Published online Aug 21, 2017. doi: 10.3748/wjg.v23.i31.5764
Peer-review started: April 8, 2017
First decision: April 26, 2017
Revised: May 11, 2017
Accepted: July 22, 2017
Article in press: July 24, 2017
Published online: August 21, 2017
Processing time: 140 Days and 22.4 Hours
To elucidate the effect of expression of doublecortin and CaM kinase-like-1 (DCLK1) in patients with pancreatic ductal adenocarcinoma (PDAC).
Tumor specimens were obtained from 136 patients with pancreatic cancer who had undergone resection without preoperative therapy between January 2000 and December 2013 at the Department of Surgical Oncology, Osaka City University. The resected specimens were analyzed for associations with clinicopathological data, including DCLK1 expression, epithelial mesenchymal transition (EMT) marker expression, and cancer stem cell (CSC) marker expression. Univariate and multivariate survival analyses were performed and we assessed the association between DCLK1 expression and clinicopathological factors, including the EMT marker and CSC marker.
In total, 48.5% (66/136) of the pancreatic cancer samples were positive for DCLK1. Patients with DCLK1-positive tumors had significantly shorter survival times than those with DCLK1-negative tumors (median, 18.7 mo vs 49.5 mo, respectively; P < 0.0001). Positive DCLK1 expression correlated with histological grade (P = 0.0290), preoperative CA19-9 level (P = 0.0060), epithelial cell adhesion molecule (EpCAM) expression (P = 0.0235), and the triple-positive expression of CD44/CD24/EpCAM (P = 0.0139). On univariate survival analysis, five factors were significantly associated with worse overall survival: histological grade of G2 to G4 (P = 0.0091), high preoperative serum SPan-1 level (P = 0.0034), R1/2 (P < 0.0001), positive expression of DCLK1 (P < 0.0001) or CD44 (P = 0.0245). On multivariate survival analysis, R1/2 [odds ratio (OR) = 2.019, 95% confidence interval (CI): 1.380-2.933; P = 0.0004] and positive DCLK1 expression (OR = 1.848, 95%CI: 1.2854-2.661; P = 0.0009) were independent prognostic factors.
DCLK1 expression was found to be an independent prognostic factor and it may play a crucial prognostic role by promoting acquisition of stemness.
Core tip: Doublecortin and CaM kinase-like-1 (DCLK1) is a microtubule - associated kinase and has recently attracted much attention as an important cancer stem cell marker. DCLK1 expression is correlated with aggressiveness in various cancers. However, there have been few investigations correlating DCLK1 expression with survival in pancreatic ductal adenocarcinoma (PDAC). PDAC patients with DCLK1-positive tumors had significantly shorter survival times than those with DCLK1-negative tumors. DCLK1 expression was an independent prognostic factor by multivariate survival analysis. Furthermore, DCLK1-positive expression was correlated to EpCAM expression and triple-positive expression of CD44/CD24/EpCAM. These findings suggest DCLK1 may have a crucial prognostic role in acquisition of stemness.
- Citation: Nishio K, Kimura K, Amano R, Nakata B, Yamazoe S, Ohira G, Miura K, Kametani N, Tanaka H, Muguruma K, Hirakawa K, Ohira M. Doublecortin and CaM kinase-like-1 as an independent prognostic factor in patients with resected pancreatic carcinoma. World J Gastroenterol 2017; 23(31): 5764-5772
- URL: https://www.wjgnet.com/1007-9327/full/v23/i31/5764.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i31.5764
Although the number of treatment strategies has increased for pancreatic ductal adenocarcinoma (PDAC), the disease still has a poor prognosis. Malignancy of PDAC is devastating, with a 5-year overall survival rate of approximately 5%[1]. The high mortality rate associated with PDAC is known to be due to extensive invasion into the surrounding tissues and early metastasis to distant organs; however, the molecular mechanisms of the highly aggressive nature of PDAC remain unclear.
Doublecortin and CaM kinase-like-1 (DCLK1) is a microtubule-associated kinase that has recently attracted much attention as an important cancer stem cell (CSC) marker. DCLK1 contains two doublecortin domains in the N terminus, which are involved in the regulation of microtubule polymerization, and a serine/threonine protein kinase domain in the C terminus. Between the N and C termini, there is a serine/proline-rich domain that mediates multiple protein-protein interactions[2]. DCLK1 is predominantly expressed in the low two-thirds of the intestinal crypt epithelium and occasionally in crypt-based columnar cells[3]. Originally, DLCK1 was reported as a putative intestinal and pancreatic stem cell marker[4,5]. More recently, however, DCLK1 has been demonstrated as expressed in CSCs but to be undetectable in normal stem cells[6]. Knockdown of DCLK1 in pancreatic cancer cells resulted in tumor growth arrest and the downregulation of Snail, Slug and Twist, which inhibit epithelial mesenchymal transition (EMT)[7-9]. As such, DCLK1 has become the focus of research into its potential as a candidate therapeutic target for various cancers.
Several studies have demonstrated that DCLK1 expression is correlated with cancer aggressiveness in colorectal[10], esophageal[11], breast[12], and renal cell[9] carcinomas. However, only few studies have investigated the correlation of DCLK1 expression with survival in PDAC. The aim of this study was to elucidate the effect of DCLK1 expression on the survival of patients with PDAC. Moreover, the correlations of clinicopathological features, including expression of EMT and CSC markers, with DCLK1 expression were investigated.
The current study used tissue samples from 136 patients who underwent pancreatic resection for PDAC at our institution. All patients were histologically confirmed to have a common type of invasive ductal carcinoma of the pancreas. Patients with neuroendocrine carcinomas, mucinous cystic carcinomas, or intraductal papillary mucinous carcinomas were excluded. Moreover, we excluded patients who had undergone neoadjuvant therapy. Clinical records were reviewed to examine clinical features, including demographic data (age and sex) and therapeutic data (chemotherapy performed after surgery, and interval from surgical resection to death). This study was approved by the ethics committee of Osaka City University and was in compliance with the Declaration of Helsinki. Each patient provided informed consent before tissue samples were obtained.
Surgery involved standard or subtotal stomach-preserving pancreaticoduodenectomy in 76 (55.9%) patients, distal pancreatectomy in 54 (39.7%) patients, and total pancreatectomy in 6 (4.4%) patients. Regional lymph node dissection was performed in all patients. The resected specimens were fixed in 10% formalin at room temperature, and the size and gross appearance of each tumor were recorded. The pathologic stage of all tumor specimens was determined using the staging system of the American Joint Committee on Cancer (AJCC), 7th edition[13]. Tumor differentiation was classified according to the classification of tumors of the World Health Organization as well-differentiated (G1), moderately differentiated (G2), poorly differentiated (G3), or undifferentiated (G4)[14].
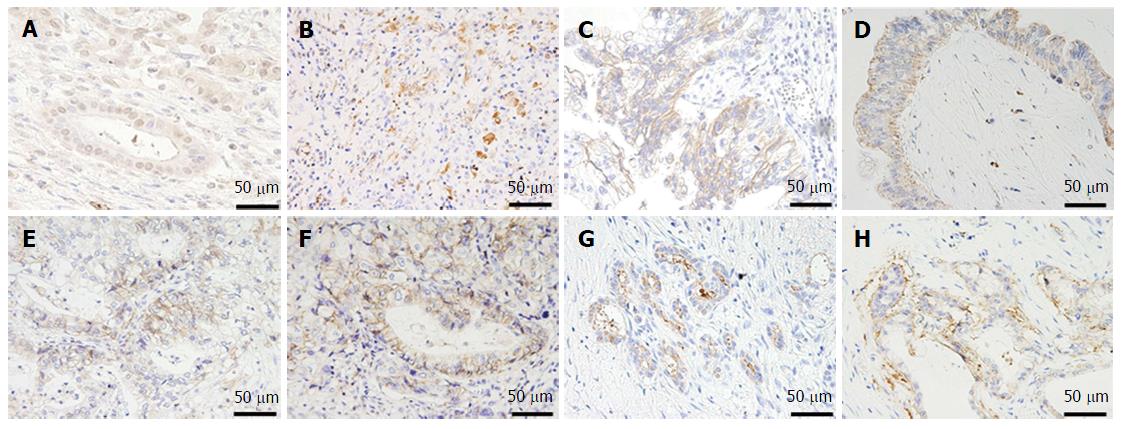
Formalin-fixed, paraffin-embedded tumor tissue was cut into 4-μm thick sections and immunohistochemistry was performed using a protocol previously reported by our group but with some modifications[15]. The most representative section of tumor for each case was selected for analysis. We analyzed not only DCLK1 expression, but also the expression of E-cadherin, N-cadherin, vimentin, and Snail as EMT markers, and CD24, CD44, CD133, and epithelial cell adhesion molecule (EpCAM) as CSC markers. The primary antibodies used for immunohistochemistry were: rabbit polyclonal anti-DCLK1 antibody (1:80 dilution; Abcam, Cambridge, MA, United States); rabbit polyclonal anti-Snail antibody (1:80 dilution; Abcam); mouse monoclonal anti-E-cadherin antibody (1:50 dilution; Dako Co., Carpinteria, CA, United States); rabbit monoclonal anti-vimentin antibody (1:100 dilution; Cell Signaling, Danvers, MA, United States); rabbit polyclonal anti-N-cadherin antibody (1:100 dilution; Abcam); goat polyclonal anti-CD24 antibody (1:20 dilution; Santa Cruz Biotechnology, Dallas, TX, United States); mouse monoclonal anti-CD44 antibody (1:50 dilution; Dako Co); mouse monoclonal anti-CD133 antibody (1:10 dilution; Miltenyi Biotec, Gladbach, Germany); and mouse monoclonal anti-EpCAM antibody (1:500 dilution; Cell Signaling).
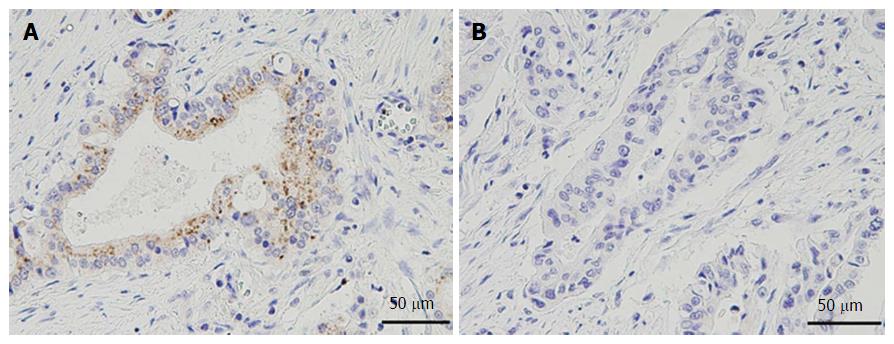
Intensity of the immunohistochemical staining (staining score) of each marker in a cancerous lesion of each sample was determined using a scoring system that ranged from 0 to 3 (0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining). Cytoplasmic staining was estimated for the analysis of DCLK1 and vimentin expression. Nuclear staining was estimated for the analysis of Snail expression. Membranous staining was estimated for the analysis of E-cadherin, N-cadherin, CD24, CD44, CD133 and EpCAM expression.
An example of the expression of each marker is shown in Figures 1 and 2. The staining score of each sample represents the average score of five randomly selected fields in the cancerous lesion. As there are no definitive standards that could be used to define positive and negative staining in this study, we defined a score of more than 2 as “positive staining” to roughly divide the samples into positive- and negative-staining groups. The scoring was performed by two surgeons (Nishio K, Kimura K) in a blinded fashion. Differences in scoring were resolved by validation of the two surgeons.
The demographic and clinical variables included age, sex, tumor location, tumor size, surgery, histological grade, AJCC classification, lymph node metastasis, adjuvant therapy, resection margin status, preoperative serum CA19-9 level, preoperative serum SPan-1 level, and expression of DCLK-1, Snail, E-cadherin, N-cadherin, vimentin, CD24, CD44, CD133 and EpCAM. For patients with preoperative jaundice, we used the preoperative serum CA19-9 data that were obtained after the jaundice had been reduced. At our medical center, endoscopic or percutaneous bile duct drainage is usually performed in patients with jaundice. For all patients, the CA19-9 level that was used in the analysis was measured when the total bilirubin level was < 5 mg/dL.
Categorical variables were compared using the χ2 test or Fisher’s exact test. Survival was calculated using the Kaplan-Meier method, and comparisons between groups were carried out by the log-rank test. P values < 0.05 were considered to be statistically significant. Variables with a significance of P < 0.05 on univariate analysis were included in the multivariate regression analysis to identify factors associated with survival after surgery. Statistical analyses were performed using SAS version 11.0 software (SAS Institute, Inc., Cary, NC, United States).
Characteristics of the patients who underwent surgery for PDAC are shown in Table 1. All patients were followed for survival, and the median follow-up period was 21.0 mo (range, 2.3-175.2 mo). The median overall survival time (MST) was 27.1 mo. The actuarial 3- and 5-year survival rates were 39.9% and 26.6%, respectively. Of the 136 total patients, 96 underwent adjuvant chemotherapy (5-fluorouracil: n = 1; tegafur-uracil: n = 28; gemcitabine: n = 46; S-1: n = 21).
| Characteristic | n |
| Age | |
| Median (range) | 70 (34-85) |
| Sex | |
| Male | 66 |
| Female | 70 |
| Location | |
| Head | 80 |
| Body-tail | 56 |
| Tumor size in cm | |
| Median (range) | 3 (1-18) |
| Surgery | |
| Pancreaticoduodenectomy | 76 |
| Distal pancreatectomy | 54 |
| Total pancreatectomy | 6 |
| Histological differentiation | |
| G1 | 20 |
| G2 | 89 |
| G3 | 17 |
| G4 | 10 |
| AJCC staging system | |
| IA | 5 |
| IB | 15 |
| IIA | 46 |
| IIB | 56 |
| III | 3 |
| IV | 11 |
| Lymph node | |
| N0 | 70 |
| N1 | 66 |
| Adjuvant therapy | |
| Yes | 96 |
| No | 40 |
| Resection margin status | |
| R0 | 86 |
| R1 | 35 |
| R2 | 15 |
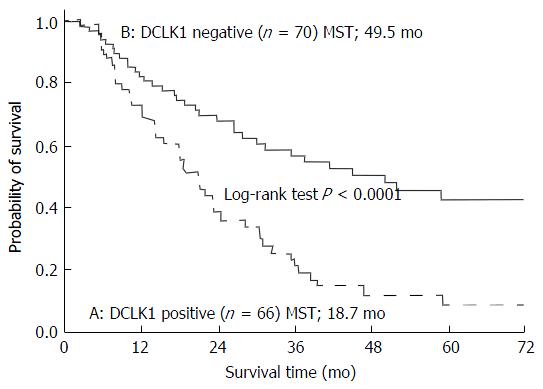
Of the 136 total patients, 66 (48.5%) were positive for DCLK1 expression and 70 (51.5%) were negative for DCLK1 expression. The MST of the DCLK1-positive patients was 18.7 mo, while that of that DCLK1-negative patients was 49.5 mo. The MST of the DCLK1-positive patients was significantly shorter than that of the DCLK1 -negative patients (P < 0.0001; Figure 3).
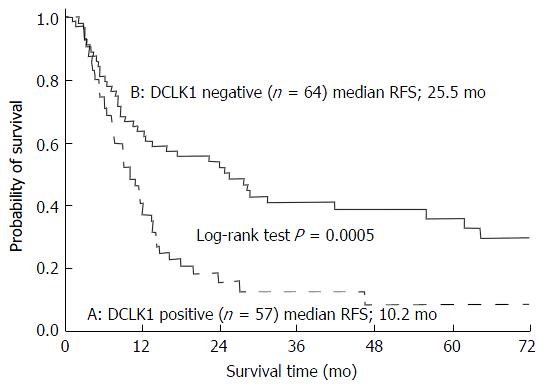
We examined relapse-free survival (RFS) and recurrent patterns of 121 patients, excluding patients with macroscopic residual tumor. Among these 121 patients, 57 were positive for DCLK1 expression and 64 patients were negative for DCLK1 expression. The DCLK1-positive patients relapsed more frequently, with 46 (80.7%) compared to the 40 (62.5%) DCLK1-negative patients who relapsed (P = 0.0438). The DCLK1-positive patients also had significantly shorter RFS than the DCLK-negative patients (P = 0.0005; Figure 4). Furthermore, of the 46 recurrent patients with DCLK1-positive tumors, 17 (37.0%) had local recurrence and 29 (63.0%) had distant metastasis. Of the 40 recurrent patients with DCLK1-negative tumors, 16 (40.0%) had local recurrence and 24 (60.0%) had distant metastasis. These results did not show significant difference for the recurrent pattern (P = 0.82).
Table 2 shows the association of DCLK1 expression with clinicopathological factors, including EMT and CSC markers. The factors showing a significant correlation with positive DCLK1 expression were histological grade (P = 0.0290), high preoperative serum CA19-9 level (P = 0.0060), and EpCAM expression (P = 0.0235). Furthermore, referring to past literature[16], we examined the combination of CSC markers and found that triple-positive CD44/CD24/EpCAM expression was significantly correlated with DCLK1 expression (P = 0.0139).
| Characteristic | DCLK-1, n = 136 | P value | |
| Positive, n = 66 | Negative, n = 70 | ||
| Tumor size in cm | 0.2962 | ||
| < 2 | 11 (16.7) | 17 (24.3) | |
| ≥ 2 | 55 (83.3) | 53 (75.7) | |
| Histological grade | 0.0290 | ||
| G1 | 5 (7.6) | 15 (21.4) | |
| G2-4 | 61 (92.4) | 55 (78.6) | |
| T category | 0.4326 | ||
| T1/T2 | 14 (21.2) | 19 (27.1) | |
| T3/T4 | 52 (78.8) | 51 (72.9) | |
| N category | 1.00 | ||
| N0 | 34 (51.5) | 37 (52.9) | |
| N1 | 32 (48.5) | 33 (47.1) | |
| Serum CA19-9 level | 0.0060 | ||
| Normal | 14 (21.2) | 31 (44.3) | |
| Elevated | 52 (78.8) | 39 (55.7) | |
| Serum SPan-1 level | |||
| Normal | 23 (34.8) | 34 (48.6) | 0.1198 |
| Elevated | 43 (65.2) | 36 (51.4) | |
| Residual tumor | 0.2148 | ||
| R0 | 38 (57.6) | 48 (68.6) | |
| R1/2 | 28 (42.4) | 22 (31.4) | |
| Snail | 0.1237 | ||
| Positive | 41 (62.1) | 34 (48.6) | |
| Negative | 25 (37.9) | 36 (51.4) | |
| E-cadherin | 0.1151 | ||
| Positive | 31 (47.0) | 23 (32.9) | |
| Negative | 35 (53.0) | 47 (67.1) | |
| N-cadherin | 0.5967 | ||
| Positive | 23 (34.8) | 28 (40.0) | |
| Negative | 43 (65.2) | 42 (60.0) | |
| Vimentin | 0.5232 | ||
| Positive | 6 (9.1) | 4 (5.7) | |
| Negative | 60 (90.9) | 66 (94.3) | |
| CD24 | 0.3758 | ||
| Positive | 27 (40.9) | 23 (32.9) | |
| Negative | 39 (59.1) | 47 (67.1) | |
| CD44 | 1.00 | ||
| Positive | 49 (74.2) | 52 (74.3) | |
| Negative | 17 (25.8) | 18 (25.7) | |
| EpCAM | 0.0235 | ||
| Positive | 46 (69.7) | 35 (50.0) | |
| Negative | 20 (30.3) | 35 (50.0) | |
| CD133 | 0.8301 | ||
| Positive | 12 (18.2) | 14 (20.0) | |
| Negative | 54 (81.8) | 56 (80.0) | |
| CD24+CD44+EpCAM+ | 0.0139 | ||
| Positive | 18 (27.3) | 7 (10.0) | |
| Negative | 48 (72.7) | 63 (90.0) | |
Table 3 shows the results of the univariate and multivariate survival analyses. Tumor size, histological grade, tumor grade (T) category, node (N) category, preoperative serum CA19-9 level, preoperative serum SPan-1 level, adjuvant therapy, resection margin status, and the expression of DCLK1, Snail, E-cadherin, N-cadherin, vimentin, CD24, CD44, CD133, and EpCAM were evaluated. On univariate analysis, five factors were significantly associated with worse overall survival: histological grade of G2 to G4 (P = 0.0091), high preoperative serum SPan-1 level (P = 0.0034), R1/2 factor (P < 0.0001), positive expression of DCLK1 (P < 0.0001) or CD44 (P = 0.0245). On multivariate analysis, R1/2 (OR = 2.019, 95%CI: 1.380-2.933; P = 0.0004) and positive DCLK1 expression (OR = 1.848; 95%CI: 1.2854-2.661; P = 0.0009) were independent factors of poor prognosis.
| Variable | Comparison | Univariate analysis | Multivariate analysis | ||||
| n | MST in mo | P value | Hazard ratio | 95%CI | P value | ||
| Tumor size in cm | < 2 | 28 | 36.6 | 0.0735 | |||
| ≥ 2 | 108 | 23.8 | |||||
| Histological grade | G1 | 20 | Not reached | 0.0091 | 1.362 | 0.844-2.318 | 0.2130 |
| G2-4 | 116 | 23.8 | |||||
| T category | T1/T2 | 33 | 30.3 | 0.7279 | |||
| T3/T4 | 103 | 28.27 | |||||
| N category | N0 | 71 | 35.4 | 0.0505 | |||
| N1 | 65 | 21.2 | |||||
| Serum CA19-9 | Normal | 45 | 31.37 | 0.2057 | |||
| Elevated | 91 | 24.43 | |||||
| Serum SPan-1 | Normal | 57 | 36.6 | 0.0034 | 1.286 | 0.905-1.840 | 0.1608 |
| Elevated | 79 | 21.2 | |||||
| Adjuvant therapy | No | 30 | 23.3 | 0.21 | |||
| Yes | 96 | 30.3 | |||||
| Resection margin status | R0 | 86 | 36.6 | < 0.0001 | 2.019 | 1.380-2.933 | 0.0004 |
| R1/2 | 50 | 17.9 | |||||
| DCLK1 | Negative | 70 | 50.1 | < 0.0001 | 1.848 | 1.2854-2.661 | 0.0009 |
| Positive | 66 | 21.0 | |||||
| Snail | Negative | 61 | 30.3 | 0.2377 | |||
| Positive | 75 | 26.57 | |||||
| E-cadherin | Negative | 82 | 26.57 | 0.5448 | |||
| Positive | 54 | 30.3 | |||||
| N-cadherin | Negative | 85 | 26.57 | 0.4568 | |||
| Positive | 51 | 36.03 | |||||
| Vimentin | Negative | 126 | 30.07 | 0.0659 | |||
| Positive | 10 | 17.9 | |||||
| CD24 | Negative | 86 | 24.43 | 0.6359 | |||
| Positive | 50 | 30.3 | |||||
| CD44 | Negative | 35 | 45.1 | 0.0245 | 1.267 | 0.855-1.919 | 0.2414 |
| Positive | 101 | 24.43 | |||||
| CD133 | Negative | 110 | 23.37 | 0.1485 | |||
| Positive | 26 | 36.6 | |||||
| EpCAM | Negative | 55 | 23.37 | 0.6580 | |||
| Positive | 81 | 30.07 | |||||
In the present study, we performed immunohistochemistry to analyze the expression of DCLK1 and clinicopathological variables, including EMT and CSC markers, to determine their correlation with survival in 136 patients with PDAC. DCLK1 expression was found to be significantly associated with the expression of EpCAM and the triple-positive expression of CD44/CD24/EpCAM. Moreover, DCLK1 expression was identified as an independent prognostic factor in resected PDAC. These findings suggest that DCLK1 may have a crucial prognostic role in the acquisition of stemness.
DCLK1, a putative marker of intestinal and pancreatic stem cells, is upregulated in various solid tumors, including colorectal, pancreatic, breast and prostate cancers, when compared to paired normal tissues[8-17]. Furthermore, recent reports indicated that DCLK1 can be used as a prognostic factor in colorectal cancer[18,19]. However, no reports have yet indicated a correlation between DCLK1 expression and the prognosis of patients with PDAC.
Cells with positive DCLK1 expression showed CSC properties in pre-invasive pancreatic cancer[20]. In addition, small interfering (si)RNA-mediated knockdown of DCLK1 resulted in the upregulation of microRNA (miR)-200a, an inhibitor of EMT, and the corresponding upregulation of E-cadherin following the downregulation of ZEB1 and ZEB2 in both human pancreatic and colorectal cancer cells[7-17]. Weygant et al[9] demonstrated that siRNA-mediated knockdown of DCLK1 in clear cell renal carcinoma cells results in decreased expression of EMT and pluripotency factors, and significantly reduces the invasion, migration, focal adhesion, drug-resistance and clonogenic capacities of cells. Chandrakesan et al[10] reported that DCLK1 is critically involved in facilitating intestinal tumorigenesis by enhancing pluripotency and EMT factors in adenomatous polyposis coli (APC) mutant intestinal tumors. Sureban et al[21] indicated that XMD8-92 treatment of pancreatic tumors resulted in the inhibition of DCLK1 and downstream oncogenic pathways (i.e., EMT, pluripotency, angiogenesis and anti-apoptotic pathways). These findings suggest that DCLK1 expression in human pancreatic cancer might directly regulate EMT, pluripotency and angiogenesis, and is significantly associated with survival. Although the abovementioned investigations on the molecular mechanisms of DCLK1 are all very important, we felt that there was a lack of reports on DCLK1 expression and its prognostic value in clinical samples of PDAC. As such, we investigated this topic, and found that DCLK1 over-expression had a significant impact on survival.
The results of the current study also indicated that the expression of EMT markers, such as E-cadherin, N-cadherin, vimentin and Snail, was not associated with poor prognosis. Cates et al[22] reported that the expression of EMT markers in PDAC was not associated with the duration of survival. In contrast, Yamada et al[23] reported that EMT markers predicted the prognosis of pancreatic cancer and that the EMT markers were associated with portal vein invasion and lymph node metastasis. Other researchers have also indicated that the expression of ZEB1 in pancreatic cancer is associated with poor prognosis[24,25].
Furthermore, we investigated stem cell markers, such as CD24, CD44, CD133 and EpCAM, to see whether they were associated with poor prognosis. Only the expression of CD44 showed an association with worse prognosis in the univariate analysis, but this association was not seen in the multivariate analysis. Pancreatic CSCs were first described by Li et al[5] in 2007. In our study, pancreatic cancer cells with triple-positive expression of CD44/CD24/EpCAM comprised only 0.2% to 0.8% of all the pancreatic cancer cells, but they had a 100-fold higher tumorigenic potential than the non-tumorigenic cancer cells[5]. In addition, Ohara et al[16] reported that triple-positive CD44/CD24/EpCAM expression was not correlated with poor prognosis, but overlapped with poorly differentiated cells and possessed high proliferative potential in clinical pancreatic cancer. Furthermore, they showed that the presence of double-positive CD44/CD24 expression appeared to be correlated with poor prognosis[16]. Finally, Akita et al[26] reported that EpCAM was a significant prognostic factor in pancreatic cancer.
To elucidate the role of DCLK1, we also analyzed the association between DCLK1 expression and clinicopathological factors, including EMT and CSC markers. Our results showed that positive EpCAM expression was significantly correlated with positive DCLK1 expression. Furthermore, examination of the combination of stem cell markers showed that the triple-positive expression of CD44/CD24/EpCAM was significantly correlated with positive DCLK1 expression in PDAC. In contrast, positive EMT marker expression was not associated with positive DCLK1 expression. These results suggest that PDAC with DCLK1 expression may gain biological malignant potential by acquiring stemness. Some investigators have demonstrated DCLK1 expression on tumor stem cells that continuously produce tumor progeny in the polyps of APCMin/+ mice, with ablation of these DCLK1-positive tumor stem cells resulting in a marked regression of polyps without any apparent damage to the normal intestine[6]. These findings suggest that DCLK1 exists in pancreatic tumor cells with stemness and that targeting DCLK1-positive cells may be a very effective advanced therapy. Recently, Westphalen et al[27] reported that there is a possibility of DCLK1 positive cells being the origin of PDAC. Accordingly, it has been postulated that DCLK1 is not only associated with stemness but also with carcinogenesis. Further studies are needed to fully elucidate the role of DCLK1 in PDAC.
There are some limitations to the present study which must be considered when interpreting the findings. This study was conducted at a single institution, and it had a small sample size. Moreover, it was a retrospective evaluation. A prospective investigation with a larger sample size is needed to confirm the significance of DCLK1 expression.
In conclusion, positive DCLK1 expression was identified as an independent prognostic factor in PDAC. The expression of DCLK1 was found to be associated with the triple-positive expression of CD44/CD24/EpCAM as well as EpCAM expression. Collectively, these findings indicate that DCLK1 may play a crucial prognostic role in the acquisition of stemness.
Doublecortin and CaM kinase-like-1 (DCLK1) is a microtubule-associated kinase and has recently attracted much attention due to its recognized importance as a cancer stem cell (CSC) marker. Several studies have demonstrated that DCLK1 expression is correlated with aggressiveness in various cancers. However, only few studies have investigated the correlation of DCLK1 expression with survival in pancreatic ductal adenocarcinoma (PDAC). It is therefore worthwhile to elucidate the effect of DCLK1 expression on the survival of patients with PDAC.
It has been reported that knockdown of DCLK1 in pancreatic cancer cells resulted in tumor growth arrest and the downregulation of epithelial mesenchymal transition (EMT), pluripotency and angiogenesis. Furthermore, several studies have demonstrated that DCLK1 expression is correlated with aggressiveness in various cancers. By using this marker in clinical samples of PDAC, the authors were able to identify DCLK1 expression as an independent prognostic factor in resected PDAC.
The authors found that DCLK1 over-expression had a significant impact on survival in resected PDAC, using clinical samples of PDAC. Furthermore, their findings suggest the possibility that PDAC with DCLK1 expression may gain biological malignant potential by acquiring stemness.
The results of the present study suggest that DCLK1 exists in pancreatic tumor cells with stemness, and that targeting DCLK1-positive cells may be very effective advanced therapy.
DCLK1 is a microtubule-associated kinase and has recently attracted much attention as an important CSC marker, and has been reported as a putative intestinal and pancreatic stem cell marker. Recently, it has been reported that DCLK1 expression in human pancreatic cancer might directly regulate EMT, pluripotency, and angiogenesis.
Although this study is retrospective in design, it is well structured and the subject is very interesting. The manuscript is correctly written and the conclusions are justified by the data.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Huan C, Kang KM S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8970] [Article Influence: 690.0] [Reference Citation Analysis (0)] |
| 2. | Ohmae S, Takemoto-Kimura S, Okamura M, Adachi-Morishima A, Nonaka M, Fuse T, Kida S, Tanji M, Furuyashiki T, Arakawa Y. Molecular identification and characterization of a family of kinases with homology to Ca2+/calmodulin-dependent protein kinases I/IV. J Biol Chem. 2006;281:20427-20439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | May R, Sureban SM, Hoang N, Riehl TE, Lightfoot SA, Ramanujam R, Wyche JH, Anant S, Houchen CW. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells. 2009;27:2571-2579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26:630-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 223] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2428] [Article Influence: 134.9] [Reference Citation Analysis (0)] |
| 6. | Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet. 2013;45:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 330] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 7. | Sureban SM, May R, Qu D, Weygant N, Chandrakesan P, Ali N, Lightfoot SA, Pantazis P, Rao CV, Postier RG. DCLK1 regulates pluripotency and angiogenic factors via microRNA-dependent mechanisms in pancreatic cancer. PLoS One. 2013;8:e73940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Sureban SM, May R, Lightfoot SA, Hoskins AB, Lerner M, Brackett DJ, Postier RG, Ramanujam R, Mohammed A, Rao CV. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res. 2011;71:2328-2338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | Weygant N, Qu D, May R, Tierney RM, Berry WL, Zhao L, Agarwal S, Chandrakesan P, Chinthalapally HR, Murphy NT. DCLK1 is a broadly dysregulated target against epithelial-mesenchymal transition, focal adhesion, and stemness in clear cell renal carcinoma. Oncotarget. 2015;6:2193-2205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Chandrakesan P, Weygant N, May R, Qu D, Chinthalapally HR, Sureban SM, Ali N, Lightfoot SA, Umar S, Houchen CW. DCLK1 facilitates intestinal tumor growth via enhancing pluripotency and epithelial mesenchymal transition. Oncotarget. 2014;5:9269-9280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Vega KJ, May R, Sureban SM, Lightfoot SA, Qu D, Reed A, Weygant N, Ramanujam R, Souza R, Madhoun M. Identification of the putative intestinal stem cell marker doublecortin and CaM kinase-like-1 in Barrett’s esophagus and esophageal adenocarcinoma. J Gastroenterol Hepatol. 2012;27:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Liu YH, Tsang JY, Ni YB, Hlaing T, Chan SK, Chan KF, Ko CW, Mujtaba SS, Tse GM. Doublecortin-like kinase 1 expression associates with breast cancer with neuroendocrine differentiation. Oncotarget. 2016;7:1464-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors . AJCC cancer staging manual (7th ed). New York, NY: Springer; 2010; . |
| 14. | Fred T, Bosman ES, Sunil R, Ohgaki H. WHO Classification of Tumours of the Digestive System. International Agency for Research on Cancer. 2010;. |
| 15. | Murata A, Amano R, Yamada N, Kimura K, Yashiro M, Nakata B, Hirakawa K. Prognostic predictive values of gemcitabine sensitivity-related gene products for unresectable or recurrent biliary tract cancer treated with gemcitabine alone. World J Surg Oncol. 2013;11:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Ohara Y, Oda T, Sugano M, Hashimoto S, Enomoto T, Yamada K, Akashi Y, Miyamoto R, Kobayashi A, Fukunaga K. Histological and prognostic importance of CD44(+) /CD24(+) /EpCAM(+) expression in clinical pancreatic cancer. Cancer Sci. 2013;104:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Sureban SM, May R, Mondalek FG, Qu D, Ponnurangam S, Pantazis P, Anant S, Ramanujam RP, Houchen CW. Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144 and inhibits colorectal cancer tumor growth via a Notch-1 dependent mechanism. J Nanobiotechnology. 2011;9:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Gagliardi G, Goswami M, Passera R, Bellows CF. DCLK1 immunoreactivity in colorectal neoplasia. Clin Exp Gastroenterol. 2012;5:35-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Gao T, Wang M, Xu L, Wen T, Liu J, An G. DCLK1 is up-regulated and associated with metastasis and prognosis in colorectal cancer. J Cancer Res Clin Oncol. 2016;142:2131-2140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N, Matsui W. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 249] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 21. | Sureban SM, May R, Weygant N, Qu D, Chandrakesan P, Bannerman-Menson E, Ali N, Pantazis P, Westphalen CB, Wang TC. XMD8-92 inhibits pancreatic tumor xenograft growth via a DCLK1-dependent mechanism. Cancer Lett. 2014;351:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Cates JM, Byrd RH, Fohn LE, Tatsas AD, Washington MK, Black CC. Epithelial-mesenchymal transition markers in pancreatic ductal adenocarcinoma. Pancreas. 2009;38:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Yamada S, Fuchs BC, Fujii T, Shimoyama Y, Sugimoto H, Nomoto S, Takeda S, Tanabe KK, Kodera Y, Nakao A. Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery. 2013;154:946-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Bronsert P, Kohler I, Timme S, Kiefer S, Werner M, Schilling O, Vashist Y, Makowiec F, Brabletz T, Hopt UT. Prognostic significance of Zinc finger E-box binding homeobox 1 (ZEB1) expression in cancer cells and cancer-associated fibroblasts in pancreatic head cancer. Surgery. 2014;156:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Kurahara H, Takao S, Maemura K, Mataki Y, Kuwahata T, Maeda K, Ding Q, Sakoda M, Iino S, Ishigami S. Epithelial-mesenchymal transition and mesenchymal-epithelial transition via regulation of ZEB-1 and ZEB-2 expression in pancreatic cancer. J Surg Oncol. 2012;105:655-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Akita H, Nagano H, Takeda Y, Eguchi H, Wada H, Kobayashi S, Marubashi S, Tanemura M, Takahashi H, Ohigashi H. Ep-CAM is a significant prognostic factor in pancreatic cancer patients by suppressing cell activity. Oncogene. 2011;30:3468-3476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Westphalen CB, Takemoto Y, Tanaka T, Macchini M, Jiang Z, Renz BW, Chen X, Ormanns S, Nagar K, Tailor Y. Dclk1 Defines Quiescent Pancreatic Progenitors that Promote Injury-Induced Regeneration and Tumorigenesis. Cell Stem Cell. 2016;18:441-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |