Published online Oct 28, 2019. doi: 10.3748/wjg.v25.i40.6145
Peer-review started: June 3, 2019
First decision: July 21, 2019
Revised: August 1, 2019
Accepted: September 9, 2019
Article in press: September 9, 2019
Published online: October 28, 2019
Processing time: 147 Days and 13.2 Hours
The current epidemiology of inflammatory bowel disease (IBD) in the multi-ethnic United Kingdom is unknown. The last incidence study in the United Kingdom was carried out over 20 years ago.
To describe the incidence and phenotype of IBD and distribution within ethnic groups.
Adult patients (> 16 years) with newly diagnosed IBD (fulfilling Copenhagen diagnostic criteria) were prospectively recruited over one year in 5 urban catchment areas with high South Asian population. Patient demographics, ethnic codes, disease phenotype (Montreal classification), disease activity and treatment within 3 months of diagnosis were recorded onto the Epicom database.
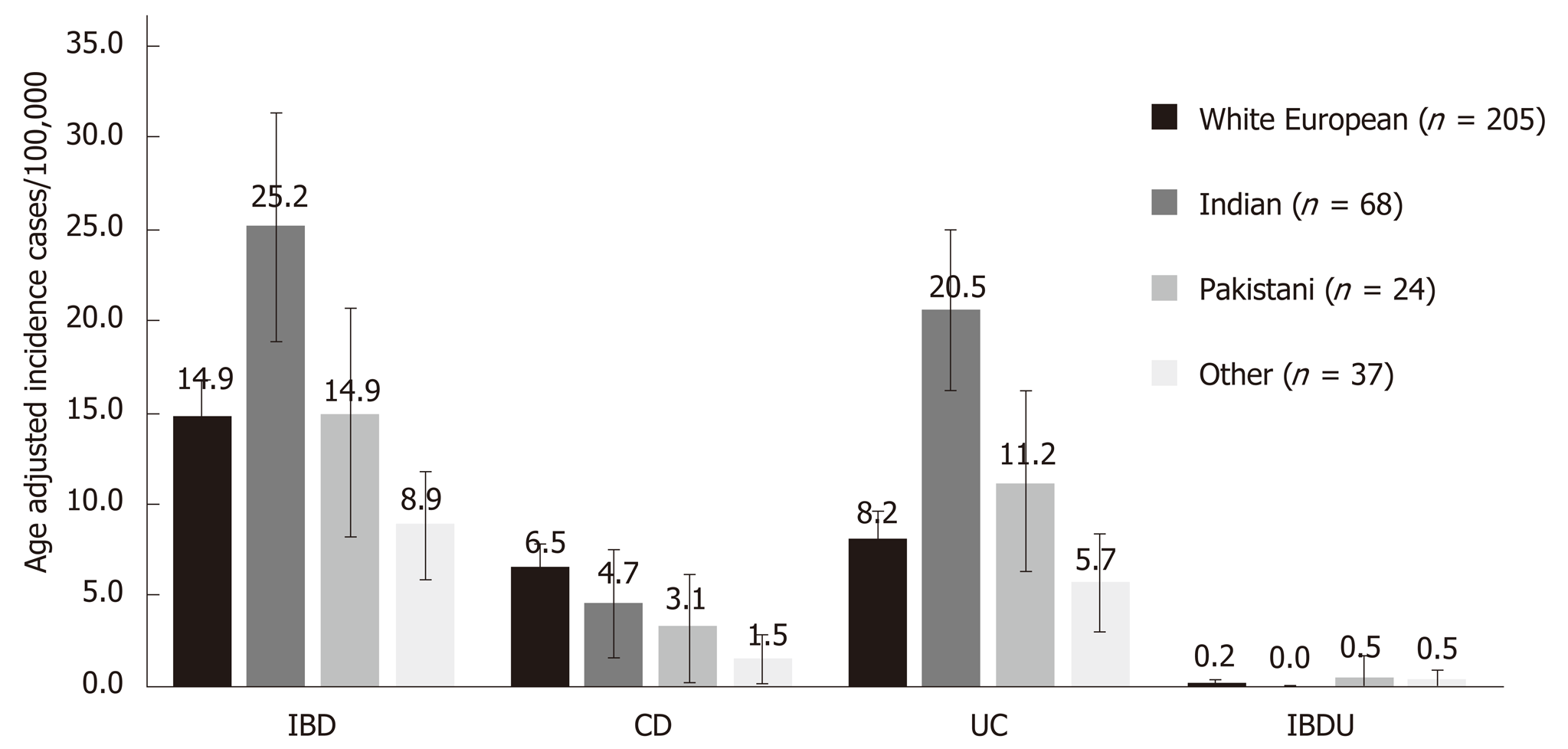
Across a population of 2271406 adults, 339 adult patients were diagnosed with IBD over one year: 218 with ulcerative colitis (UC, 64.3%), 115 with Crohn's disease (CD, 33.9%) and 6 with IBD unclassified (1.8%). The crude incidence of IBD, UC and CD was 17.0/100000, 11.3/100000 and 5.3/100000 respectively. The age adjusted incidence of IBD and UC were significantly higher in the Indian group (25.2/100000 and 20.5/100000) compared to White European (14.9/100000, P = 0.009 and 8.2/100000, P < 0.001) and Pakistani groups (14.9/100000, P = 0.001 and 11.2/100000, P = 0.007). The Indian group were significantly more likely to have extensive disease than White Europeans (52.7% vs 41.7%, P = 0.031). There was no significant difference in time to diagnosis, disease activity and treatment.
This is the only prospective study to report the incidence of IBD in an ethnically diverse United Kingdom population. The Indian ethnic group showed the highest age-adjusted incidence of UC (20.5/100000). Further studies on dietary, microbial and metabolic factors that might explain these findings in UC are underway.
Core tip: We performed a United Kingdom multicentre prospective cohort study to describe the incidence of inflammatory bowel disease and differences within ethnic groups. Seven urban centres with high ethnic background population recruited 339 cases over a 1 year period. Patients of Indian ethnicity were almost three times more likely to have UC than White Europeans. The impact of diet and environmental factors on this high risk population requires further study.
- Citation: Misra R, Limdi J, Cooney R, Sakuma S, Brookes M, Fogden E, Pattni S, Sharma N, Iqbal T, Munkholm P, Burisch J, Arebi N. Ethnic differences in inflammatory bowel disease: Results from the United Kingdom inception cohort epidemiology study. World J Gastroenterol 2019; 25(40): 6145-6157
- URL: https://www.wjgnet.com/1007-9327/full/v25/i40/6145.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i40.6145
The incidence of inflammatory bowel disease (IBD) is highest in industrialised regions of North America and Europe[1]. Incidence rates in Europe and North America are stable unlike less industrialised countries where they are increasing. Recent epidemiologic studies report rates of IBD incidence rates of 15.2/100000, 24.5/100000 and 0.5-3.4/100000 for Europe, Australia and South East Asia respectively[2,3]. The last incidence study in the United Kingdom comes from a population of 135723, which reported an incidence of 13.9/100000 for ulcerative colitis (UC) and 8.3/100000 for Crohn’s disease (CD) in a predominantly White European population (98%)[4]. Some regions in the United Kingdom have a large and increasing South Asian (SA) population extending back to a migrant flow in the 1950s[5]. Early studies of this migrant population suggested a higher UC incidence than European populations (10.8/100000 vs 5.3/100000) with an increase over time between 1980 and 1990[6-8]. However, the disease phenotype in the SA population was not homogenous. For instance the Bangladeshi population were less likely than White Europeans to have UC and presented with aggressive CD[9,10]. Differences in incidence and phenotype have also been reported for African-Americans, Hispanics and East Asians in the United States[11-14]. The reasons for these differences are unclear. An environmental trigger on genetically susceptible individuals is likely, given that migrants tend to adopt the disease incidence of their adopted country rather than country of origin[15]. Further studies on migrant populations may offer new insights into disease pathogenesis[16]. The prevailing reference point for the epidemiology of IBD in the United Kingdom is drawn from retrospective, single centre studies carried out almost two decades ago[17]. We aimed to describe incidence and phenotype of IBD in the United Kingdom across ethnic groups as an update on the previous literature and to justify future explanatory studies.
The study populations were determined by examining the latest United Kingdom census data from 2011[5]. All United Kingdom residents are legally bound to complete the national survey sent out every 10 years. Over 94% of the population completed the census survey in 2011, which required respondents to select their ethnic group from a list of standardised categories set up and defined by the National Statistics Harmonisation group[18]. These are White, Mixed ethnic group, Asian, Black/African/Caribbean and Other ethnic groups. The Asian category is subdivided into Indian, Pakistani, Bangladeshi, Chinese and Asian Other. White and two SA groups (Indian and Pakistani, 44.3% of ethnic groups) offered the largest population sizes and comparisons between these three were undertaken.
High ethnically diverse populations were identified by examining the census data and selecting regions where more than 10% of the background population was non-White European. The census areas are divided into local authorities. The selection of recruiting hospitals was based on matching the hospital catchment area to the local authority area. For example, Birmingham is the largest single local authority area in England with a population of over one million. Therefore, three hospitals serving central Birmingham were recruited to cover the relevant area. Seven centres covering a population of 2271406 with a SA population of 443303 participated: Northwest of England (Pennine NHS Trust, Northeast Manchester), East Midlands (University Hospitals of Leicester), four in the West Midlands (University Hospital Birmingham, Heart of England NHS Trust, South West Birmingham NHS Trust and Royal Wolverhampton NHS Trust) and North West London (London North West NHS Trust. Each centre has its own defined catchment area and the investigators at each centre formed the United Kingdom Inception Epidemiology study group. To ensure patients came from the background population, patients living outside the designated uptake area at the time of diagnosis were excluded.
All new diagnoses of adult IBD aged 16 years over a one-year period commencing February 1st, 2016 and living in the predefined catchment areas by postcode were prospectively included. Cases were required to meet the Copenhagen Diagnostic Criteria for CD and UC to conform with the case definitions used in the previously validated Epi-IBD study[19,20].
Copenhagen diagnostic criteria for CD (at least two of the criteria present): (1) History of abdominal pain, weight loss and/or diarrhoea for more than three months; (2) Characteristic endoscopic findings of ulceration (aphthous lesions, snail track ulceration) or cobble stoning or radiological features of stricture or cobble stoning; (3) Histopathology consistent with CD (epithelioid granuloma of Langerhans type or transmural discontinuous focal or patchy inflammation); and (4) Fistula and/or abscess in relation to affected bowel segments.
Copenhagen diagnostic criteria for UC (all three of the criteria present): (1) History of diarrhoea and/or rectal bleeding and pus for more than one week or repeated episodes; (2) Characteristic endoscopic findings of continuous ulceration, vulnerability or granulated mucosa; and (3) Histopathology consistent with UC (neutrophils within epithelial structures, cryptitis, crypt distortion, crypt abscesses).
Cases with endoscopic and histologic evidence of chronic IBD without fulfilment of diagnostic criteria for UC and CD but in need of treatment were classified as IBD unclassified (IBDU). Each case was then examined by the author (RM) to ensure the diagnostic criteria were fulfilled.
In the United Kingdom, IBD patients are diagnosed and managed in secondary care by a dedicated gastroenterology team. Incident IBD cases were identified through three pathways: (1) New referrals from primary care with signs and symptoms of IBD; (2) New IBD patients referred by the attending internal physician or surgeon at the time of hospitalisation; and (3) Patients referred from endoscopy with IBD. Endoscopy, histology and radiology reports were searched on a weekly basis for key words: UC, colitis, CD and proctitis to enhance case capture. Participating centres were asked to screen endoscopy, radiology and histology reports on a weekly basis to identify incident cases. Centres were given expected incidence targets calculated based on the background population. Each centre was required to submit their recruitment figures monthly. Centres falling behind the expected recruitment target were quickly identified. The recruitment methodology was checked and advice given to ensure all methods of a case capture were being utilised. Cases could still be entered on the database if discovered after the recruitment period up to 6 months after the end of recruitment; this gave additional time for cases which were initially missed and subsequently identified to be included in the study. Case report forms were provided to the centres for data collection. IBD phenotype was classified according to Montreal classification[21]. All treatments commenced within the first three months after diagnosis either single or in combination were defined as reported. A surgical intervention was defined as a surgical procedure related to IBD within three months of diagnosis.
Data were collected using standardised case report forms at the first patient visit within three months of diagnosis and entered onto the validated Epicom database[22]. The database was modified to include ethnicity reporting, country of birth and time living in country. The data entry schemes included diagnostic criteria scheme comprising data on diagnosis, disease extent, behaviour and patient demographic details. Smoking status and family history of IBD were also recorded. Disease activity at diagnosis was recorded by the physician using Harvey–Bradshaw index (HBI) for CD and Simple Clinical Colitis Activity Index (SCCAI) for UC [23,24]. Patients were provided with an environmental questionnaire designed by the International Organisation of IBD. Participants were asked to complete their responses and return the questionnaire to their local centre.
A monthly remote monitoring exercise was performed to verify captured cases. Random selection of a patient number was followed by request for relevant participating site to send anonymised copies of endoscopy, histology and clinic reports. These were cross checked with the corresponding information on the database by the authors (RM and SS). Written feedback was given to centres to correct any errors identified. Missing data were highlighted and relevant centres informed. Data input was monitored in real time.
The study was approved by the London Northwest NHS Trust and National Research Ethics Service (REC number14/EM/1290). Study recruitment was supported by National Institute of Health Research Clinical Research Network United Kingdom with dedicated research nurses at each centre.
The statistical review of the study was by a statistican, Paul Bassett of St. Marks Hospital and Academic Institute.
Crude incidence rates were calculated for IBD, CD, and UC based on the number of patients diagnosed compared with the total population at risk. Incidence rates were age standardised using the European age standardised rate and reported with 95% confidence intervals (CI), assuming a Poisson distribution[25]. Data from the Indian and Pakistani cohorts were compared with the White European cohort using the Mann–Whitney U-test for continuous variables such as age at diagnosis, and the chi-squared test for categorical variables. P values < 0.05 were considered statistically significant.
Across a population of 2271406 adults from five regions, 339 patients were diagnosed with IBD over the year study period: 192 (56.6%) were male. In total 218 patients were diagnosed with UC (64.3%), 115 with CD (33.9%) and 6 with IBDU (1.76%). The absolute population sizes of the White European, Indian, Pakistani and Other ethnic groups were 1387047, 239814, 152264 and 492461 respectively.
The crude incidence of IBD, UC and CD for the total population was 17.0/100000, 11.3/100,000 and 5.3/100000 respectively. The distribution of UC in the total population was 23.7% proctitis (n = 51), 34.4% left sided disease (n = 74) and 41.9% with extensive involvement (n = 90). The overall CD disease location showed ileal involvement in 34.4% (n = 40), colonic disease 31.2% (n = 36) and ileo-colonic disease in 31.0% (n = 35). Disease behaviour was inflammatory in 75.9% (n = 88) and stricturing 16.4% (n = 19). Perianal involvement was noted in 7.8% (n = 14) of cases at diagnosis.
The demography of the main three populations is shown in Table 1. Ethnicity data was missing for 5/339 cases. The median age at diagnosis for CD was lowest in the Other ethnic groups (19 years) and highest in the White European group, however there were no statistical differences between the groups. There was a greater male predisposition to IBD in Indian (61.8%) and Pakistani (66.7%) groups compared with White Europeans (50.2%) although not significant. Indians, Pakistanis and other ethnic groups with UC were significantly more likely to be non-smokers than White Europeans (P = 0.0001, P = 0.01, P = 0.0001 respectively). The majority of patients of Indian ethnicity (85.2%) were born in the United Kingdom. There was no significant difference in family history of IBD between ethnic groups (5.7% Whites, 6.6% Indians and 11.1% Pakistanis).
| White European (n = 205) | Indian(n = 68) | Pakistani (n = 24) | Other (n = 37) | |
| Male sex, n (%) | 109 (53.2) | 42 (61.8) | 16 (66.7) | 25 (83.3) |
| Median age, yr (IQR range) | ||||
| CD | 35 (25-54) | 32 (29-35) | 29 (23-44) | 19 (16-27) |
| UC | 40 (28-59) | 38 (29-50) | 29 (23-37) | 34 (25-45) |
| Median time from symptom onset to diagnosis, mo (IQR) | ||||
| CD | 2.9 (0.9-8.5) | 3.0 (2.0-6.0) | 3.5 (2.0-5.3) | 3 (2.0-3.2) |
| UC | 2.3 (1.0-6.0) | 2.5 (0.98-4.0) | 2.7 (2.0-6.2) | 2.5 (1.7-3.4) |
| Smoking history, n (%) | ||||
| CD | ||||
| Never | 15 (22.0) | 3 (33.3) | 2 (40) | 3 (60) |
| Ex-smoker | 35 (51.4) | 3 (33.3) | 1 (20) | 1 (20) |
| Current | 18 (26.4) | 3 (33.3) | 2 (40) | 1 (20) |
| UC | ||||
| Never | 35c (38.0) | 28a (75.7) | 7b (77.8) | 17a (85.0) |
| Ex-smoker | 35 (38.0) | 7 (19.0) | 1 (11.1) | 2 (10.5) |
| Current | 22 (24.0) | 2 (5.4) | 1 (11.1) | 1 (5.3) |
| Born in the United Kingdom (%) | 91.2 | 85.2 | 66.6 | 89.9 |
| Family history of IBD, n (%) | 9 (5.7) | 4 (6.6) | 2 (11.1) | 1 (3.3) |
The crude and age adjusted incidence rates are shown in Figure 1 and Table S1. The age adjusted incidence of IBD and UC was higher in the Indian group (25.2/100000 and 20.5/100000) compared with White European and Pakistani groups. Most Indian UC cases (45/55: 81.8%) were diagnosed in Leicester and North West London.
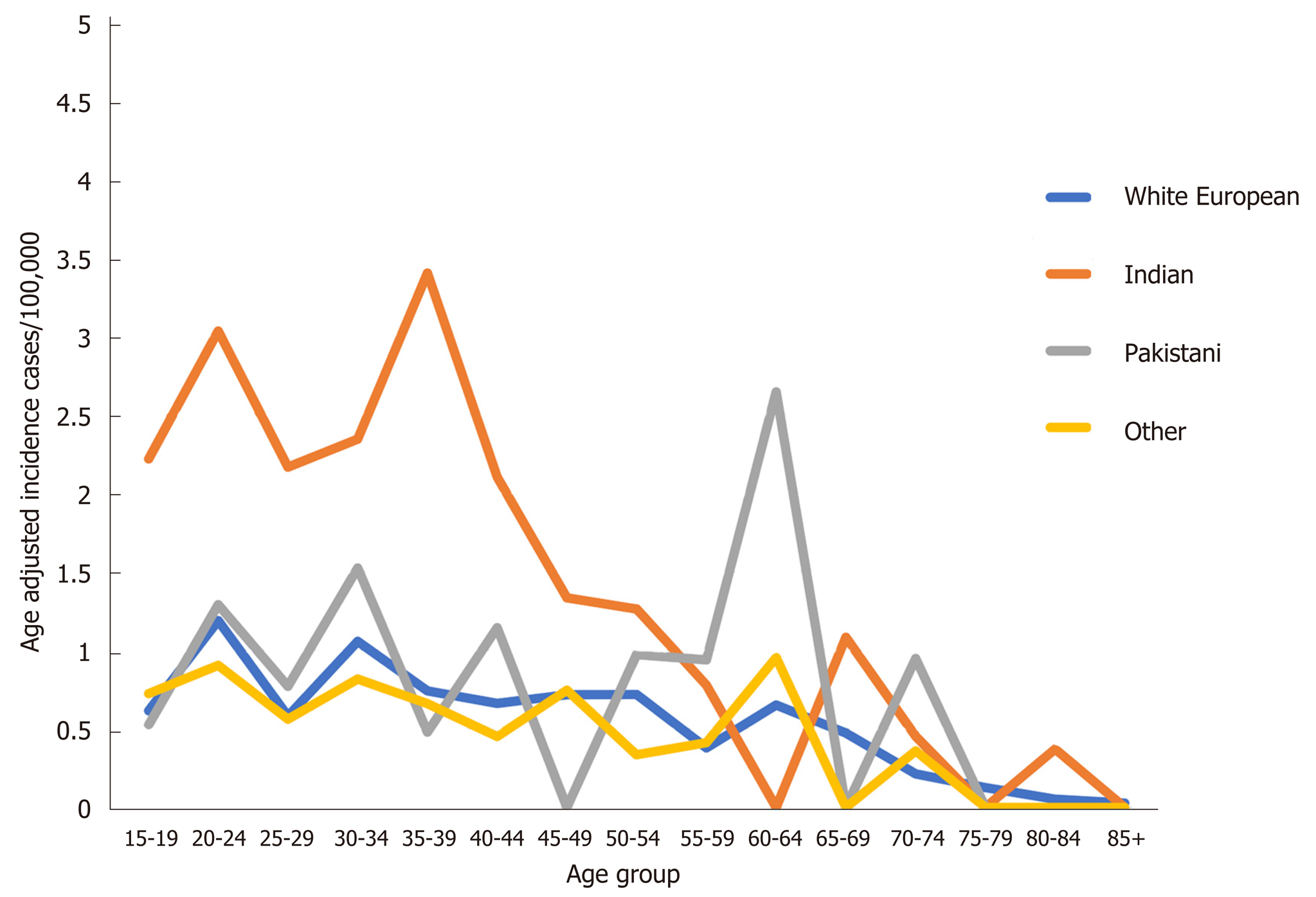
The variation in incidence rate by age is shown in Figure 2. There is a consistently higher incidence of UC in the Indian group between 15-40 years compared to the other groups. In comparison the pattern of CD incidence was similar across ethnic groups (Figure S1).
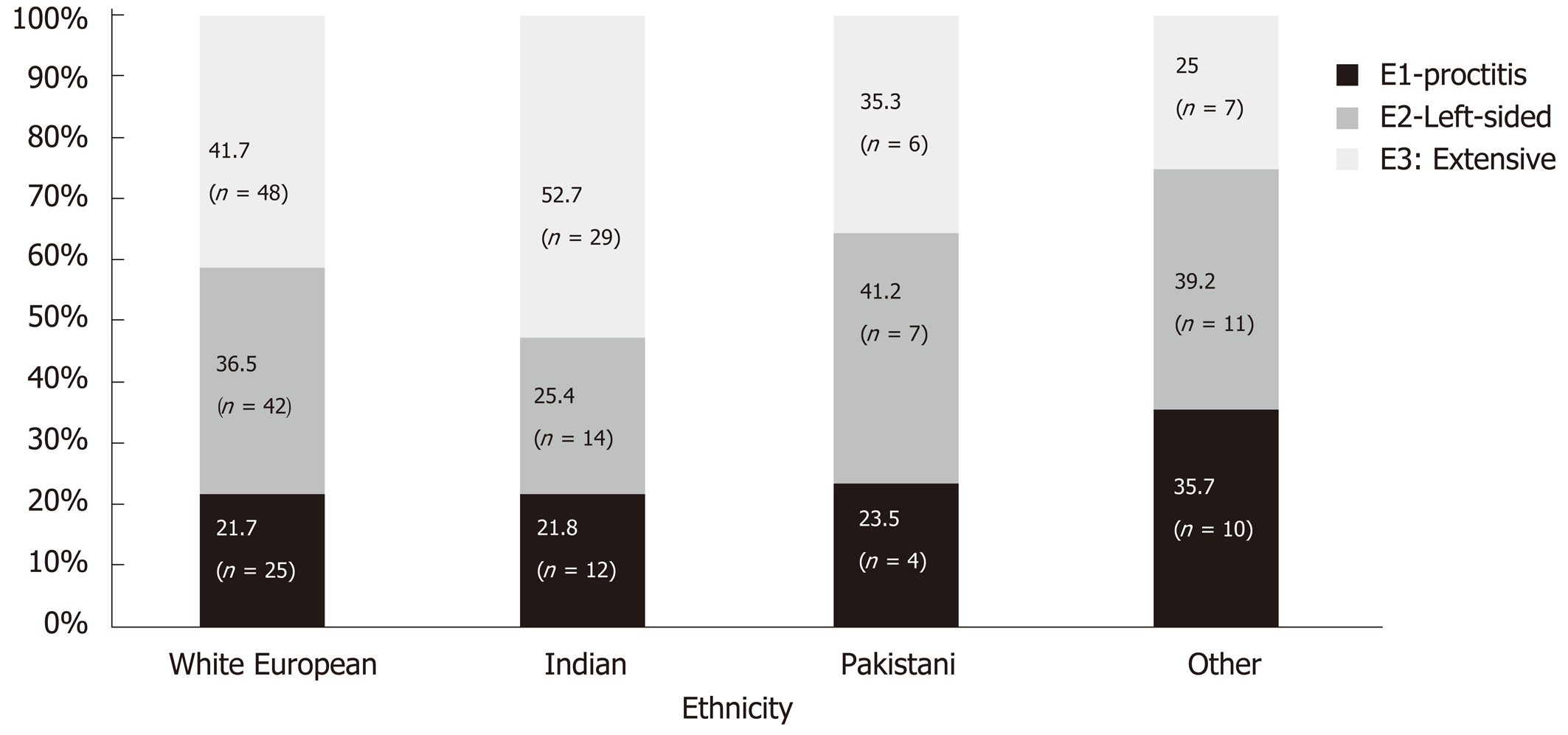
Disease location was similar between White Europeans and Indians for proctitis (21.7% vs 21.8%) and left sided (36.5% vs 25.4) however Indians were significantly more likely to have extensive disease (41.7% vs 52.7%, P = 0.03) (Figure 3). Left sided disease was the predominant disease extent in Pakistani and other ethnic groups (41.2% and 39.2%).
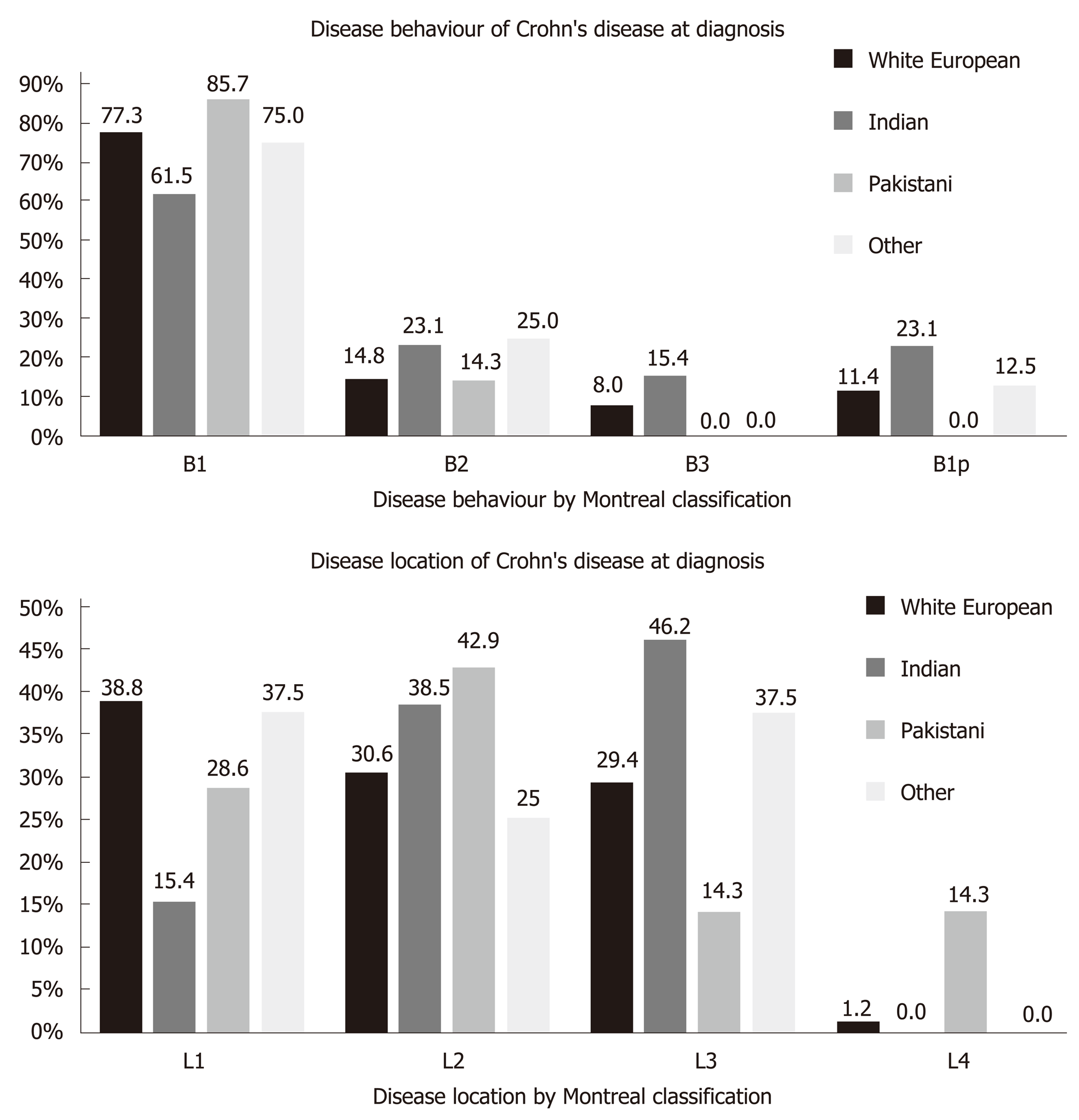
Ileal disease was the predominant phenotype in the White European group (38.8%) whereas colonic disease was most common in the Indian group (46.2%) (Figure 4). There was no significant difference in disease location. There were insufficient cases in the other ethnic groups for meaningful comparison. Inflammatory disease behaviour (B1) was the most common presentation in all ethnic groups. Perianal disease was more common in Indians (23.1%) compared with Whites (11.4%) although these differences were not significant.
The mean SCCAI and HBI scores at diagnosis are shown in Table S2 . Compared to the White European group, mean SCCAI scores were highest in the Indian group (6.3 vs 5.1). The Pakistani group had the highest HBI scores (6.8 vs 6.2). There were no significant differences in disease activity indexes at presentation between groups.
Treatment data was available for 183/218 UC patients and 105/115 CD patients (Table 2). 5-aminosalicylic acid treatment was the most common initial treatment for UC in White European and Indian patients (81.5% and 89.4%). Steroid use was comparable between the two groups. (33.3% and 34.0%). Nine White European patients (8.3%) required rescue therapy at diagnosis with ciclosporin or infliximab. No patients from the other groups required rescue therapy. Two White Europeans patients (1.9%) underwent surgery during their index presentation. 5-aminosalicylic acids, steroid and immunomodulator treatment were the most common therapies used at diagnosis for White Europeans with CD. Biologic therapy was utilised in 14.6% of cases and surgery required at index presentation in 3.7%. The small population of Indians and Pakistanis with CD make it difficult to make valid comparisons of the data.
| White European, n (%) | Indian, n (%) | Pakistani, n (%) | Other, n (%) | Total, n (%) | |
| UC | |||||
| None | 5 (4.6) | 3 (6.4) | 1 (7.7) | 2 (9.5) | 11 (5.8) |
| 5-ASA | 88 (81.5) | 42 (89.4) | 12 (92.3) | 12 (57.1) | 154 (81.4) |
| Steroids | 36 (33.3) | 16 (34.0) | 2 (15.4) | 4 (19.0) | 58 (30.7) |
| IM | 0 | 0 | 0 | 0 | 0 |
| Biologics | 7 (6.5) | 0 | 0 | 3 (14.3) | 10 (5.2) |
| Ciclosporin | 2 (1.9) | 0 | 0 | 0 | 2 (1.0) |
| Surgery | 2 (1.9) | 0 | 0 | 2 (9.5) | 4 (2.1) |
| CD | |||||
| None | 7 (8.5) | 2 (16.7) | 0 | 0 | 9 |
| 5-ASA | 31 (37.8) | 6 (50.0) | 2 (22.2) | 2 (28.6) | 43 |
| Steroids | 34 (41.5) | 6 (50.0) | 6 (66.7) | 4 (57.1) | 50 |
| IM | 19 (23.2) | 2 (16.7) | 4 (44.4) | 3 (42.9) | 28 |
| Biologics | 12 (14.6) | 0 | 1 (11.1) | 1 (14.3) | 14 |
| Other | 1 (1.2) | 0 | 0 | 0 | 1 |
| Surgery | 3 (3.7) | 0 | 0 | 0 | 3 |
This is the first prospective IBD inception cohort study in England. The incidence of UC in the overall population was higher than CD and comparable to the most recent data from Western Europe (IBD 18.5/100000, UC 9.8/100000 CD 6.3/100000 and IBDU 2.4/100000)[2]. We observed differential incidence rates between ethnic groups whereby Indians showed a UC incidence rate of 20.5/100000 double that of the Pakistani group (11.2/100000) and almost three times the White European population (7.5/100000).
The high incidence of UC in SA is well recognised, however the incidence observed in our Indian sub-group is the highest reported. This could be because many of the previous studies looked at the SA group collectively rather than sub-groups, despite recognised cultural and lifestyle difference between people of Indian, Pakistani and Bangladeshi origin. In Leicester, where 76.2% of the SA population is of Indian origin, the UC incidence rate for SAs in the 1980s was 10.3/100000 compared to our results for Indians living in Leicester where the incidence was 31/100000[5,6]. These are the only results that can be compared to show a change in incidence of IBD in the SA population in England.
Canada also hosts a large SA population. In the province of Ontario, SAs are predominantly Indian origin, however, in contrast to our findings they showed a lower UC incidence rate compared with non-immigrants (5/100000 vs 11.3/10000)[15]. However, it should be noted that the SA community studied was predominantly first generation immigrants unlike our cohort where 78% were born in the United Kingdom. More akin to our population is the study from British Columbia, where UC incidence rate reported for second generation SAs born in Canada was higher than for non-SAs (6.7/100000 and 0.96/100000)[26]. These findings suggest that environmental factors related to the country of birth might influence the onset of UC in SA populations.
Analysis of comparative incidence rates according to country of birth for our Indian population, would have been useful to support the hypothesis were it not for the small proportion born outside the United Kingdom introducing risk of bias. Lower incidence rates for Indians living in India would also support the role of environmental factors. However, there is very little, and archaic data from India. Using house to house questionnaires to record symptoms of UC, a study in 2003 reported an incidence rate of 6.02/100000[27] for a background population of 51910, much smaller that our Indian subgroup population of 239614 people.
The reasons for the higher incidence of UC compared with other groups living in the same environment as well as Indians living in India, remain speculative at this stage. Early-life exposure to western environmental triggers for a cohort with genetic susceptibility to IBD is plausible, particularly as North Indians harbour unique susceptibility genes for UC and the United Kingdom Indian population is predominantly of North Indian origin[6,28-30]. These susceptibility genes may be switched on by the ‘Western’ environment in the form of change in diet, different microbial exposure or environmental pollutants with genes that incur heightened susceptibility over those living in the West. This theory is supported by the observation of a younger age of onset in the Indian population demonstrated in the incidence by age group analysis.
Dietary habits differ between the SA sub-groups and might explain some of the observed differences. A recent study of IBD patients in India suggested vegetarianism maybe protective in this cohort[31]. As vegetarianism is higher in Gujarati populations (North Indians), who form the predominant ethnic group in North West London and Leicester, we would have expected a lower not higher UC incidence rates for these regions[32,33]. The unexpected incidence is likely to be due to the change to a western diet. Hindu UC patients living in Leicester reported a significant deviation from their traditional diet[34]. Dietary patterns in turn affect the gut microbiome[35]. Consumption of a Western diet was associated with a pro-inflammatory microbial profile with increases in Escherichia coli and reduced protective bacteria in mouse models[36]. The microbiome is also affected by smoking which protects against UC[37]. Seventy-two percent of the Indians in our study had never smoked compared with 36% of White European. Loss of this protective effect may have predisposed this sub-group to UC. Extensive disease phenotype was significantly more common in the Indian group, findings which are consistent with reports from other studies[8,38-40].
Our study has several strengths. The United Kingdom National Health Service (NHS) offers standard referral pathway from primary to secondary care across the country with access to free healthcare readily available at diagnosis and for long-term care minimising the risk of missing patients from lack of access to healthcare. We used standardised methods for pre-defined case ascertainment and reviewed this throughout the study. Furthermore, the prospective study design and real time data auditing reduced the probability of missing data and avoided coding errors inherent to retrospective studies.
We also recognise the limitations. We may have missed patients diagnosed in the private sector however the number are expected to be small given the low incidence of IBD and small % of population covered by private healthcare[41,42]. Moreover, most patients transfer their care to the NHS since health insurance cover precludes long-term care. Missed cases are unlikely to impact our conclusions of a higher UC incidence rate instead they would be expected to increase the incidence rate further rather than reducing it. Nevertheless, we tried to capture these patients to include all diagnosis over one year even if referred to hospital after the year recruitment. We may have also missed patients who sought medical attention and diagnosed in hospitals outside their catchment area; this would underestimate rather than overestimate our reported incidence. From an audit of newly diagnosed IBD cases across North West London, only three new UC cases living in the catchment area of London North West NHS trust were identified at other sites over the recruitment period. Another limitation is the un-adjusted analyses for confounding variables, such as occupation, education and socioeconomic status. Lastly, the epidemiology description for the ethnic population depended on standardised categories used in national census data rather than the sub-population genetic predisposition. Heterogeneity within the Indian population is well described with genetic and linguistic categories attributed to different ancestral origins as well as the remapping of boundaries between India, Pakistan and Bangladesh[43,44]. Although we did not analyse genetic predisposition in our study, most of the population in the high incidence regions are of Gujarati speaking and of North Indian origin[32,33].
In conclusion, this is the first prospective study reporting the incidence of IBD in five ethnically diverse populations in the England. SAs of Indian origin had the highest age-adjusted incidence rates for UC compared with other Asians (Pakistanis) and White Europeans in the United Kingdom. The majority of SAs were born in the United Kingdom suggesting that the complex interactions with the exposome may trigger disease expression in genetically predisposed individuals. Further studies on Indian populations may provide clues to how genetics and environment interact to predispose to UC.
Epidemiological studies have described the difference in inflammatory bowel disease (IBD) epidemiology in ethnic groups. However, the studies are predominantly single centre and retrospective. In the United Kingdom the last IBD incidence study was performed over 20 years ago.
To describe the incidence and phenotype of IBD in the United Kingdom by ethnic group.
A prospective inception cohort study over the course of one year in seven urban centres in the United Kingdom was performed. Standardised methods across all centres were employed for case ascertainment. Data was entered real time on the Epicom database.
Of 339 patients were diagnosed with IBD over the year study period across a population of 2271406 adults. The crude incidence of IBD, ulcerative colitis (UC) and Crohn’s disease for the total population was 17.0/100000, 11.3/100000 and 5.3/100000 respectively. The age adjusted incidence of UC in the Indian subgroup was almost three times the White European population (20.5/100000 and 7.5/100000 respectively). Indians were significantly more likely to have extensive disease (41.7% vs 52.7%, P = 0.03)
The Indian subgroup are particularly at risk of developing UC with a pan-colonic phenotype. These findings support the hypothesis of an environmental trigger in a genetically susceptible population. Financial planning and the provision of healthcare services should be reflexive to the local population and may differ according to the ethnic make-up of the background population.
Future studies should incorporate detailed dietary assessment prior to diagnosis to identify modifiable dietary risk factors. Studying early environmental factors in the paediatric population may yield clues to disease pathogenesis in the Indian migrant group. A prospective incidence study in India is required to provide a comparator to the observed trends.
The authors wish to thank the National Institute for Health Research for help in recruitment and Paul Bassett for statistical analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hosoe N, M'Koma AE, Kalaitzakis E, Pallav K, Tarnawski AS S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL
| 1. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4079] [Article Influence: 509.9] [Reference Citation Analysis (110)] |
| 2. | Burisch J, Pedersen N, Čuković-Čavka S, Brinar M, Kaimakliotis I, Duricova D, Shonová O, Vind I, Avnstrøm S, Thorsgaard N, Andersen V, Krabbe S, Dahlerup JF, Salupere R, Nielsen KR, Olsen J, Manninen P, Collin P, Tsianos EV, Katsanos KH, Ladefoged K, Lakatos L, Björnsson E, Ragnarsson G, Bailey Y, Odes S, Schwartz D, Martinato M, Lupinacci G, Milla M, De Padova A, D'Incà R, Beltrami M, Kupcinskas L, Kiudelis G, Turcan S, Tighineanu O, Mihu I, Magro F, Barros LF, Goldis A, Lazar D, Belousova E, Nikulina I, Hernandez V, Martinez-Ares D, Almer S, Zhulina Y, Halfvarson J, Arebi N, Sebastian S, Lakatos PL, Langholz E, Munkholm P; EpiCom-group. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63:588-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 288] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 3. | Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, Li MF, Ng KK, Kamm MA, Studd C, Bell S, Leong R, de Silva HJ, Kasturiratne A, Mufeena MNF, Ling KL, Ooi CJ, Tan PS, Ong D, Goh KL, Hilmi I, Pisespongsa P, Manatsathit S, Rerknimitr R, Aniwan S, Wang YF, Ouyang Q, Zeng Z, Zhu Z, Chen MH, Hu PJ, Wu K, Wang X, Simadibrata M, Abdullah M, Wu JC, Sung JJY, Chan FKL; Asia–Pacific Crohn's and Colitis Epidemiologic Study (ACCESS) Study Group. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology. 2013;145:158-165.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 595] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 4. | Rubin GP, Hungin AP, Kelly PJ, Ling J. Inflammatory bowel disease: epidemiology and management in an English general practice population. Aliment Pharmacol Ther. 2000;14:1553-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 197] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Office for National Statistics. Census 2011. Available from: https://www.nomisweb.co.uk/census/2011/lc2109ewls. |
| 6. | Probert CS, Jayanthi V, Pinder D, Wicks AC, Mayberry JF. Epidemiological study of ulcerative proctocolitis in Indian migrants and the indigenous population of Leicestershire. Gut. 1992;33:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 185] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Tsironi E, Feakins RM, Probert CS, Rampton DS, Phil D. Incidence of inflammatory bowel disease is rising and abdominal tuberculosis is falling in Bangladeshis in East London, United Kingdom. Am J Gastroenterol. 2004;99:1749-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Carr I, Mayberry JF. The effects of migration on ulcerative colitis: a three-year prospective study among Europeans and first- and second- generation South Asians in Leicester (1991-1994). Am J Gastroenterol. 1999;94:2918-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Probert CS, Jayanthi V, Wicks AC, Carr-Locke DL, Garner P, Mayberry JF. Epidemiological study of abdominal tuberculosis among Indian migrants and the indigenous population of Leicester, 1972-1989. Gut. 1992;33:1085-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Goodhand JR, Kamperidis N, Joshi NM, Wahed M, Koodun Y, Cantor EJ, Croft NM, Langmead FL, Lindsay JO, Rampton DS. The phenotype and course of inflammatory bowel disease in UK patients of Bangladeshi descent. Aliment Pharmacol Ther. 2012;35:929-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Cross RK, Jung C, Wasan S, Joshi G, Sawyer R, Roghmann MC. Racial differences in disease phenotypes in patients with Crohn's disease. Inflamm Bowel Dis. 2006;12:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Sofia MA, Rubin DT, Hou N, Pekow J. Clinical presentation and disease course of inflammatory bowel disease differs by race in a large tertiary care hospital. Dig Dis Sci. 2014;59:2228-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Wang YR, Loftus EV, Cangemi JR, Picco MF. Racial/Ethnic and regional differences in the prevalence of inflammatory bowel disease in the United States. Digestion. 2013;88:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Nguyen GC, Chong CA, Chong RY. National estimates of the burden of inflammatory bowel disease among racial and ethnic groups in the United States. J Crohns Colitis. 2014;8:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 15. | Benchimol EI, Mack DR, Guttmann A, Nguyen GC, To T, Mojaverian N, Quach P, Manuel DG. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am J Gastroenterol. 2015;110:553-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 16. | Juyal G, Amre D, Midha V, Sood A, Seidman E, Thelma BK. Evidence of allelic heterogeneity for associations between the NOD2/CARD15 gene and ulcerative colitis among North Indians. Aliment Pharmacol Ther. 2007;26:1325-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Misra R, Faiz O, Munkholm P, Burisch J, Arebi N. Epidemiology of inflammatory bowel disease in racial and ethnic migrant groups. World J Gastroenterol. 2018;24:424-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (2)] |
| 18. | Government Statistical Service. Harmonised concepts and questions for social data sources, GSS Harmonised Principle: Ethnic group, 2017. Available from: https://gss.civilservice.gov.uk/wp-content/uploads/2017/08/Ethnic-Group-June-17.pdf. |
| 19. | Vind I, Riis L, Jess T, Knudsen E, Pedersen N, Elkjaer M, Bak Andersen I, Wewer V, Nørregaard P, Moesgaard F, Bendtsen F, Munkholm P; DCCD study group. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003-2005: a population-based study from the Danish Crohn colitis database. Am J Gastroenterol. 2006;101:1274-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 389] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 20. | Burisch J. Epi-IBD Group. Available from: http://www.epi-ibd.org/. |
| 21. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, Jewell DP, Karban A, Loftus EV, Peña AS, Riddell RH, Sachar DB, Schreiber S, Steinhart AH, Targan SR, Vermeire S, Warren BF. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2148] [Cited by in RCA: 2366] [Article Influence: 215.1] [Reference Citation Analysis (0)] |
| 22. | Burisch J, Cukovic-Cavka S, Kaimakliotis I, Shonová O, Andersen V, Dahlerup JF, Elkjaer M, Langholz E, Pedersen N, Salupere R, Kolho KL, Manninen P, Lakatos PL, Shuhaibar M, Odes S, Martinato M, Mihu I, Magro F, Belousova E, Fernandez A, Almer S, Halfvarson J, Hart A, Munkholm P. Construction and validation of a web-based epidemiological database for inflammatory bowel diseases in Europe An EpiCom study. J Crohns Colitis. 2011;5:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980;1:514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1940] [Cited by in RCA: 2185] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 24. | Higgins PD, Schwartz M, Mapili J, Krokos I, Leung J, Zimmermann EM. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut. 2005;54:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. | Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJ, Lozano R, Inoue M. Age standardization of rates: A new WHO standard. Available from: http://www.who.int/healthinfo/paper31.pdf. |
| 26. | Pinsk V, Lemberg DA, Grewal K, Barker CC, Schreiber RA, Jacobson K. Inflammatory bowel disease in the South Asian pediatric population of British Columbia. Am J Gastroenterol. 2007;102:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Sood A, Midha V, Sood N, Bhatia AS, Avasthi G. Incidence and prevalence of ulcerative colitis in Punjab, North India. Gut. 2003;52:1587-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 195] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2019] [Cited by in RCA: 1880] [Article Influence: 134.3] [Reference Citation Analysis (2)] |
| 29. | Pugazhendhi S, Santhanam S, Venkataraman J, Creveaux I, Ramakrishna BS. NOD2 gene mutations associate weakly with ulcerative colitis but not with Crohn's disease in Indian patients with inflammatory bowel disease. Gene. 2013;512:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Juyal G, Negi S, Sood A, Gupta A, Prasad P, Senapati S, Zaneveld J, Singh S, Midha V, van Sommeren S, Weersma RK, Ott J, Jain S, Juyal RC, Thelma BK. Genome-wide association scan in north Indians reveals three novel HLA-independent risk loci for ulcerative colitis. Gut. 2015;64:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Amarapurkar AD, Amarapurkar DN, Rathi P, Sawant P, Patel N, Kamani P, Rawal K, Baijal R, Sonawane A, Narawane N, Kolekar S, Totla N. Risk factors for inflammatory bowel disease: A prospective multi-center study. Indian J Gastroenterol. 2018;37:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Roberts-Thomson T. The Diversity of Leicester-A Demographic Profile. 2008; Available from: http://www.lsr-online.org/static/lsr/legacy/documents/research/Diversity%20of%20Leicester%20-%20DRAFT.pdf. |
| 33. | Kalka I. The politics of the ‘community’ among Gujarati Hindus in London. J Ethn Migr Stud. 1991;17:377-385. [DOI] [Full Text] |
| 34. | Probert CS, Bhakta P, Bhamra B, Jayanthi V, Mayberry JF. Diet of South Asians with inflammatory bowel disease. Arq Gastroenterol. 1996;33:132-135. [PubMed] |
| 35. | David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5625] [Cited by in RCA: 6800] [Article Influence: 566.7] [Reference Citation Analysis (0)] |
| 36. | Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet P, Bernalier-Donadille A, Denis S, Hofman P, Bonnet R, Billard E, Barnich N. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci Rep. 2016;6:19032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 320] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 37. | Parkes GC, Whelan K, Lindsay JO. Smoking in inflammatory bowel disease: impact on disease course and insights into the aetiology of its effect. J Crohns Colitis. 2014;8:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 38. | Walker DG, Williams HR, Kane SP, Mawdsley JE, Arnold J, McNeil I, Thomas HJ, Teare JP, Hart AL, Pitcher MC, Walters JR, Marshall SE, Orchard TR. Differences in inflammatory bowel disease phenotype between South Asians and Northern Europeans living in North West London, UK. Am J Gastroenterol. 2011;106:1281-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Hilmi I, Singh R, Ganesananthan S, Yatim I, Radzi M, Chua AB, Tan HJ, Huang S, Chin KS, Menon J, Goh KL. Demography and clinical course of ulcerative colitis in a multiracial Asian population: a nationwide study from Malaysia. J Dig Dis. 2009;10:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Carroll MW, Hamilton Z, Gill H, Simkin J, Smyth M, Espinosa V, Bressler B, Jacobson K. Pediatric Inflammatory Bowel Disease Among South Asians Living in British Columbia, Canada: A Distinct Clinical Phenotype. Inflamm Bowel Dis. 2016;22:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Statista. Share of respondents with private health insurance in addition to compulsory social security in the United Kingdom [Internet]. 2017. Available from: https://www.statista.com/statistics/681534/individuals-with-private-health-insurance-in-the-united-kingdom-by-region. |
| 42. | Kings Fund. Commission on the Future of Health and Social Care in England: The UK private health market, 2014. Available from: https://www.kingsfund.org.uk/.../commission-appendix-uk-private-health-market.pdf. |
| 43. | Metspalu M, Romero IG, Yunusbayev B, Chaubey G, Mallick CB, Hudjashov G, Nelis M, Mägi R, Metspalu E, Remm M, Pitchappan R, Singh L, Thangaraj K, Villems R, Kivisild T. Shared and unique components of human population structure and genome-wide signals of positive selection in South Asia. Am J Hum Genet. 2011;89:731-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 44. | Ali M, Liu X, Pillai EN, Chen P, Khor CC, Ong RT, Teo YY. Characterizing the genetic differences between two distinct migrant groups from Indo-European and Dravidian speaking populations in India. BMC Genet. 2014;15:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |