Published online Feb 28, 2016. doi: 10.3748/wjg.v22.i8.2636
Peer-review started: September 19, 2015
First decision: November 5, 2015
Revised: November 19, 2015
Accepted: December 8, 2015
Article in press: December 8, 2015
Published online: February 28, 2016
Processing time: 160 Days and 2.8 Hours
Condyloma acuminatum (CA) is a common sexually transmitted disease caused by human papilloma virus infection. Not all individuals develop persistent, progressive disease, but careful follow up is required with moderate-to-severe dysplasia to prevent progression to malignancy. Standard therapies include surgical treatments (trans-anal resection and trans-anal endoscopic microsurgery) and immunotherapeutic and topical methods (topical imiquimod); however, local recurrence remains a considerable problem. Here, we report a case with superficial CA of the anal canal, treated with endoscopic submucosal dissection (ESD). A 28-year-old man presented with a chief complaint of hematochezia. Digital exam did not detect a tumor. Screening colonoscopy revealed 10-mm long, whitish condyles extending from the anal canal to the lower rectum. The lesion covered almost the whole circumference, and only a small amount of normal mucosa remained. Magnifying endoscopy with narrow band imaging showed brownish hairpin-shaped, coiled capillaries. Although histopathological diagnosis by biopsy revealed CA, accurate histological differentiation between CA, papilloma, and squamous cell carcinoma can be difficult with a small specimen. Therefore, we performed diagnostic ESD, which provides a complete specimen for precise histopathological evaluation. The pathological diagnosis was CA, with moderate dysplasia (anal intraepithelial neoplasia 2). There was no recurrence at 16 mo after the initial ESD. Compared to surgical treatment, endoscopic diagnosis and resection could be performed simultaneously and the tumor margin observed clearly with a magnifying chromocolonoscopy, resulting in less recurrence. These findings suggest that endoscopic resection may be an alternative method for CA that prevents recurrence.
Core tip: We report a case of condyloma acuminatum (CA) in a patient who underwent endoscopic submucosal dissection (ESD). Although most cases of CA have been treated with surgery or immunotherapeutic techniques, the rate of local recurrence is high because of difficulty detecting a precise margin of the lesion, especially those located in the anal canal. ESD enables detection of the lesion with magnification chromoendoscopy and treatment in the endoscopic visual field; thereby achieving en-bloc resection. ESD provides precise histopathological evaluation and could be an alternative method for CA that prevents recurrence.
- Citation: Sasaki A, Nakajima T, Egashira H, Takeda K, Tokoro S, Ichita C, Masuda S, Uojima H, Koizumi K, Kinbara T, Sakamoto T, Saito Y, Kako M. Condyloma acuminatum of the anal canal, treated with endoscopic submucosal dissection. World J Gastroenterol 2016; 22(8): 2636-2641
- URL: https://www.wjgnet.com/1007-9327/full/v22/i8/2636.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i8.2636
Condyloma acuminatum (CA) is one of the most common viral sexually transmitted diseases globally[1], affecting as many as 30 million individuals. It is caused by human papilloma virus (HPV) infection, and has an incubation period ranging from 3 wk to 8 mo[2]. Perianal HPV infection is the most common anorectal infection affecting homosexual young men[3] and results in several diseases from asymptomatic infection to genital warts to invasive cancer[4].
Recurrence is a significant issue for CA treatment, ranging from 4.6% to > 70% depending on the treatment modality[5,6]. Inferior visualization of the operative field in surgical treatments, such as trans-anal resection (TAR) and trans-anal endoscopic microsurgery, may contribute to these rates; with these procedures, the margins of rectal lesions spreading to the dentate line are not clearly observed[7]. It is difficult to differentiate neoplasms from non-neoplasms with a small biopsy specimen; diagnostic resection is feasible.
Compared with surgical treatments, endoscopic resection-particularly endoscopic submucosal dissection (ESD)-enables en-bloc resection. A high-definition, magnified view with narrow band imaging (NBI) and indigo carmine dye spray is beneficial for precise tumor margin determination and subsequent reduced recurrence[7]. In addition, transmission or persistence of HPV in the tissues and coexistence with visible warts might increase the risk of recurrence. Therefore, ESD is particularly useful.
Here, we report a case of anal CA treated with ESD.
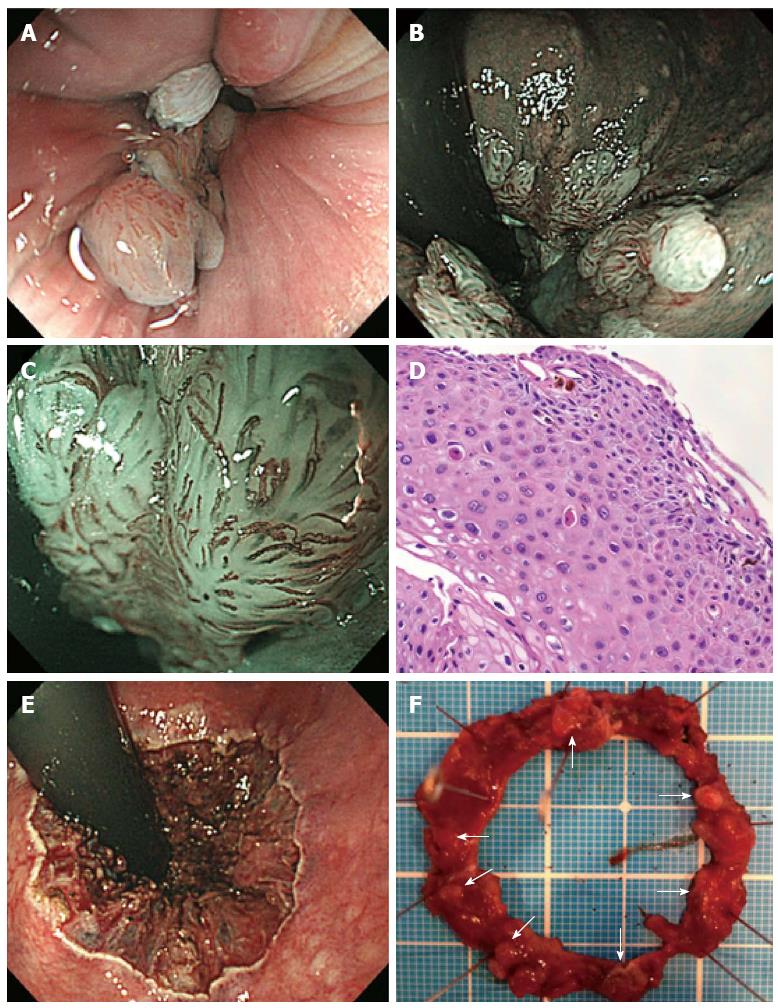
A 28-year-old man presented to the gastroenterology outpatient department after a 6-mo episode of hematochezia. He had a male partner. Physical examination was unremarkable; the digital exam did not reveal a tumor. Laboratory investigations revealed a hemoglobin level of 15.6 g/L, erythrocyte sedimentation rate of 1 mm/h, and normal liver and kidney profiles. Hepatitis B surface antigen, hepatitis C virus antibody, syphilis, and HIV antibody tests were negative. Colonoscopy revealed 10-mm long, whitish condyles extending from the anal canal to the lower rectum (Figure 1A and B). The lesion was situated almost circumferentially; little normal intestinal membrane remained in the proximal-to-distal site, as observed with white light and the NBI system (Figure 1A and B). A single small wart coexisted in the mucosa around the anus. Dilated red vessels on the surface of the lesion in the anal canal as brownish capillaries with a hairpin shape or coil were observed using magnifying endoscopy with the NBI system (Figure 1C). Colonic biopsy specimens revealed acanthotic squamous epithelium with hyperpapillomatosis (Figure 1D). The epithelial cells indicated koilocytosis, single cell keratinization, and mild-to-moderate dysplasia of the squamous epithelium, confirming the diagnosis of CA related to HPV infection. Because the lesion was circumferential and had poor visibility for endoscopic mucosal resection, ESD was performed (Figure 1E and F).
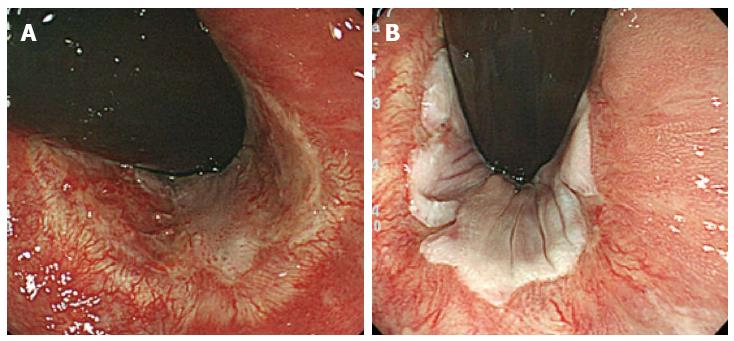
The procedures were primarily performed using a dual-knife or insulation-tipped (IT) knife (Olympus Co, Tokyo, Japan) with carbon dioxide insufflation instead of air insufflation to reduce patient discomfort[8]. Lesion margins were delineated before ESD using 0.4% indigo-carmine dye spray. To prevent pain, 1% lidocaine (100 mg/10 mL) was injected on the anal side of the lesion. Following Glyceol® (Chugai Pharmaceutical Co., Tokyo, Japan; 10% glycerol and 5% fructose in normal saline solution)[9] and sodium hyaluronate acid injection into the submucosal layer[10], a circumferential incision was made using the dual knife. ESD was then performed using both the dual and IT knives. The entire submucosal dissection took 160 min, without any adverse event (Figure 1E and F). The patient complained of anal pain for 3 d, without massive bleeding. We applied corticosteroid ointment to the lesion to prevent stenosis. In addition, the ablation treatment was performed for the other wart on the anus (3 mm in size). Five months post-ESD, colonoscopy revealed no recurrence. He had no pain, bleeding, or dyschezia by stenosis (Figure 2A). Follow-up colonoscopy performed 16 mo post-ESD also showed no apparent recurrence or stenosis (Figure 2B).
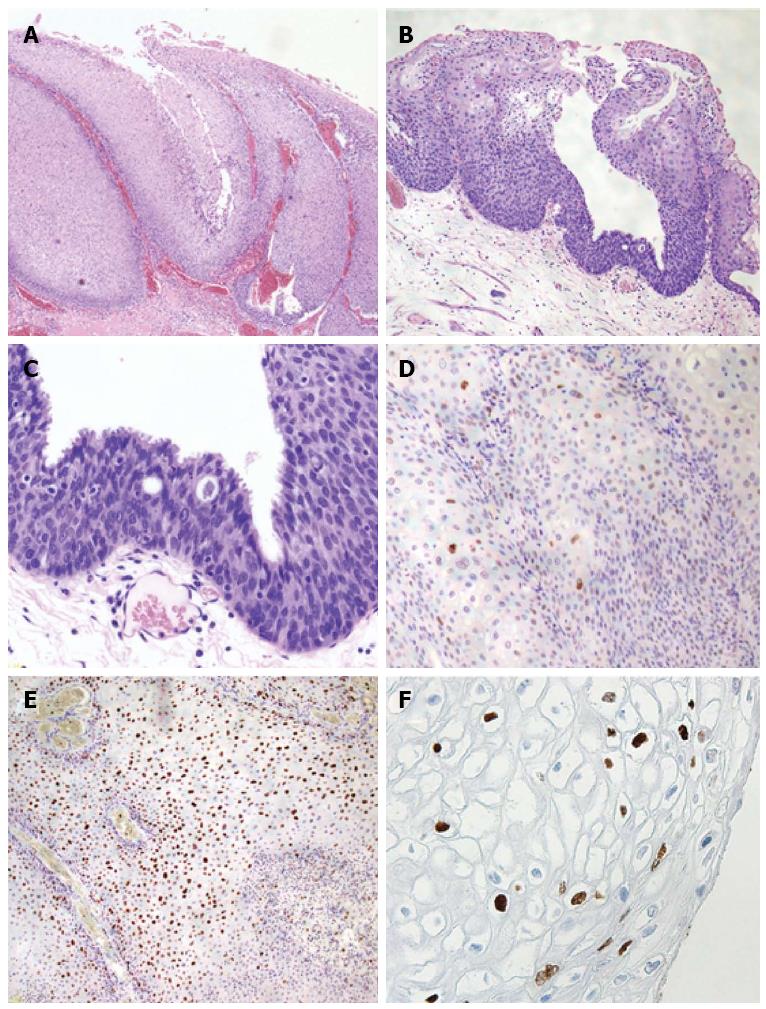
The pathological diagnosis was CA, with moderate dysplasia [anal intraepithelial neoplasia 2 (AIN2); Figure 3A-C]. Immunohistochemical peroxidase staining for p53 protein showed cells were negative (Figure 3D), and the Ki-67 stain showed 40% of cells were positive through the epithelial layer (Figure 3E). Immunohistochemical studies for papillomavirus common antigen were positive, confirming the presence of HPV (Figure 3F). The specimen did not demonstrate any malignancy.
The previously reported standard treatment for CA includes surgical, palliative, or immunotherapeutic techniques, such as topical imiquimod, each with limitations. Post-TAR recurrence for non-invasive rectal tumors varies (2.8%-30%) and is considerably higher than post-ESD recurrence[7]. The present case indicates that ESD is an alternative method with high curability for CA of the anal canal to the lower rectum. This method enables detection of the lesion margin with magnification chromoendoscopy and precise marking in the endoscopic visual field; therefore, en-bloc resection can be achieved.
A qualitative diagnosis can be challenging for rectal lesions spreading to the dentate line. ESD enables a precise pathological diagnosis with safe and en-bloc R0 resection. Piecemeal resection of colorectal lesions generally results in a higher recurrence rate than does ESD, especially in lesions > 20 mm[11,12]. In 122 patients with perianal warts who underwent surgical excisions between 2000 and 2004, local recurrence occurred in 59 patients (48%) within 6 mo after surgery, except for 1 case[13]. Multiple perianal lesions, the presence of an anal canal lesion, and bleeding were significant risk factors for postoperative recurrence after surgical therapy. Warts may also recur after treatment because of the activation of a latent virus in healthy skin adjacent to the lesion[14]. Thus, immunotherapy, such as topical imiquimod, is recommended after surgical therapy. However, topical imiquimod is contraindicated for anal canal lesions because of its toxicity. Topical imiquimod is an immune modulator that induces interferon and cytokine release by the host tissues. Because it causes severe erosion and ulcers, its indication has been limited to pudendal and perianal lesions.
In addition to the many merits of ESD, the method is further improved with magnifying colonoscopy[15]. Although current efforts aim at removing or destroying all visible warts, little is known about subsequent transmission or persistence of papillomavirus in the tissues[16]. Oono reported that latent infection could occur under the seemingly normal epithelium between visible warts[17], as demonstrated histopathologically with endoscopic findings, and suggested that iodine staining and autofluorescence imaging could detect latent infection in the tissues; an unstained lesion with a clear margin was detected via a colonoscopy after iodine staining. These findings may help detect underlying lesions.
Three case reports have been published regarding CA treatment with endoscopy (Table 1)[15,17,18]. In the first case, a lesion of one-third circumference treated with ESD showed AIN2 histopathologically, while the second case with an approximately 15-mm ESD also showed AIN2 histopathologically. In both cases, no obvious recurrence was observed at 3-4 mo follow-up. The third case, with a lesion of 30 mm in diameter that was originally diagnosed as a laterally spreading tumor, was successfully treated with ESD[18]. In the present case, there was no visible recurrence at 16 mo post-ESD. The risk of recurrence is considerably reduced if it does not occur more than 6 mo after CA treatment[13]. In addition to removing the visible warts, underlying lesions should be detected and addressed to reduce the risk of relapse. Careful endoscopic observation with image-enhanced endoscopy may be beneficial.
| Case 1 | Case 2 | Case 3 | Our case | |
| Age (yr) | 76 | 63 | 63 | 28 |
| Sex | F | F | F | M |
| Country | Japan | Japan | Italy | Japan |
| Chief complaint | Anemia | Occult blood | Occult blood | Hematochezia |
| HIV Ab antigen sensitivity | Negative | Negative | Unknown | Negative |
| Location | Lower rectum to anal canal | Lower rectum to anal canal | Lower rectum to anal canal | Anal canal and skin |
| Size | One-third circumference | 15 mm | 30 mm | Almost circumferential |
| Treatment | ESD | ESD | ESD | ESD |
| Pathology | AIN2 | AIN2 | AIN1 | AIN2 |
| Recurrence | No (4 mo f/u) | Unknown | Unknown | No (16 mo f/u) |
In conclusion, endoscopic therapy can be an alternative method for CA in the anal canal. We believe this method will reduce the CA recurrence rate and protect the younger generation from anorectal cancers and the spread of infections. Further studies are needed to confirm our findings that ESD treatment can be useful for anal condyloma acuminatum.
A 28-year-old man, who had a male partner, presented to the gastroenterology outpatient department after a 6-mo episode of hematochezia.
Physical examination was unremarkable; the digital exam did not reveal a tumor.
Squamous cell carcinoma, rectal polyp, and Bowenoid papulosis.
A hemoglobin level of 15.6 g/L, erythrocyte sedimentation rate of 1 mm/h, and normal liver and kidney profiles. Hepatitis B surface antigen, hepatitis C virus antibody, syphilis, and HIV antibody tests were negative.
Colonoscopy revealed 10-mm long, whitish condyles extending from the anal canal to the lower rectum almost circumferentially.
The pathological diagnosis was condyloma acuminatum (CA) with moderate dysplasia, with immunohistochemical staining negative for p53, papillomavirus common antigen was positive, and the Ki67 index was 40% higher than is normal.
The patient was treated with endoscopic submucosal dissection (ESD).
The local recurrence rate of CA is high because of the difficulty of detecting a precise margin of the lesion, especially those located in the anal canal; use of ESD potentially reduces the recurrence of CA.
Immunohistochemical staining is based on antigen-antibody reactions that detect whether there is a target antigen in cells or tissue.
This case report presents a case of CA treated with ESD.
This is a case report on CA, treated with ESD, and it was well-written one and had enough importance for readers.
P- Reviewer: Kobayashi N, Kouraklis G S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Chang GJ, Welton ML. Human papillomavirus, condylomata acuminata, and anal neoplasia. Clin Colon Rectal Surg. 2004;17:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Burk RD, Kelly P, Feldman J, Bromberg J, Vermund SH, DeHovitz JA, Landesman SH. Declining prevalence of cervicovaginal human papillomavirus infection with age is independent of other risk factors. Sex Transm Dis. 1996;23:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 138] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Sohn N, Robilotti JG. The gay bowel syndrome. A review of colonic and rectal conditions in 200 male homosexuals. Am J Gastroenterol. 1977;67:478-484. [PubMed] |
| 4. | Blomberg M, Friis S, Munk C, Bautz A, Kjaer SK. Genital warts and risk of cancer: a Danish study of nearly 50 000 patients with genital warts. J Infect Dis. 2012;205:1544-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Abcarian H, Sharon N. Long-term effectiveness of the immunotherapy of anal condyloma acuminatum. Dis Colon Rectum. 1982;25:648-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Swerdlow DB, Salvati EP. Condyloma acuminatum. Dis Colon Rectum. 1971;14:226-231. [PubMed] |
| 7. | Kiriyama S, Saito Y, Matsuda T, Nakajima T, Mashimo Y, Joeng HK, Moriya Y, Kuwano H. Comparing endoscopic submucosal dissection with transanal resection for non-invasive rectal tumor: a retrospective study. J Gastroenterol Hepatol. 2011;26:1028-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Kozu T, Saito D. A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc. 2007;65:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Uraoka T, Fujii T, Saito Y, Sumiyoshi T, Emura F, Bhandari P, Matsuda T, Fu KI, Saito D. Effectiveness of glycerol as a submucosal injection for EMR. Gastrointest Endosc. 2005;61:736-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Yamamoto H, Sekine Y, Higashizawa T, Kihira K, Kaneko Y, Hosoya Y, Ido K, Saito K, Sugano K. Successful en bloc resection of a large superficial gastric cancer by using sodium hyaluronate and electrocautery incision forceps. Gastrointest Endosc. 2001;54:629-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 12. | Nakajima T, Saito Y, Tanaka S, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc. 2013;27:3262-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 13. | Toyonaga T, Matsushima M, Katori R, Takahashi T, Kiryu K, Sogawa N, Kanyama H, Matsumura N, Shimojima Y, Nozawa M. Factors affecting recurrence after surgical excision for perianal warts. J Jpn Soc Coloproctol. 2006;59:259-264. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Gearhart PA, Randall TC, Buckley RM Jr, Higgins RV. Human papillomavirus. Accessed August 26. 2014; Available from: http://emedicine.medscape.com/article/219110-overview. |
| 15. | Suzuki K, Suzuki T, Fujita N, Noda Y, Hirasawa D, Obana T, Sugawara T, Ohira T, Harada Y, Maeda Y. Anorectal condyloma acuminatum treated with endoscopic submucosal dissection. Gastroenterol Endosc. 2013;55:281-286. [DOI] [Full Text] |
| 16. | Auborn KJ, Carter TH. Treatment of human papillomavirus gynecologic infections. Clin Lab Med. 2000;20:407-422. [PubMed] |
| 17. | Oono Y. Condyloma acuminatum in the anal canal, report of a case. Stomach Int. 2010;45:1699-1707. |
| 18. | Azzolini F, Cecinato P, Iori V, De Marco L, Zecchini R, Fodero C, Tioli C, Sassatelli R. Endoscopic submucosal dissection of an unusual flat rectal neoplasm. Gut. 2015;64:180, 183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |