Ning JW, Zhang Y, Yu MS, Gu ML, Xu J, Usman A, Ji F. Emodin alleviates intestinal mucosal injury in rats with severe acute pancreatitis via the caspase-1 inhibition. Hepatobiliary Pancreat Dis Int. 2017;16:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Deng P, Li X, Wei Y, Liu J, Chen M, Xu Y, Dong B, Zhu L, Chai L. The herbal decoction modified Danggui Buxue Tang attenuates immune-mediated bone marrow failure by regulating the differentiation of T lymphocytes in an immune-induced aplastic anemia mouse model. PLoS One. 2017;12:e0180417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Yang R, Tenhunen J, Tonnessen TI. HMGB1 and Histones Play a Significant Role in Inducing Systemic Inflammation and Multiple Organ Dysfunctions in Severe Acute Pancreatitis. Int J Inflam. 2017;2017:1817564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Gerasimenko JV, Gryshchenko O, Ferdek PE, Stapleton E, Hébert TO, Bychkova S, Peng S, Begg M, Gerasimenko OV, Petersen OH. Ca2+ release-activated Ca2+ channel blockade as a potential tool in antipancreatitis therapy. Proc Natl Acad Sci USA. 2013;110:13186-13191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 22. | Rosado JA, Diez R, Smani T, Jardín I. STIM and Orai1 Variants in Store-Operated Calcium Entry. Front Pharmacol. 2015;6:325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Liu G, Zhang J, Chen H, Wang C, Qiu Y, Liu Y, Wan J, Guo H. Effects and mechanisms of alveolar type II epithelial cell apoptosis in severe pancreatitis-induced acute lung injury. Exp Ther Med. 2014;7:565-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Su S, Liang T, Zhou X, He K, Li B, Xia X. Qingyi decoction attenuates severe acute pancreatitis in rats via inhibition of inflammation and protection of the intestinal barrier. J Int Med Res. 2019;47:2215-2227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 133985] [Article Influence: 5582.7] [Reference Citation Analysis (1)] |
| 26. | Gong AG, Lau KM, Zhang LM, Lin HQ, Dong TT, Tsim KW. Danggui Buxue Tang, Chinese Herbal Decoction Containing Astragali Radix and Angelicae Sinensis Radix, Induces Production of Nitric Oxide in Endothelial Cells: Signaling Mediated by Phosphorylation of Endothelial Nitric Oxide Synthase. Planta Med. 2016;82:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Bulava A, Skvarilová M, Marek O, Lukl J. [Electrocardiographic changes in patients with acute pancreatitis. Case report and review of the literature]. Vnitr Lek. 2001;47:407-410. [PubMed] |
| 28. | Zhang H, Liu D, Wang X, Chen X, Long Y, Chai W, Zhou X, Rui X, Zhang Q, Wang H, Yang Q. Melatonin improved rat cardiac mitochondria and survival rate in septic heart injury. J Pineal Res. 2013;55:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | An R, Zhao L, Xi C, Li H, Shen G, Liu H, Zhang S, Sun L. Melatonin attenuates sepsis-induced cardiac dysfunction via a PI3K/Akt-dependent mechanism. Basic Res Cardiol. 2016;111:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Piquereau J, Godin R, Deschênes S, Bessi VL, Mofarrahi M, Hussain SN, Burelle Y. Protective role of PARK2/Parkin in sepsis-induced cardiac contractile and mitochondrial dysfunction. Autophagy. 2013;9:1837-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 31. | Zhang XM, Ma PA, Sun JW, Duan CN, Hou XH, Zhang YC. [Effect of Qingyi Chengqi Decoction on severe acute pancreatitis patients: a clinical study]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34:31-34. [PubMed] |
| 32. | Landesberg G, Levin PD, Gilon D, Goodman S, Georgieva M, Weissman C, Jaffe AS, Sprung CL, Barak V. Myocardial Dysfunction in Severe Sepsis and Septic Shock: No Correlation With Inflammatory Cytokines in Real-life Clinical Setting. Chest. 2015;148:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Xiping Z, Hua T, Hanqing C, Li C, Zhiwei W, Keyi W, Wei Y, Yun L, Qingyu L, Qing H, Fei W. The protecting effects and mechanisms of Baicalin and Octreotide on heart injury in rats with SAP. Mediators Inflamm. 2007;2007:19469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Harper SJ, Cheslyn-Curtis S. Acute pancreatitis. Ann Clin Biochem. 2011;48:23-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |