Published online Aug 21, 2017. doi: 10.3748/wjg.v23.i31.5732
Peer-review started: March 14, 2017
First decision: May 16, 2017
Revised: June 14, 2017
Accepted: July 12, 2017
Article in press: July 12, 2017
Published online: August 21, 2017
Processing time: 158 Days and 22.9 Hours
To explore the relationship between such a construct and an existing continence score.
A retrospective study of incontinent patients who underwent anal physiology (AP) was performed. AP results and Cleveland Clinic Continence Scores (CCCS) were extracted. An anal physiology score (APS) was developed using maximum resting pressures (MRP), anal canal length (ACL), internal and external sphincter defects and pudendal terminal motor latency. Univariate associations between each variable, APS and CCCS were assessed. Multiple regression analyses were performed.
Of 508 (419 women) patients, 311 had both APS and CCCS measured. Average MRP was 51 mmHg (SD 23.2 mmHg) for men and 39 mmHg (19.2 mmHg) for women. Functional ACL was 1.7 cm for men and 0.7 cm for women. Univariate analyses demonstrated significant associations between CCCS and MRP (P = 0.0002), ACL (P = 0.0006) and pudendal neuropathy (P < 0.0001). The association between APS and CCCS was significant (P < 0.0001) but accounted for only 9.2% of the variability in CCCS. Multiple regression showed that the variables most useful in predicting CCCS were external sphincter defect, pudendal neuropathy and previous pelvic surgery, but only improving the scores predictive ability to 12.5%.
This study shows that the ability of AP tests to predict continence scores improves when considered collectively, but that a constructed summation model before and after multiple regression is poor at predicting the variability in continence scores.
Core tip: This study explored the relationship between a hypothesized anal physiology score combining rankings from maximal manometric resting pressures and anal canal length, ultrasound proportions of anal canal length of internal and external anal sphincters which were intact, and bilateral pudendal nerve terminal motor latencies; with the Cleveland Clinic Continence Score. The association between physiology and continence scores was significant but accounted for only 9.2% of the variability. The most useful variables predicting continence score were proportion of external sphincter intact, pudendal neuropathy and previous pelvic surgery. This study shows anal physiology tests predict continence scores better when considered collectively.
- Citation: Young CJ, Zahid A, Koh CE, Young JM. Hypothesized summative anal physiology score correlates but poorly predicts incontinence severity. World J Gastroenterol 2017; 23(31): 5732-5738
- URL: https://www.wjgnet.com/1007-9327/full/v23/i31/5732.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i31.5732
The extent of anal incontinence often determines the most appropriate treatment and facilitates monitoring treatment response[1]. Measuring anal incontinence severity is complex and requires considering the separate elements of gas, liquid, and solid, the frequency, the circumstances (e.g., diarrhea), and impact on lifestyle and pad use[2-4]. Since the first grading system described by Browning and Parks, numerous grading scores have been developed, each with varying utility[5,6]. The importance of subjective assessments in a symptom-based condition such as incontinence is well established[1,6].
The preponderance of available continence scores indicates that none is perfect. Incontinence is a heterogeneous condition including groups of patients with soiling rather than frank liquid stool incontinence, yet few continence scores make that distinction between the two[6,7]. Though continence scores are often taken as surrogate measures of anorectal function with higher continence scores associated with worse anorectal function, they usually correlate poorly with anorectal physiology[6,8]. Continence scores reflect patient perception of the severity and burden of incontinence, but are often unhelpful in guiding treatment choice[8].
An objective measure that quantifies anal function and could be extrapolated to incontinence was considered as being desirable in providing clinicians with an alternative grading scale, which could also be useful in guiding treatment selection or predicting treatment response. Since no single anal physiology (AP) test is capable of thoroughly assessing anorectal function, such a measure could be derived using several different anal physiology tests. It is important to note that such a measure does not replace continence scores but serves to complement in the assessment of incontinence.
It was hypothesized that a summation measure that considered anal physiology tests collectively would correlate with continence scores better than when they were considered individually.
A retrospective study of consecutive patients presenting for anal physiology with a single colorectal surgeon for anal incontinence at the Royal Prince Alfred Hospital between 1999 and 2006 was carried out. All patients routinely underwent anorectal manometry, endoanal ultrasound (EUS) and pudendal terminal motor latency (PNTML).
Anal physiology study results and continence scores were extracted from a prospectively maintained electronic database and medical records. Data collection began after written approval from the local human research and ethics committee was obtained.
Anorectal manometry was performed using a Stryker manometer (Stryker Corp, San Jose, CA, United States) with a station pull-through technique. After a thorough inspection of perineum for surgical scars or asymmetry in skin corrugations, the catheter is gently introduced into the anal canal before digital examination to avoid pressure artifacts. Six resting pressures were measured at 1 cm intervals in a craniocaudal direction. The point with the highest resting pressure was taken as the site of the high-pressure zone and this was the position at which squeeze pressures were measured.
EUS was performed using a 10 MHz Bruel and Kjaer rotating endoprobe with the patient in left lateral position. Sphincter defects were graded according to the longitudinal extent of sphincter defect. Sphincter quality is graded as either homogeneous or heterogeneous. Hard copies of sonographic images at the level of puborectalis, upper anal canal, mid anal canal and lower anal canal were also obtained so that sphincter thickness could be measured.
PNTML was measured using a glove mounted St. Mark’s electrode with the patient in left lateral position. Three readings on each side with a satisfactory electrical tracing were taken with the shortest latency being recorded as the PNTML. Prolonged latency was defined as PNTML ≥ 2.2 ms.
The hypothesized anal physiology score (APS) consisted of variables derived from manometry, EUS and PNTML. Manometry, EUS, and PNTML were chosen because these are the most commonly used anorectal physiology tests for incontinence investigation and the most likely to be informative and incorporated into clinical practice[8,9].
The five variables in the hypothesized APS were maximum resting pressure, anal canal length, internal sphincter defects, external sphincter defects and PNTML (Table 1). Individual variables were rated from zero to four; giving a possible APS value that ranged from zero to twenty where zero was hypothesized as associated with continence and twenty with incontinence. The worse the resting pressure, the shorter the anal canal length, the worse the sphincter defects and the worse the neuropathy, the higher the APS and therefore the worse the incontinence was anticipated to be.
| Maximum resting pressure (mmHg) | Anal canal length (cm) | Internal sphincter defect | External sphincter defect | PNTML | |
| 0 | > 40 | > 3 | Intact | Intact | Normal |
| 1 | > 30, ≤ 40 | > 2, ≤ 3 | ≤ ¼ defect | ≤ ¼ defect | - |
| 2 | > 20, ≤ 30 | > 1, ≤ 2 | > ¼, ≤ ½ | > ¼, ≤ ½ | Unilateral neuropathy |
| 3 | > 10, ≤ 20 | > ½, ≤ 1 | > ½, ≤ ¾ | > ½, ≤ ¾ | - |
| 4 | ≤ 10 | ≤ ½ | > ¾ | > ¾ | Bilateral neuropathy |
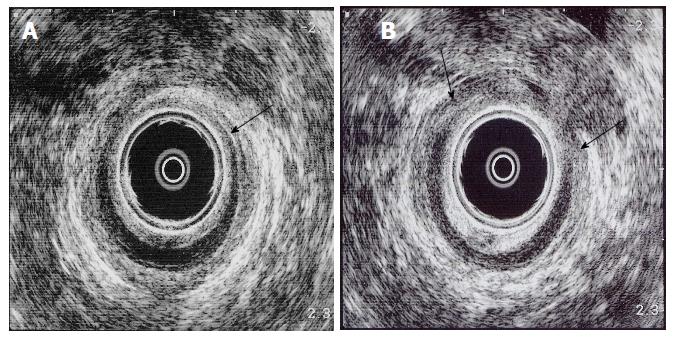
Anal canal length was calculated using the technique described by Hool et al[10] using 30 mmHg as a cutoff. Although a study by Morgado et al[11] found mean pressures to be a better measure than maximum pressures, using mean resting pressures and anal canal length as variables would have led to repeated use of the same measurements. Choices of variables with EUS were sphincter defects, sphincter thickness, and sphincter morphology. Internal and external sphincter defects were chosen as the third and fourth variables because the main strength of EUS is its ability to detect sphincter defects[3,12]. All internal and external anal sphincter lengths found on EUS, specifically designation as fully intact or the proportion intact and proportion non-intact due to defects, were recorded by Author (Young CJ) at the time of EUS (Figure 1). This was measured relative to the length of anal canal found at EUS, whether intact or not, from the lower anal canal to puborectalis, allowing for rank classification of 0-4 as shown in Table 1. External anal sphincter thickness was explored as a potential variable but was considered difficult because the outer boundary of the external sphincter is often difficult to discern as it merges with surrounding fibro-fatty tissue. Its utility remains controversial[13-17]. Sphincter morphology was not chosen because the value of sphincter morphology remains to be determined in the available literature[18]. Finally, PNTML was included to complement manometry and EUS in the global assessment of anal function.
The analysis was performed using SAS (SAS Instutitute Inc, 2005). APS variables were expressed as means with standard deviations and medians with inter-quartile ranges. Univariate associations between individual APS variables and CCCS were first analyzed using an ANOVA procedure. The association between APS and CCCS was assessed next. In order to determine whether different combinations of variables could provide a more predictive model and to adjust for potential confounding variables, multiple regression was undertaken. All variables (including clinical information) that were significant or near significant (P < 0.1) in univariate analyses were included in the full base model. A manual backwards stepwise approach was then used to eliminate variables from the model in order of least significance. R2 values were used to calculate the proportion of variability in CCCS that was explained by the model. Gender differences were analyzed using Wilcoxon rank sum test for the continuous variables and chi-square tests for categorical variables.
There were 508 (419 females) patients over the eight-year study period with a mean age of 57.8 for men and 60.3 for women (Table 3). The prevalence of urinary incontinence was similar between men and women in our cohort and was 10%. Women were much more likely to have had pelvic surgery with the most common procedure being hysterectomy followed by a range of gynecological procedures mainly for pelvic prolapse. Men were much more likely to have had anorectal surgery with hemorrhoidectomy being the most common procedure (Table 3).
| Men | Women | |
| n | 89 (17.5) | 419 (82.5) |
| mean Age | 57.8 (SD 15.1) | 60.3 (SD 14.1) |
| Diabetes | ||
| Yes | 10 (11.2) | 17 (4.1) |
| No | 72 (80.9) | 362 (86.4) |
| Irritable bowel syndrome | ||
| Yes | 9 (10.1) | 16 (3.8) |
| No | 73 (82.0) | 363 (86.6) |
| Urinary incontinence | ||
| Yes | 9 (10.1) | 42 (10.0) |
| No | 73 (82.0) | 365 (87.0) |
| Previous pelvic surgery | ||
| Yes | 13 (14.6) | 197 (47.0) |
| No | 70 (78.7) | 184 (43.9) |
| Hysterectomy | - | 148 (35.3) |
| Anterior resection | 8 (9.0) | 11 (2.6) |
| Rectopexy | 3 (3.4) | 15 (3.6) |
| Zaccharin's procedure | - | 45 (10.7) |
| Bladder procedure | - | 45 (10.7) |
| Previous OSR | - | 12 (2.9) |
| Other gynae surgery | - | 48 (11.5) |
| Prostatectomy | 6 (6.7) | - |
| Anorectal surgery | ||
| Yes | 37 (41.6) | 85 (20.3) |
| No | 45 (50.6) | 373 (89.0) |
| Hemorrhoidectomy | 17 (19.1) | 34 (8.1) |
| Sphincterotomy | 4 (4.5) | 9 (2.1) |
| Anal dilatation | 0 (0) | 6 (1.4) |
| Fistulotomy | 8 (9.0) | 13 (3.1) |
Manometry and EUS results were available in all 508 patients. PNTML was unavailable in 34 patients due to faulty software resulting in PNTML being available in 474 patients. CCCS was available in 397 patients. Of 508 patients, 311 patients had adequate data to derive both APS and CCCS. The distribution for each APS variable is shown in Table 4. All APS variables were significantly different between men and women, except for internal sphincter defect. Almost 80% of men had normal pudendal nerve function while only 46% of women did. Women were at least four times more likely than men to have unilateral or bilateral neuropathy.
| Men | Women | P value | Mean reference range | |
| Maximum RP (mmHg) | Men: 50-80 | |||
| Mean | 51.0 (SD 23.2) | 38.8 (SD 15.6) | < 0.0001 | Women: 30-60 |
| Median | 52.0 (IQR 33.5) | 38.0 (IQR 22.5) | ||
| ACL (cm) | Men: 2.5-3.5 | |||
| Mean | 1.7 (SD 1.4) | 0.7 (SD 0.8) | < 0.0001 | Women: 2.0-3.0 |
| Median | 2.0 (IQR 2.8) | 0.5 (IQR 1.0) | ||
| IAS defect | ||||
| Intact | 61 (68.5) | 286 (68.3) | 0.7 | - |
| ≤ ¼ defect | 0 (0.0) | 4 (1) | (c2 = 2.4, df = 4 ) | |
| > ¼, ≤ ½ | 12 (13.5) | 49 (11.7) | ||
| > ½, ≤ ¾ | 2 (2.2) | 21 (5.0) | ||
| > ¾ | 14 (15.7) | 59 (14.1) | ||
| EAS defect | ||||
| Intact | 85 (95.5) | 305 (73.0) | < 0.0001 | - |
| ≤ ¼ defect | 0 (0.0) | 2 (0.5) | (c2 = 21.7, df = 4) | |
| > ¼, ≤ ½ | 2 (2.2) | 62 (14.8) | ||
| > ½, ≤ ¾ | 1 (1.1) | 36 (8.6) | ||
| > ¾ | 1 (1.1) | 13 (3.1) | ||
| Pudendal Neuropathy | ||||
| Normal | 70 (78.7) | 191 (45.6) | < 0.0001 | |
| Unilateral | 3 (3.4) | 67 (16.0) | (c2 = 43.1, df=2) | 2.20 ms |
| Bilateral | 6 (6.7) | 137 (32.7) | ||
| Mean CCCS | 5.9 | 8 | 0.001 | - |
Univariate associations between each APS variable and CCCS is shown in Table 5. Maximum resting pressures, anal canal length, and pudendal neuropathy were all significantly associated with CCCS but the associations between internal or external sphincter defects and CCCS were not statistically significant.
| F value | df | P1 value | Proportion variability in CCCS explained | |
| Maximum RP (mmHg) | 13.8 | 1, 395 | 0.0002 | 3.10% |
| ACL | 12.1 | 1, 395 | 0.0006 | 2.70% |
| IAS defect | 2.2 | 4, 392 | 0.07 | 1.20% |
| EAS defect | 2.3 | 4, 392 | 0.06 | 1.30% |
| Pudendal neuropathy | 11.7 | 2, 394 | < 0.0001 | 5.10% |
The mean APS value was 7.2 (SD 4.1). The association between the derived APS and CCCS was also highly significant (P < 0.0001) with a Pearson correlation coefficient of 0.3. However, the R2 was 0.092 meaning that APS was only able to explain 9.2% of the variability in CCCS.
Following multiple regression modeling, only external sphincter defect, pudendal neuropathy and previous pelvic surgery were significant and independent predictors of CCCS (Table 6). After adjusting for the other variables in the model maximum resting pressure and anal canal length were not significantly associated with CCCS, whereas external sphincter became significant. In combination, external sphincter defect, pudendal neuropathy and previous pelvic surgery accounted for 12.5% of the variability in CCCS.
| Variable | Classification | Change in CCCS | P value |
| EAS defect | Intact | - | 0.01 |
| ¼ | 4 | ||
| ½ | 0.3 | ||
| ¾ | 1.5 | ||
| > ¾ | 5.1 | ||
| PNTML | Normal | - | < 0.0001 |
| Unilateral neuropathy | 1.2 | ||
| Bilateral neuropathy | 2.6 | ||
| Previous pelvic surgery | Yes | - | 0.0007 |
| No | -1.6 |
This study shows that anal physiology tests can predict continence scores better when considered collectively rather than individually. While aiming to develop an objective score quantifying anal function and thereby incontinence, we showed that the APS only accounted for 9.2% of the variability in CCCS. The remaining variability suggests that there are many other factors in the anal incontinent population to account for it.
The five APS variables were chosen based on our understanding of the strengths and weaknesses of each variable, and because they are the most commonly used tests in our unit. Anal manometry squeeze pressures rely on patient effort and the ability of patients to squeeze their sphincters and pelvic floor. Many were found to adjunct this with “buttock squeeze”, and potentially the measurement obtained may not be adequately representative of the actual sphincter function. The large overlap in squeeze pressures between incontinent and healthy individuals also means that squeeze pressures were inadequately discriminatory and therefore not chosen as a variable[19]. Maximum resting pressure reflects pressure generated by the high-pressure zone, and has been shown to be significantly different between incontinent and continent subjects[20]. Functional anal canal length was chosen due to its importance as a marker of continence[10,11].
Normal continence is a complex interaction of several factors including the rectum, pelvic nerves, pelvic floor, anal sphincters, colonic motility and stool consistency[1]. Anorectal function and incontinence can only be fully assessed when each of these elements has been evaluated and included within the APS model respective to their importance to continence. The APS model may then appear very sphincter-centric because manometry, EUS, and PNTML each assess a separate aspect of the sphincter. If APS is found to be helpful in guiding treatment selection or predicting treatment outcome, it is more likely to be incorporated into routine clinical practice as the tests are commonly performed. Further, it is fruitless to subject patients to time-consuming and potentially uncomfortable or embarrassing and costly investigations (such as defecography, colonic transit study) if clinical assessment can provide the required answer and when their relative importance to sphincter function is unknown. The role of stool consistency in aggravating incontinence or causing incontinence in an individual with compromised anorectal function is well known[21]. While information on stool consistency using the Bristol chart was collected, this data was only available for 20% of the subjects and could not be included for analysis[22]. Because each physiology parameter has contributed less than 5% each to the predictive ability of CCCS, we doubt that inclusion of stool consistency to the APS would have made a dramatic difference to the predictive ability of the APS.
Because of the poor predictive ability of APS, analysis of variables that may influence continence was carried out using multiple regression. These included gender, age, parity and previous pelvic surgery[23-25]. Using multiple regression, we found that external sphincter defect, pudendal neuropathy, and previous pelvic surgery were most useful in predicting CCCS. Although external sphincter defect was not found to be significantly associated with continence scores on univariate associations, it was significant using multiple regression. This apparent initial lack of association between external sphincter defect and continence scores is assumed to be due to masking by other variables. Conversely, maximum resting pressures and anal canal length were significantly associated with continence scores on univariate associations but not multiple regression. This apparent effect was the result of confounding due to the differences in the distribution of external sphincter defect, pudendal neuropathy and previous pelvic surgery between men and women. Similarly, the strong apparent gender influence was also a confounder due to the much higher prevalence of external sphincter defect, pelvic surgery and pudendal neuropathy amongst females. The predictive ability of this new model improved marginally to account for 12.5% of the variability in CCCS.
The association between bilateral pudendal neuropathy and continence scores has previously been shown by Ricciardi et al[26] but not the relationship between unilateral neuropathy and continence score. This study demonstrated an almost linear relationship between neuropathy and continence scores. The same could not be demonstrated for external sphincter defect and is likely related to the small sample size in this study, and the results should be confirmed using a larger sample size. The role of pelvic surgery in incontinence had been controversial. Hysterectomy was the most widely studied pelvic procedure, and conflicting results have been reported[27,28]. Due to the relatively small numbers of each procedure except hysterectomy, all those involving pelvic viscera (uterus, rectum) and the pelvic floor were classified as pelvic surgery. The results of this study indicate that previous pelvic surgery was associated with greater incontinence.
In this study, the gender difference in CCCS was highly significant suggesting that women had more severe incontinence than men. A meta-analysis by Pretlove et al[23] reported that more women experienced severe incontinence than men because of the higher prevalence of solid and liquid incontinence among women. All APS variables were significantly different between men and women, except for internal sphincter defect, and this may be a clue as to why there is a gender difference in CCCS.
Given that the majority of CCCS is unexplained by clinical or anal physiology data, we believe that although continence scores and anorectal physiology are both used in incontinence, they measure different aspects of incontinence. The clinical utility of this newly developed measure in guiding treatment selection or predicting treatment response needs to be determined in a prospective clinical study.
In conclusion, anal physiology tests correlate better with continence scores when considered collectively rather than individually. Our APS model was only able to predict 9.2% of the variability in CCCS, suggesting that continence scores and physiology measure different aspects of incontinence. These aspects are likely to be complementary in the assessment of incontinence, with the clinical utility of the APS derived using multiple regression requiring prospective assessment in a clinical setting.
Anal physiology tests involving manometry, ultrasound and electromyography are routine in the investigation of fecal incontinence. Continence scores are also routinely assessed. This study aimed to research the connection between anal physiology and continence score as a means of correlating and subsequently applying the findings to the diagnosis, management and understanding of fecal incontinence.
The ongoing relevance of anal physiology tests and their role in the diagnosis and management of fecal incontinence continues to require clarification and validation, to bring a clearer understanding of when and why they are beneficial.
The innovation of this study is the way the anal physiology data has been combined to create a summative anal physiology score, much in the same vain as a continence score, which can shed new insights into the diagnosis and management of fecal incontinence.
The novel summative anal physiology score presented and studied in this research can be used for future research projects involving the diagnosis and management of fecal incontinence. That will potentially open an entire new field of assessment of fecal incontinence in line with quality of life measures also.
There are no terms that the authors do not believe are unfamiliar to most readers.
Authors established the anal physiology score model and it is helpful to assess the incontinence.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Yu CG S- Editor: Qi Y L- Editor: A E- Editor: Xu XR
| 1. | Saldana Ruiz N, Kaiser AM. Fecal incontinence - Challenges and solutions. World J Gastroenterol. 2017;23:11-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 107] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (2)] |
| 2. | Stanley TH, Webster LR. Anesthetic requirements and cardiovascular effects of fentanyl-oxygen and fentanyl-diazepam-oxygen anesthesia in man. Anesth Analg. 1978;57:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 223] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2089] [Cited by in RCA: 1967] [Article Influence: 61.5] [Reference Citation Analysis (1)] |
| 4. | de la Portilla F, Calero-Lillo A, Jiménez-Rodríguez RM, Reyes ML, Segovia-González M, Maestre MV, García-Cabrera AM. Validation of a new scoring system: Rapid assessment faecal incontinence score. World J Gastrointest Surg. 2015;7:203-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 5. | Parks AG. Royal Society of Medicine, Section of Proctology; Meeting 27 November 1974. President's Address. Anorectal incontinence. Proc R Soc Med. 1975;68:681-690. [PubMed] |
| 6. | Takács L, Debreczeni LA. Circulatory regulation in pregnant rats: pregnancy and haemorrhagic hypotension. Acta Physiol Acad Sci Hung. 1972;42:345-365. [PubMed] |
| 7. | O’Brien PE, Skinner S. Restoring control: the Acticon Neosphincter artificial bowel sphincter in the treatment of anal incontinence. Dis Colon Rectum. 2000;43:1213-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Whitehead WE, Wald A, Norton NJ. Treatment options for fecal incontinence. Dis Colon Rectum. 2001;44:131-142; discussion 142-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Terra MP, Deutekom M, Dobben AC, Baeten CG, Janssen LW, Boeckxstaens GE, Engel AF, Felt-Bersma RJ, Slors JF, Gerhards MF. Can the outcome of pelvic-floor rehabilitation in patients with fecal incontinence be predicted? Int J Colorectal Dis. 2008;23:503-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Hool GR, Lieber ML, Church JM. Postoperative anal canal length predicts outcome in patients having sphincter repair for fecal incontinence. Dis Colon Rectum. 1999;42:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Morgado PJ Jr, Wexner SD, Jorge JM. Discrepancies in anal manometric pressure measurement--important or inconsequential? Dis Colon Rectum. 1994;37:820-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Andromanakos N, Filippou D, Skandalakis P, Papadopoulos V, Rizos S, Simopoulos K. Anorectal incontinence. pathogenesis and choice of treatment. J Gastrointestin Liver Dis. 2006;15:41-49. [PubMed] |
| 13. | Gantke B, Schäfer A, Enck P, Lübke HJ. Sonographic, manometric, and myographic evaluation of the anal sphincters morphology and function. Dis Colon Rectum. 1993;36:1037-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Schäfer R, Heyer T, Gantke B, Schäfer A, Frieling T, Häussinger D, Enck P. Anal endosonography and manometry: comparison in patients with defecation problems. Dis Colon Rectum. 1997;40:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Voyvodic F, Rieger NA, Skinner S, Schloithe AC, Saccone GT, Sage MR, Wattchow DA. Endosonographic imaging of anal sphincter injury: does the size of the tear correlate with the degree of dysfunction? Dis Colon Rectum. 2003;46:735-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Prichard D, Harvey DM, Fletcher JG, Zinsmeister AR, Bharucha AE. Relationship Among Anal Sphincter Injury, Patulous Anal Canal, and Anal Pressures in Patients With Anorectal Disorders. Clin Gastroenterol Hepatol. 2015;13:1793-1800.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Gurland B, Hull T. Transrectal ultrasound, manometry, and pudendal nerve terminal latency studies in the evaluation of sphincter injuries. Clin Colon Rectal Surg. 2008;21:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Lam TJ, Kuik DJ, Felt-Bersma RJ. Anorectal function evaluation and predictive factors for faecal incontinence in 600 patients. Colorectal Dis. 2012;14:214-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | McHugh SM, Diamant NE. Effect of age, gender, and parity on anal canal pressures. Contribution of impaired anal sphincter function to fecal incontinence. Dig Dis Sci. 1987;32:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 157] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Osterberg A, Graf W, Påhlman L. The longitudinal high-pressure zone profile in patients with fecal incontinence. Am J Gastroenterol. 1999;94:2966-2971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 21. | Read NW, Bartolo DC, Read MG. Differences in anal function in patients with incontinence to solids and in patients with incontinence to liquids. Br J Surg. 1984;71:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 59] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1858] [Cited by in RCA: 2105] [Article Influence: 75.2] [Reference Citation Analysis (1)] |
| 23. | Pretlove SJ, Radley S, Toozs-Hobson PM, Thompson PJ, Coomarasamy A, Khan KS. Prevalence of anal incontinence according to age and gender: A systematic review and meta-regression analysis. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Bharucha AE, Zinsmeister AR, Locke GR, Seide BM, McKeon K, Schleck CD, Melton LJ 3rd. Risk factors for fecal incontinence: a population-based study in women. Am J Gastroenterol. 2006;101:1305-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Lunniss PJ, Gladman MA, Hetzer FH, Williams NS, Scott SM. Risk factors in acquired faecal incontinence. J R Soc Med. 2004;97:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Ricciardi R, Mellgren AF, Madoff RD, Baxter NN, Karulf RE, Parker SC. The utility of pudendal nerve terminal motor latencies in idiopathic incontinence. Dis Colon Rectum. 2006;49:852-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Forsgren C, Zetterström J, Lopez A, Nordenstam J, Anzen B, Altman D. Effects of hysterectomy on bowel function: a three-year, prospective cohort study. Dis Colon Rectum. 2007;50:1139-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Altman D, Zetterström J, López A, Pollack J, Nordenstam J, Mellgren A. Effect of hysterectomy on bowel function. Dis Colon Rectum. 2004;47:502-508; discussion 508-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |