Published online Mar 14, 2017. doi: 10.3748/wjg.v23.i10.1920
Peer-review started: October 28, 2016
First decision: December 19, 2017
Revised: January 5, 2017
Accepted: January 18, 2017
Article in press: January 18, 2017
Published online: March 14, 2017
Processing time: 137 Days and 22.2 Hours
Gastrointestinal stromal tumors (GISTs) represent the most common mesenchymal tumors of the alimentary tract. These tumors may have different clinical and biological behaviors. Malignant forms usually spread via a hematogenous route, and lymph node metastases rarely occur. Herein, we report a patient with a jejunal GIST who developed supraclavicular lymph node metastasis. We conclude that lymphatic diffusion via the mediastinal lymphatic station to the supraclavicular lymph nodes can be a potential metastatic route for GISTs.
Core tip: Unlike gastrointestinal carcinomas, lymph node metastases rarely develop in patients with malignant gastrointestinal stromal tumors (GISTs). We report a patient with a jejunal GIST who developed supraclavicular lymph nodes metastasis and review the related literature. We conclude that lymphatic diffusion via mediastinal lymphatic station to the supraclavicular lymph nodes can be a potential metastatic route of GISTs.
- Citation: Ma C, Hao SL, Liu XC, Nin JY, Wu GC, Jiang LX, Fancellu A, Porcu A, Zheng HT. Supraclavicular lymph node metastases from malignant gastrointestinal stromal tumor of the jejunum: A case report with review of the literature. World J Gastroenterol 2017; 23(10): 1920-1924
- URL: https://www.wjgnet.com/1007-9327/full/v23/i10/1920.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i10.1920
Gastrointestinal stromal tumors (GISTs) represent the most common mesenchymal tumors of the alimentary tract. These tumors may have different clinical and biological behavior. Malignant forms usually spread via hematogenous route, and lymph nodes metastases rarely occur. Herein, we report a patient with jejunal GIST who developed supraclavicular lymph nodes metastasis.
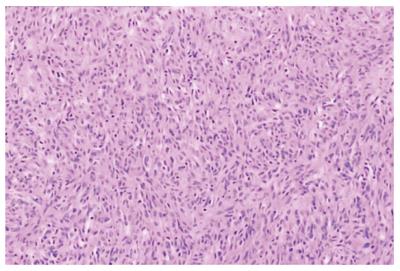
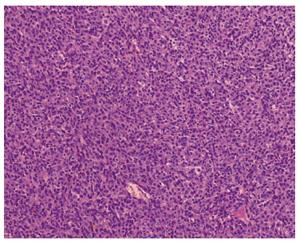
A 56-year-old man with a 24-h history of melena was admitted to the gastrointestinal department of the Yuhuangding Hospital affiliated to Qingdao University, China. Laboratory assessment revealed a hemoglobin level of 8 g/dL. Urgent gastroduodenoscopy, colonoscopy, and enhanced computed tomography (CT) did not reveal any source of bleeding. During the next 24 h, the patient had further episodes of melena and became hemodynamically unstable after receiving a transfusion of 5 units of packed red blood cells and hemostatic agents. Therefore, emergency laparotomy was performed. Upon surgical exploration, a bleeding solid mass was found in the jejunum. Resection of a small bowel loop measuring 20 cm in length was performed. Gross examination revealed a nodular well-encapsulated tumor measuring 2 cm. Histologic sections showed a GIST infiltrating through all bowel layers, and it had features of mixed spindle and epithelioid types of cells. The mitotic index was > 5/50 high-power fields (HPFs). There was no infiltration of the surgical margins, and two harvested lymph nodes were free of metastases (Figure 1). CD117, CD34, and Dog-1 were positive in immunohistochemical studies; the Ki67 index was 20%. A diagnosis of GIST of the small intestine with high-grade malignancy was established based on the modified NIH GIST criteria[1]. Postoperative total body CT scan and positron emission tomography-computed tomography (PET-CT) were negative for metastatic disease. Adjuvant imatinib therapy was prescribed, but he declined it due to family and economic reasons.
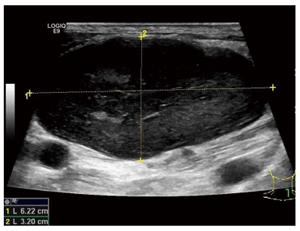
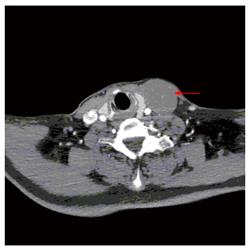
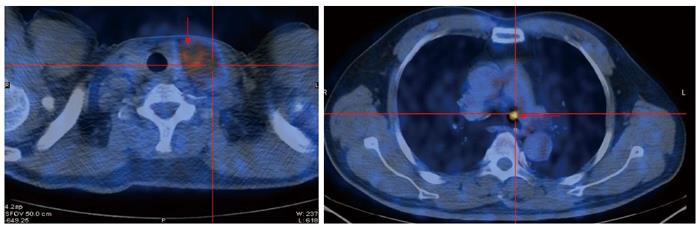
One year later, the patient was admitted to the thyroid department at same hospital complaining of a left cervical mass that had been gradually enlarging over one month. Ultrasound revealed a hypoechoic mass above the left clavicle, measuring 3.1 cm x 4.6 cm; this mass was unenhanced in a contrast-enhanced CT scan (Figures 2 and 3). Interestingly, there were no alterations of the thyroid in imaging studies. Core needle biopsy was performed, and histopathological examination with hematoxylin-eosin staining revealed lymph node metastasis from a GIST. In immunohistochemical studies, CD117, CD34, and vim were positive, whereas calponin, estrogen, progesterone, and thyroglobulin were negative. A PET-CT scan showed uneven 18F-fluorodeoxyglucose (FDG) uptake in the cervical mass and multiple lymph nodes in the mediastinum (Figure 4). The patient underwent surgical removal of the cervical mass. Gross examination of the excised lymph node demonstrated that it measured 5 cm x 6 cm x8 cm and appeared irregular, smooth, and well encapsulated. Histopathologic examination again confirmed a metastasis from a GIST (Figure 5). Immunohistochemical studies of the resected lymph node showed positivity for CD117 and CD34 and negativity for Dog-1 and S-100. The Ki67 index was 30%. Insertion of GCC TAT in exon 9 of the c-KIT gene was identified with mutation analysis. Exons 11, 13, and 17 and the PDGFRα gene were wild type. After the second operation, the patient was regularly given imatinib at a dose of 400 mg per day. At 1 year following the surgery, the patient was asymptomatic, and there were no signs of tumor recurrence or progression (Table 1).
| Ref. | Primary site | HPF | T size (cm) | Treatment | LNM site | LNM time | Gene mutation |
| Sato et al[7] | Gastric | 4 | Proximal gastrectomy | Right cardia | Pre | deletion mutation in exon 11 | |
| Gastric | 2.5 | Wedge resection + partial hepatectomy | Adjacent to the tumor | Pre | No mutation | ||
| El Demellawy et al[8] | Small bowel | Mesenteric | Pre | ||||
| Hu et al[9] | Hepatic | 4/10 | 15 × 10 | Right hepatic lobectomy | Hilar | Post | |
| Canda et al[12] | Gastric | 25/50 | 8 × 8 × 4 | Distal gastrectomy + perigastric LN dissection | Perigastric | Pre | No mutation |
| Kong et al[13] | Small intestinal | 2/50 | 6 × 7 | Partial resection of the ileum | Peri-intestine | Pre | deletion 559-569 in exon 11 |
| Small intestinal | 2/50 | 5 × 5 | Partial resection of the ileum | Peri-intestine | Pre | Deletion 559-565 in exon 11 | |
| Zhang et al[14] | Gastric | Distal gastrectomy, perigastriclymphadenectomy and hepatectomy | Inguinal LN | Post | deletion 557/558 in exon 11 | ||
| Yamada et al[15] | Gastric | > 5/50 | 4.5 × 3.5 | Gastrectomy + lymph node dissection | Perigastric | Pre | |
| Catani et al[19] | Gastric | Gastrectomy + resection of the tail of the pancreas, the spleen, and the transverse colon | Perigastric | Pre | |||
| Masuda et al[16] | Esophagus | 15/50 | 9.5 | Subtotal esophagectomy | Periesophagus | Pre | |
| Shafizad et al[17] | Gastric | 8 | Total gastrectomy and omentectomy | Perigastric | Pre | ||
| Vassos et al[18] | Ileum | Partial resection of the ileum | Inguinal | Pre | |||
| Gastric | Extended gastrectomy, atypical liver resection, splenectomy | Auxiliary | Post | ||||
| Sakurai et al[10] | Esophagus | Middle and lower esophagectomy | Multiple | Post | |||
| Asakage et al[11] | Gastric | Total gastrectomy with distal pancreatosplenectomy and segmental liver resection | Perigastric | Pre | |||
| Tashiro et al[5] | Gastric | 1-5 | No mutation | ||||
| Gastric | Ki67 10% | 2.5 | Proximal gastrectomy with sampling of the regional LNs | Exon 11 |
GISTs represent the most common neoplasms of mesenchymal origin of the gastrointestinal tract. GISTs may have different clinical and biological behavior, ranging from small benign tumors to aggressive forms that have a dismal prognosis. Approximately 20%-25% of GISTs are located in the stomach, and 40%-50% of those located in the small intestine were malignant neoplasms with features such as local recurrence after surgical removal, intraperitoneal dissemination and distant metastases[2]. However, unlike gastrointestinal carcinomas, lymph node metastases (LNMs) rarely develop in patients with malignant GISTs. The mainstay of treatment for GISTs is complete surgical resection without a regional lymph adenectomy[3,4].
The rates of LNMs from GISTs range from 0% to 5%[3,5,6]. A few studies reporting on this subject are summarized in Table 1[5,7-19]. Most of the reported cases are peritumoral lymph nodes metastases, which have occasionally been discovered with histopathological examination of surgical specimens. We found only 3 cases that could be defined as distant LNMs[14,18], including 2 inguinal lymph nodes and 1 axillary lymph node. In our case, LNMs developed in the left supraclavicular and mediastinal basins. This behavior is similar to that observed in malignant gastrointestinal tumors of an epithelial origin. To the best of our knowledge, this is the first case report of the lymphatic spread of a gastric GIST to supraclavicular and mediastinum lymph nodes. This might indicate that a particular subgroup of GISTs has biological characteristics similar to carcinomas.
According to the modified NIH GIST criteria, our case was a high-grade malignancy[1]. Ki67 expression changed from 20% in the primary tumor to 30% in the supraclavicular metastasis. Interestingly, Dog-1 was negative in the LNM, whereas it was positive in primary tumor. It could be speculated that the Ki67 and Dog-1 levels may be markers of a primary tumor de-differentiation tendency.
Activating mutations of the c-kit gene (especially exons 11 and 9) are present in most GISTs and probably play a fundamental role in the development of these tumors. Among the reported cases of LNMs from GISTs, few gene detection results have been described, most of which are exon 11 mutations[5,7,13,14]. In the study by Kong et al[13], the exon 11 mutation was linked to the likelihood of LNMs. However, in our case, we found an exon 9 mutation. This genetic mutation in the LNMs from GISTs has not been reported to date. The relationship between gene mutations and LNMs is still not clear, but many authors have stated that KIT exon 9-mutant tumors developed imatinib resistance more frequently than exon 11-mutant tumors[20,21]. Cases with exon 9-mutant tumors should be treated with increased imatinib doses. Because the patient declined imatinib treatment after the first surgery, he was treated with 400 mg of imatinib per day after the second operation.
In conclusion, complete surgical resection remains the mainstay of treatment for resectable GISTs. Imatinib is currently indicated for the first-line treatment of patients with metastatic or unresectable KIT-positive GISTs. Adjuvant therapy with imatinib was deemed necessary for this patient following complete resection of a primary jejunual tumor because it was an aggressive, high-risk form of GIST. Unfortunately, he did not take imatinib after his first operation, and distant lymph node metastases occurred after 12 months. Following the second operation, the patient received imatinib treatment and had survived without disease progression at the 1-year follow up.
This case confirms that LNMs in the mediastinum and supraclavicular lymph nodes is a potential metastatic route for malignant GISTs. Further studies are needed to clarify the mechanism of lymph node metastases in patients with GISTs.
The patient was admitted to hospital, complaining of a left cervical mass, which had been diagnosed as a jejunum gastrointestinal stromal tumor (GIST) and cured by surgery 1 year before.
For the differential diagnoses of thyroid tumor, lymphoma, or metastatic carcinoma, the patient underwent computed tomography (CT), ultrasound (US), positron emission tomography-computed tomography (PET-CT) and biopsy. US, CT and PET-CT revealed a hypoechoic, unenhanced and uneven FDG uptake mass above the left clavicle measuring 3.1 cm × 4.6 cm.
After a biopsy of the cervical mass, this patient was diagnosed as having supraclavicular lymph node metastases from GISTs.
Core needle biopsy was carried out, and the histopathological examination using hematoxylin-eosin stain showed lymph node metastasis from GIST.
The patient underwent surgical removal of the cervical mass and was regularly given imatinib 400 mg per day after the second operation.
This case confirms that LNM in the mediastinum and supraclavicular lymph nodes are a potential metastatic route of malignant GISTs. Physicians should be aware of this during operation and chemotherapy. In this case report, we tried to give some but not sufficient evidence of the possible mechanisms of the supraclavicular lymph node metastasis.
This case report is well organized and had much information including genetic analysis data on primary GIST and metastatic lesion.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Alimehmeti RH, Kim ST S- Editor: Qi Y L- Editor: Ma JY E- Editor: Wang CH
| 1. | Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 865] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 2. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58. [PubMed] |
| 4. | Pierie JP, Choudry U, Muzikansky A, Yeap BY, Souba WW, Ott MJ. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg. 2001;136:383-389. [PubMed] |
| 5. | Tashiro T, Hasegawa T, Omatsu M, Sekine S, Shimoda T, Katai H. Gastrointestinal stromal tumour of the stomach showing lymph node metastases. Histopathology. 2005;47:438-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Aparicio T, Boige V, Sabourin JC, Crenn P, Ducreux M, Le Cesne A, Bonvalot S. Prognostic factors after surgery of primary resectable gastrointestinal stromal tumours. Eur J Surg Oncol. 2004;30:1098-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 7. | Sato T, Kanda T, Nishikura K, Hirota S, Hashimoto K, Nahagawa S, Ohashi M, Hatakeyama K. Two cases of gastrointestinal stromal tumor of the stomach with lymph node metastasis. Hepatogastroenterology. 2007;54:1057-1060. [PubMed] |
| 8. | El Demellawy D, Shokry P, Ing A, Khalifa M. Polypoid gastrointestinal stromal tumor of small bowel metastasizing to mesenteric lymph nodes: a case report. Pathol Res Pract. 2008;204:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Hu X, Forster J, Damjanov I. Primary malignant gastrointestinal stromal tumor of the liver. Arch Pathol Lab Med. 2003;127:1606-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Sakurai N, Yamauchi J, Shibuma H, Ikeda E, Sasou S. [A case of recurrent GIST of the esophagus which completely responded to imatinib mesilate]. Gan To Kagaku Ryoho. 2007;34:237-240. [PubMed] |
| 11. | Asakage N, Kobayashi S, Gotou T, Sasaki M, Tsukada K, Suzuki T, Yamamoto T, Sekine M, Miyazaki E, Hirai S. [Two cases of gastrointestinal stromal tumor (GIST) of the stomach and a consideration of its malignancy potential and treatment strategy - report of two cases]. Gan To Kagaku Ryoho. 2007;34:919-923. [PubMed] |
| 12. | Canda AE, Ozsoy Y, Nalbant OA, Sagol O. Gastrointestinal stromal tumor of the stomach with lymph node metastasis. World J Surg Oncol. 2008;6:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Kong M, Wang YL, Xu LJ, Teng XD. [Gastrointestinal stromal tumor of small intestine associated with lymph node metastasis: a report of 2 cases with review of literatures]. Zhonghua Bing Li Xue Za Zhi. 2009;38:617-620. [PubMed] |
| 14. | Zhang Q, Yu JW, Yang WL, Liu XS, Yu JR. Gastrointestinal stromal tumor of stomach with inguinal lymph nodes metastasis: a case report. World J Gastroenterol. 2010;16:1808-1810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Yamada E, Oyaizu T, Miyashita T. [A case of gastrointestinal stromal tumor of the stomach with lymph node metastasis followed up for 7 years without evidence of recurrence after surgery]. Nihon Shokakibyo Gakkai Zasshi. 2010;107:743-749. [PubMed] |
| 16. | Masuda T, Toh Y, Kabashima A, Aoki Y, Harimoto N, Ito S, Taomoto J, Ikeda O, Ohga T, Adachi E. Overt lymph node metastases from a gastrointestinal stromal tumor of the esophagus. J Thorac Cardiovasc Surg. 2007;134:810-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Shafizad A, Mohammadianpanah M, Nasrolahi H, Mokhtari M, Mousavi SA. Lymph Node Metastasis in Gastrointestinal Stromal Tumor (GIST): to Report a Case. Iran J Cancer Prev. 2014;7:171-174. [PubMed] |
| 18. | Vassos N, Agaimy A, Hohenberger W, Croner RS. Extraabdominal lymph node metastasis in gastrointestinal stromal tumors (GIST). J Gastrointest Surg. 2011;15:1232-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Catani M, De Milito R, Simi M. [New orientations in the management of advanced, metastatic gastrointestinal stromal tumors (GIST): combination of surgery and systemic therapy with imatinib in a case of primary gastric location]. Chir Ital. 2005;57:127-133. [PubMed] |
| 20. | Heinrich MC, Owzar K, Corless CL, Hollis D, Borden EC, Fletcher CD, Ryan CW, von Mehren M, Blanke CD, Rankin C. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26:5360-5367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 459] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 21. | Lee JH, Kim Y, Choi JW, Kim YS. Correlation of imatinib resistance with the mutational status of KIT and PDGFRA genes in gastrointestinal stromal tumors: a meta-analysis. J Gastrointestin Liver Dis. 2013;22:413-418. [PubMed] |