Published online May 28, 2017. doi: 10.3748/wjg.v23.i20.3684
Peer-review started: January 20, 2017
First decision: February 21, 2017
Revised: February 28, 2017
Accepted: March 21, 2017
Article in press: March 21, 2017
Published online: May 28, 2017
Processing time: 126 Days and 8.3 Hours
To assess the feasibility and safety of a novel enteroscope, negative-pressure suction endoscope in examining the small intestine of a porcine model.
In vitro experiments in small intestinal loops from 20 pigs and in vivo experiments in 20 living pigs were conducted.
In in vitro experiments, a negative pressure of > 0.06 MPa was necessary for optimal visualization of the intestine, and this pressure did not cause gross or histological damage to the mucosa. For satisfactory examination of the small intestine in vivo, higher negative pressure (> 1.00 MPa) was required. Despite this higher pressure, the small intestine did not show any gross or microscopic damage in the suctioned areas. The average time of examination in the living animals was 60 ± 7.67 min. The animals did not experience any apparent ill effects from the procedure.
Small intestine endoscope was safely performed within a reasonable time period and enabled complete visualization of the intestine in most cases.
Core tip: The main component of endoscopes is an ultrafine tubular endoscope, with an added external propeller, to assist in the migration of the endoscope through the intestine.
- Citation: Liu JH, Liu DY, Wang L, Han LP, Qi ZY, Ren HJ, Feng Y, Luan FM, Mi LT, Shan SM. Animal experimental studies using small intestine endoscope. World J Gastroenterol 2017; 23(20): 3684-3689
- URL: https://www.wjgnet.com/1007-9327/full/v23/i20/3684.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i20.3684
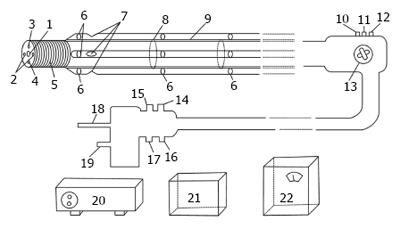
Endoscopic examination of the small intestine remains a challenge. Endoscopes currently used in the diagnosis and treatment of gastrointestinal diseases include capsule gastroscope[1], duodenoscope, double-balloon endoscope, single-balloon enteroscope, and colonoscope. The detection range of these instruments is from the mouth to the duodenum and from the anus, retrogradely, to the cecum[2-5]. Negative-pressure suction small intestine endoscope has been proposed as a solution to this problem. Its working principle is similar to the functions of suction cups of parasites, and its operating principles are similar to those of double-balloon and single-balloon endoscopes. The main component of negative-pressure endoscopes is an ultrafine tubular endoscope, with an added external propeller, to assist in the migration of the endoscope through the intestine. The endoscope is intended to enable the collection of intestinal fluids and tissue samples as well as to aid in the diagnosis and treatment of small intestinal diseases.
Small intestine endoscopes have been developed independently in China, with regard to the intellectual property rights. Three patent licenses from the Chinese State Intellectual Property Office, as well as two patent licenses from the Japanese State Intellectual Property Office, have been granted for these endoscopes[2-6].
In the present study, we aimed to assess the feasibility and safety of a negative-pressure suction endoscope in examining the small intestine of pigs.
The materials used included freshly excised small intestines, with the complete mesentery, from 20 live pigs (150 kg; 2 years of age); a negative-pressure suction small intestine endoscope (Figure 1); four electric suction units (Yuyue, 7A-23D; Nanjing, China), with pressures ranging from 0.06 to 1.00 Mpa; the cardinal machine; heatless light source; monitor (Jiangsu matt phillips photoelectric technology Co., LTD); computer; fixative solution; refrigerator; tissue sectioning instrument; and microscope.
The cardinal machine, heatless light source, monitor, and negative-pressure suction device were switched on. Under the control of an operator, the working component of the endoscope was inserted into the prepared pig small intestine, the suction tubes on the slider were pulled, and the suction cup-like inlets on the front at the sides of the endoscope were examined to determine whether they properly sucked the small intestine. The areas surrounding the inlets were smeared with methylene blue, the suction force was increased, and the suction tubes on the sliders were pulled, thus causing the small intestine to pile up behind the working component of the endoscope. After the operation, the suctioned areas, which were stained with methylene blue, were biopsied for microscopic examination.
All animal experiments were conducted according to the institutional guidelines for the care and use of animals. The animal protocol was designed to minimize pain or discomfort to the animals. The ethical standards of experiments were in accordance with the guidelines provided by the Committee for the Purpose of Control and Supervision of Experiments on Animals. Animals were fasted for 2 d prior to being anesthetized with ketamine hydrochloride injection (5 mg/kg). The negative-pressure suction device, the cardinal machine, heatless light source, and monitor were switched on. The endoscope was inserted into the mouth of the pigs, and if necessary, a small amount of air was introduced before the examination of the digestive tract. During the examination, the suction tubes on the slider were pulled, which gradually caused the intestine to pile up like sleeves around the main lens. The intestinal surface was examined as the suction tubes were being pulled. An attempt was made to reach the ileocecal junction in each animal. The diet, activity, and defecation of the pigs were monitored for 2 wk after the operation.
Data were analyzed using SPSS version 12.0 for windows (SPSS Inc., Tokyo). All data are expressed as mean ± SD. Continuous data were compared using t tests (n = 20), with P-values < 0.05 considered statistically significant.
The operator used the propelling arm of the endoscope to grasp the small intestine by applying negative-pressure suction from the electric suction unit. When the suction force was not sufficiently strong, the endoscope could not grasp the small intestine firmly; the initial pressure required for the endoscope to properly function was greater than 0.06 MPa. During the operation, the small intestine appeared to pile up in a sleeve-like manner around the main lens. Through the image acquisition window at the front of the main lens, the backward movement of the small intestine could be followed and the inner intestinal walls could be examined. In total, only 80% of the ileocecal junction were observed in four pigs, whereas a 4-m length of the small intestine was visualized in all 20 pigs. The examination required an average of 40 ± 5.47 min (95%CI: 38.8-41.1). No histological damage to the small intestine was evident in the areas where negative-pressure suction had been applied.
On the basis of the results from the in vitro experiments, the initial pressure for the in vivo examinations was set at > 0.06 MPa. When the pressure was greater than 1.00 MPa, no slippage was seen. Small intestine endoscope was successful in all the 20 pigs, and the procedure required an average of 60 ± 7.67 min (95%CI: 56.41-63.59). The average depth of insertion was 2.0 m. After the examination, all pigs were able to eat; 18 were able to defecate by the second day, and 2 by the third day. During the 2-week observation period after endoscopy, no pigs showed signs of abdominal distension, bloody stool, constipation, or other complications, and their food intake, activity levels, sleep patterns, defecation patterns, and urination were normal.
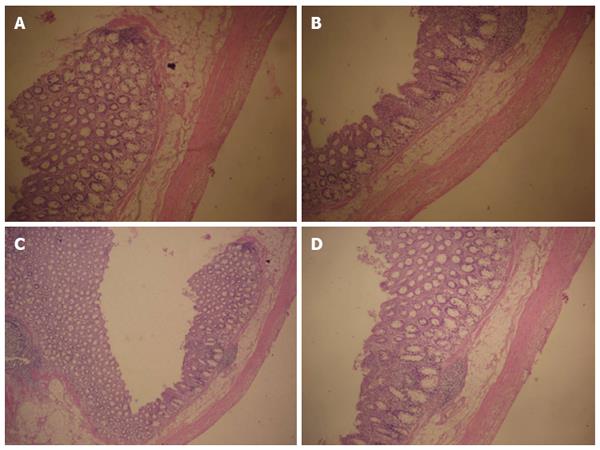
Examinations of histological sections from areas of the small intestine that were sucked by negative pressure did not show any breakage, shedding of large areas of the intestinal villi, tissue displacement, deformation, or damage to the glandular structures in the submucosal layer. The normal orderly arrangement of smooth muscle layers was maintained, and the muscle cells were clearly outlined (Figure 2).
Endoscopic examination of the small intestine has always presented a tough challenge. Small intestine endoscopy has been proposed as a technique that might help overcome this challenge. Therefore, in the present study, we evaluated the feasibility and safety of this approach through in vitro and in vivo experiments in a porcine model.
In the in vitro experiments, we established that the pig small intestine was tolerant to negative pressures that were acceptable for endoscopic examination of the intestinal wall, and indeed tolerant to even much higher pressures. At a pressure of > 0.06 MPa, the endoscope functioned smoothly, and 4-m segments of the small intestine were successfully examined in the intestines from all 20 animals studied. No visual damage was noted in the areas of the small intestine that were repeatedly sucked. Moreover, tissue biopsy specimens from those areas showed no signs of submucosal damage or breakage in the muscle layers. Therefore, we concluded that the negative pressure exerted by the suction endoscope was within the tolerance level of the small intestine. Indeed, we found that the intestine had an extremely high level of tolerance to negative pressure; even when 6 MPa of negative-pressure was exerted, no apparent damage to the sucked areas was noted. The positive results of these in vitro experiments encouraged us to proceed with in vivo experiments.
In live pigs, we found that somewhat higher negative pressure was needed, as compared to the in vitro condition, in order to prevent slippage of the bowel. Thus, we used a pressure of > 1.00 MPa, which permitted successful examination of the intestines of all 20 animals. The average time of completing the in vivo examinations was 60 ± 7.67 min.
The time for this procedure was considerably shorter than that reported for double-balloon endoscope performed in Fuji, Japan, which took an average of 3-4 h per examination. The Japanese researchers also found that slippage of the intestine occurred when the two balloons were alternated, whereas we found no slippage when the working pressure level was set as > 1.00 MPa.
We believe that it is encouraging that the negative-pressure suction endoscope did not histologically damage the small intestine, and that the animals had no evident adverse effects associated with the procedure; indeed, they resumed normal defecation within 2-3 d after the operation and had no signs of discomfort during the 2 postoperative weeks. This study in pigs indicated that small intestine endoscope is feasible and can be performed safely in a reasonable length of time. The entire small intestine was visualized in a high percentage of animals, and neither gross nor microscopic damage to the intestine was noted. We are hopeful that this promising technique for examination of the small intestine will soon be evaluated in human subjects. In addition, we are planning an experiment on human subjects, which will enable the commercial application of this type of endoscope in the small intestine.
The small intestine endoscope had four power modes, and the examination speed was greater than that with the double-balloon and single-balloon endoscope. The curve formed by the endoscope is not associated with any serious effects, and can avoid pain.
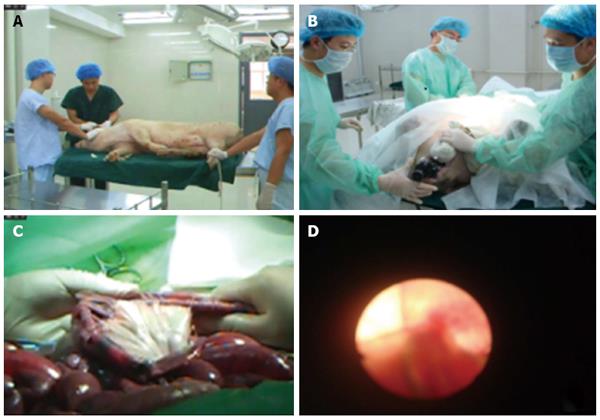
This endoscope is designed based on the ultrafine endoscope, and has four power modes. It is inserted into the cavity through the mouth while the patient is under general anesthesia, thus enabling the small intestine to pile up at the proximal end along the primary eyepiece, while the detector at the distal end can perform detection or treatment. Experimental results on animals confirmed that endoscopic examination using our newly designed endoscope has the following advantages compared to the Japanese double-balloon endoscope: (1) quicker examination; (2) high operability and ease of mastery; (3) low cost; and (4) short slippage distance[7-15]. Moreover, endoscopic examination using our newly designed endoscope is more advantageous than capsule endoscopy in terms of (1) clearer images; (2) uninterrupted examination; (3) ability to collect pathological samples or conduct endoscopic treatment; (4) short examination duration; and (5) not causing ileus[16-30]. The most important feature of small intestine endoscope is its rapid examination speed; it only takes an average of 60 ± 7.67 min to complete the examination of a 4-m-long small intestine with complete mesentery, which is 2 h less than the average examination time required for Japanese Fujinon double-balloon endoscope, as reported in the literature. In the present study, we provided evidence (Figure 3) to support the manufacture of this endoscope, which will be beneficial for patients, alleviate pain, and help ensure that no blind spots are present during examination and treatment with the small intestine endoscope.
Endoscopic examination of the small intestine remains a challenge. Endoscopes currently used in the diagnosis and treatment of gastrointestinal diseases include capsule gastroscope, duodenoscope, double-balloon endoscope, single-balloon enteroscope, and colonoscope.
The small intestine endoscope has four power modes, and the examination speed is greater than that with the double-balloon and single-balloon endoscope. The curve formed by the endoscope is not associated with any serious effects, and can avoid pain.
Small intestine endoscopes have been developed independently in China, with regard to the intellectual property rights. Three patent licenses from the Chinese State Intellectual Property Office, as well as two patent licenses from the Japanese State Intellectual Property Office, have been granted for these endoscopes.
In live pigs, they found that somewhat higher negative pressure was needed, as compared to the in vitro condition, in order to prevent slippage of the bowel. Thus, they used a pressure of > 1.00 MPa, which permitted successful examination of the intestines of all 20 animals. The average time of completing the in vivo examinations was 60 ± 7.67 min.
Small intestine endoscope is a medical device for the diagnosis and treatment of small intestine diseases.
This is an interesting study about the small intestine enteroscopy. In this study, Liu et al assessed the feasibility and safety of a novel enteroscopic technique, negative-pressure suction enteroscopy, for examining the small intestine in a porcine model. Experiments in small intestinal loops from 20 pigs, and in vivo experiments in 20 living pigs, were conducted. The authors found that the enteroscopy was safely performed within a reasonable time period and enabled complete visualization of the intestine in most cases.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Shaun C, Satya R S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Romero-Vázquez J, Argüelles-Arias F, García-Montes JM, Caunedo-Álvarez Á, Pellicer-Bautista FJ, Herrerías-Gutiérrez JM. Capsule endoscopy in patients refusing conventional endoscopy. World J Gastroenterol. 2014;20:7424-7433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Liu JH, Liu DY. Pipette type small intestine endoscope. 2007;. |
| 3. | Liu JH, Wang L, Zhang ZL, Wang SQ, Liu DY. Built-in Straw type small intestine endoscope. 2010;. |
| 4. | Liu JH, Wang L, Zhang ZL, Wang SQ, Liu DY. Catheter balloon type small intestine endoscope. 2010;. |
| 5. | Liu JH, Wang L, Zhang ZL, Wang SQ, Liu DY. Built-in Straw type small intestine endoscope. 2011;. |
| 6. | Liu JH, Wang L, Zhang ZL, Wang SQ, Liu DY. Catheter balloon type small intestine endoscope. 2011;. |
| 7. | Wada M, Lefor AT, Mutoh H, Yano T, Hayashi Y, Sunada K, Nishimura N, Miura Y, Sato H, Shinhata H. Endoscopic ultrasound with double-balloon endoscopy in the evaluation of small-bowel disease. Surg Endosc. 2014;28:2428-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Miura Y, Shinozaki S, Hayashi Y, Sakamoto H, Lefor AK, Yamamoto H. Duodenal endoscopic submucosal dissection is feasible using the pocket-creation method. Endoscopy. 2017;49:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Shimatani M, Hatanaka H, Kogure H, Tsutsumi K, Kawashima H, Hanada K, Matsuda T, Fujita T, Takaoka M, Yano T. Diagnostic and Therapeutic Endoscopic Retrograde Cholangiography Using a Short-Type Double-Balloon Endoscope in Patients With Altered Gastrointestinal Anatomy: A Multicenter Prospective Study in Japan. Am J Gastroenterol. 2016;111:1750-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Iwashita C, Miura Y, Osawa H, Takezawa T, Ino Y, Okada M, Lefor AK, Yamamoto H. Laser Imaging Facilitates Early Detection of Synchronous Adenocarcinomas in Patients with Barrett’s Esophagus. Clin Endosc. 2017;50:81-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Shinozaki S, Miura Y, Ino Y, Shinozaki K, Lefor AK, Yamamoto H. Erratum: An Ultrathin Endoscope with a 2.4-mm Working Channel Shortens the Esophagogastroduodenoscopy Time by Shortening the Suction Time. Clin Endosc. 2016;49:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Yokoyama K, Yano T, Kumagai H, Mizuta K, Ono S, Imagawa T, Yamamoto H, Yamagata T. Double-balloon Enteroscopy for Pediatric Patients: Evaluation of Safety and Efficacy in 257 Cases. J Pediatr Gastroenterol Nutr. 2016;63:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Sunada K, Shinozaki S, Nagayama M, Yano T, Takezawa T, Ino Y, Sakamoto H, Miura Y, Hayashi Y, Sato H. Long-term Outcomes in Patients with Small Intestinal Strictures Secondary to Crohn’s Disease After Double-balloon Endoscopy-assisted Balloon Dilation. Inflamm Bowel Dis. 2016;22:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Shinozaki S, Yano T, Sakamoto H, Sunada K, Hayashi Y, Sato H, Lefor AK, Yamamoto H. Long-Term Outcomes in Patients with Overt Obscure Gastrointestinal Bleeding After Negative Double-Balloon Endoscopy. Dig Dis Sci. 2015;60:3691-3696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Yamamoto H, Yano T, Ohmiya N, Tanaka S, Tanaka S, Endo Y, Matsuda T, Matsui T, Iida M, Sugano K. Double-balloon endoscopy is safe and effective for the diagnosis and treatment of small-bowel disorders: prospective multicenter study carried out by expert and non-expert endoscopists in Japan. Dig Endosc. 2015;27:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Khorsandi MA, Karimi N, Samavi S, Hajabdollahi M, Soroushmehr SM, Ward K, Najarian K. Hardware image assessment for wirelessendoscopycapsules. C. onf Proc IEEE Eng Med Biol Soc. 2016;2016:2050-2053. |
| 17. | Girelli CM, Soncini M, Rondonotti E. Implications of small-bowel transit time in the detection rate of capsule endoscopy: A multivariable multicenter study of patients with obscure gastrointestinal bleeding. World J Gastroenterol. 2017;23:697-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Ma JJ, Wang Y, Xu XM, Su JW, Jiang WY, Jiang JX, Lin L, Zhang DQ, Ding J, Chen L. Capsule endoscopy and single-balloon enteroscopy in small bowel diseases: Competing or complementary? World J Gastroenterol. 2016;22:10625-10630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Bandorski D, Kurniawan N, Baltes P, Hoeltgen R, Hecker M, Stunder D, Keuchel M. Contraindications for video capsule endoscopy. World J Gastroenterol. 2016;22:9898-9908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Barkin JA, Barkin JS. Video Capsule Endoscopy: Technology, Reading, and Troubleshooting. Gastrointest Endosc Clin N Am. 2017;27:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Liu JH, Zhang ZL, Wang L, Liu DY, Kong FH, Yu TC, Qin Y, Zhang Y. Study on the intestinal injury induced by small intestinal endoscopy. Zhongguo Weichang Waike Zazhi. 2013;5:494-495. [DOI] [Full Text] |
| 22. | Lee HS, Lim YJ, Shim KN, Moon CM, Song HJ, Kim JO, Jeon SR, Jung DY, Kim JH, Kim KO. Diagnostic Value of Small Bowel Capsule Endoscopy in Isolated Ileitis: A CAPENTRY Study. Dig Dis Sci. 2017;62:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Kakiya Y, Shiba M, Okamoto J, Kato K, Minamino H, Ominami M, Fukunaga S, Nagami Y, Sugimori S, Tanigawa T. A comparison between capsule endoscopy and double balloon enteroscopy using propensity score-matching analysis in patients with previous obscure gastrointestinal bleeding. Scand J Gastroenterol. 2017;52:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Seguí S, Drozdzal M, Pascual G, Radeva P, Malagelada C, Azpiroz F, Vitrià J. Generic feature learning for wireless capsule endoscopy analysis. Comput Biol Med. 2016;79:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Nakamura M, Sato J, Goto H. Optional technique for preventing incomplete colon capsule endoscopy: Abdomen compression method. Dig Endosc. 2016;28:757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Spada C, Pasha SF, Gross SA, Leighton JA, Schnoll-Sussman F, Correale L, González Suárez B, Costamagna G, Hassan C. Accuracy of First- and Second-Generation Colon Capsules in Endoscopic Detection of Colorectal Polyps: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1533-1543.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Friedlander JA, Liu QY, Sahn B, Kooros K, Walsh CM, Kramer RE, Lightdale JR, Khlevner J, McOmber M, Kurowski J. NASPGHAN Capsule Endoscopy Clinical Report. J Pediatr Gastroenterol Nutr. 2017;64:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Kopylov U, Carter D, Eliakim AR. Capsule Endoscopy and Deep Enteroscopy in Irritable Bowel Disease. Gastrointest Endosc Clin N Am. 2016;26:611-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Yung DE, Koulaouzidis A, Avni T, Kopylov U, Giannakou A, Rondonotti E, Pennazio M, Eliakim R, Toth E, Plevris JN. Clinical outcomes of negative small-bowel capsule endoscopy for small-bowel bleeding: a systematic review and meta-analysis. Gastrointest Endosc. 2017;85:305-317.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Takamaru H, Yamada M, Sakamoto T, Nakajima T, Saito Y, Kakugawa Y, Matsumoto M, Matsuda T, Ide D, Saito S. Dual camera colon capsule endoscopy increases detection of colorectal lesions. Scand J Gastroenterol. 2016;51:1532-1533. [PubMed] |