Published online Jul 21, 2016. doi: 10.3748/wjg.v22.i27.6328
Peer-review started: December 31, 2015
First decision: January 28, 2016
Revised: February 29, 2016
Accepted: March 18, 2016
Article in press: March 18, 2016
Published online: July 21, 2016
Processing time: 198 Days and 3.2 Hours
Bile cast nephropathy is a condition of renal dysfunction in the setting of hyperbilirubinemia. There are very few cases of this condition reported in the last decade and a lack of established treatment guidelines. While the exact etiology remains unknown, bile cast nephropathy is presumed to be secondary to multiple concurrent insults to the kidney including direct toxicity from bile acids, obstructive physiology from bile casts, and systemic hypoperfusion from vasodilation. Therapy directed at bilirubin reduction may improve renal function, but will likely need dialysis or plasmapheresis as well. We report our case of bile cast nephropathy and the therapeutic measures undertaken in a middle-aged male with chronic renal insufficiency that developed hyperbilirubinemia and drug-induced liver injury secondary to antibiotic use. He developed acute renal injury in the setting of rising bilirubin. He subsequently had a progressive decline in renal and hepatic function, requiring dialysis and plasmapheresis with some improvement, ultimately requiring transplantation.
Core tip: The role of bilirubin in causing acute renal insufficiency is not well known. Our case report is one of few documenting evidence of renal insufficiency as a result of hyperbilirubinemia. Diagnosis requires a high index of suspicion in patients with hyperbilirubinemia with concomitant acute renal insufficiency. Renal biopsy is the solitary means of definitive diagnosis. Treatment is targeted at improving hepatic dysfunction and decreasing bilirubin burden. Numerous treatment modalities to reduce bilirubin have been suggested with variable outcomes.
- Citation: Patel J, Walayat S, Kalva N, Palmer-Hill S, Dhillon S. Bile cast nephropathy: A case report and review of the literature. World J Gastroenterol 2016; 22(27): 6328-6334
- URL: https://www.wjgnet.com/1007-9327/full/v22/i27/6328.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i27.6328
Cholestatic liver disease has a number of consequences on renal function. Most of the clinical manifestations are secondary to hemodynamic changes resulting in a pre-renal state. In patients with profound hyperbilirubinemia, bile casts result in direct toxicity to the nephron. It is commonly known as bile cast nephropathy and has been described under various terms including cholemic nephrosis, biliary nephrosis and jaundice-related nephropathy. This entity has been reported as early as 1899 when patients with jaundice and renal failure were found to have bile cast deposition in their kidneys on biopsies. Only a handful of cases were reported until van Slambrouck et al[1] published a study of 44 patients with bile cast nephropathy. It is characterized by the presence of bile casts on renal biopsy in the setting of hyperbilirubinemia and renal insufficiency[1,2]. Its prevalence is likely greater than previously recognized. Early recognition and treatment are essential as they may lead to reversal of symptoms and improved prognosis. Presently, there is little literature about the disease and its etiology with only a few case reports in the past decade[3-8].
Herein, we report a case of bile cast nephropathy in a patient who developed acute renal injury in the setting of rising bilirubin and with improvement as bilirubin levels decreased.
A 54-year-old Caucasian male presented to the emergency department with a 3 wk history of progressive jaundice, intractable pruritus, and anorexia. Two weeks prior to symptom onset, he was diagnosed with chronic left heel osteomyelitis and was started on antibiotic therapy with Piperacillin/tazobactam. At the time of evaluation, signs and symptoms of hepatic dysfunction were persistent despite discontinuation of antibiotic therapy. His past medical history was significant for well-controlled diabetes mellitus (most recent Hgb A1c of 5.6), hypertension, chronic sinusitis, chronic renal insufficiency (baseline creatinine of 2.1) and hyperlipidemia. Surgical history included a distant cholecystectomy and a partial nephrectomy for a complex renal cyst. Family history was negative for any liver or kidney disease. Social history was negative for alcohol use or tobacco use, intravenous drug abuse, tattoos, history of blood transfusions or high risk sexual behavior. On physical exam, he was alert and oriented with jaundice, a distended abdomen, and a 1+ bilateral pitting edema of his lower extremities. The rest of the physical exam was unremarkable. Laboratory studies at the time of presentation were significant for a sodium of 135 mmol/L, potassium of 4.4 mmol/L, BUN of 40 mg/dL, creatinine of 2.13 mg/dL, albumin of 2.8 g/dL, AST of 121 U/L, ALT of 129 U/L, alkaline phosphatase of 851 U/L, a GFR of 33 and a total bilirubin of 19.3 mg/dL. His CBC was significant for hemoglobin of 11.5 g/dL, MCV 95.6 fl, RDW 18.2, WBC 10.56/mcl, platelet count 506/mcl and his INR was 1.4. A urinalysis was unremarkable with the exception of trace protein and presence of bile. At baseline, he had normal liver enzyme values and a creatinine of 2.1 mg/dL. An ultrasound of the abdomen revealed mild splenomegaly, ascites, and absence of biliary ductular dilation. A subsequent CT and MRCP were also negative for evidence of biliary pathology. The patient was admitted with a presumptive diagnosis of drug-induced liver injury (DILI) with the differential diagnoses including acute viral hepatitis, autoimmune liver disease, and acute decompensation of chronic liver disease. His workup was negative for HAV, HBV, HCV, EBV, and HSV. Ceruloplasmin levels and autoantibodies were also negative. He was treated supportively with intravenous hydration, initiation of ursodeoxycholic acid, cholestyramine and N-acetylcysteine for possible DILI. The patient experienced continued deterioration of his hepatic and renal function. A trans-jugular liver biopsy revealed portal and peri-portal fibrosis (stage 1-2), marked hepatocanalicular cholestasis with focal bile infarcts and multiple pseudo-ground glass inclusions within the hepatocytes consistent with DILI. Additionally, his peri-hepatic pressures were normal. The patient’s renal function continued to progressively deteriorate with increasing azotemia and oliguria. Laboratory data at this time was significant for a urine sodium less than 20 mmol/L, BUN of 81 mg/dL and creatinine of 5.1 mg/dL, suggesting a pre-renal origin with hepatorenal syndrome in the differential diagnosis. His urine analysis revealed 3+ RBCs, 5-10 WBCs, positive leukocyte esterase and presence of eosinophils. The presence of eosinophils suggested an immune-mediated process such as a vasculitis or interstitial nephritis. His ANCA, complement C3, and C4 levels however, were normal.
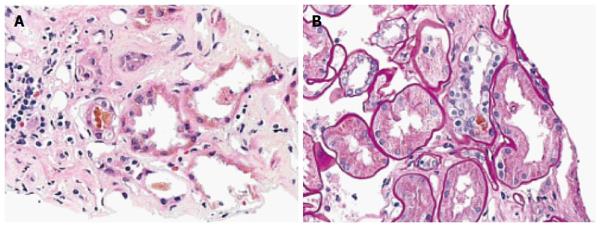
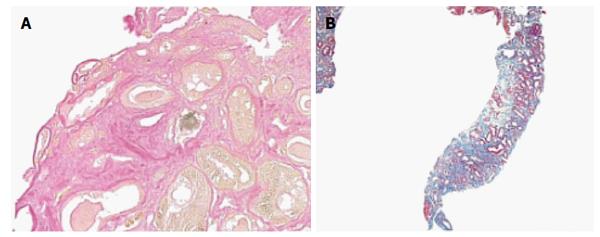
The patient began empiric therapy with levofloxacin and steroids due to concerns of a urinary tract infection, rapidly progressive glomerulonephritis, and/or interstitial nephritis. Urine cultures were positive for E. coli and his levofloxacin was changed to meropenem. During this time, his renal function continued to decline and he underwent a renal biopsy that revealed thickening of the glomerular basement membrane and sclerosis of Bowman’s capsule. In addition, both proximal and distal tubules contained pigmented bilirubin casts and droplets (Figure 1). The cross sections stained positive with Fouchet’s stain (Figure 2A). Lastly, tubular atrophy and interstitial fibrosis were seen (Figure 2B). Immunostaining was negative for IgG, IgM, C3, C4, C1q, albumin, as well as kappa and lambda light chains.
Hemodialysis (HD) was initiated while the patient continued treatment with steroids, ursodeoxycholic acid, and cholestyramine. After approximately two weeks of HD, his bilirubin persisted in the 20 mg/dL range and he remained oliguric (Table 1). Subsequently, plasmapheresis and diuretic therapy with furosemide and spironolactone were initiated with a decrease in bilirubin to 10 mg/dL after multiple treatments and improvement in oliguria. He was closely monitored for any infectious or bleeding complications and was discharged home with continued plasmapheresis as needed to maintain plasma bilirubin levels of approximately 10 mg/dL and under.
| Date | On admission | 18-Feb | 26-Feb | 3-Mar | 6-Mar | 12-Mar | 15-Mar | 25-Mar | 26-Mar | 1-Apr | 15-Apr | 20-Apr | 21-Apr | 7-May | 9-May |
| Treatment date | 0 | 28 | 36 | 41 | 44 | 50 | 53 | 63 | 64 | 70 | 84 | 89 | 90 | 106 | 108 |
| Dialysis Initiated | Plasmapheresis Initiated | CVC removed | |||||||||||||
| Creatinine | 2.13 | 2.89 | 4.18 | 4.92 | 5.47 | 1.75 | 2.58 | 3.63 | 3.49 | 3.01 | 3.6 | 4.26 | 4.43 | 5.14 | 4.73 |
| T. Bili | 19.3 | 18.2 | 22.9 | 26.1 | 29.0 | 19.5 | 17.9 | 25.3 | 20.4 | 12.6 | 10.9 | 18.0 | 29.7 | 27.1 |
He returned to the hospital 2 wk post-discharge with a central venous catheter infection, requiring removal of his temporary dialysis catheter and cessation of plasmapheresis. Despite cessation of plasmapheresis, the patient was maintaining adequate urine output of approximately 1L per day with stable electrolyte and acid-base status. However, he rapidly developed recurrent hyperbilirubinemia (T. bili 29.7 mg/dL) and progressive renal dysfunction (Cr 5.14, GFR 12-13, urine sodium 89 mmol/L, bilirubinuria and bilirubin crystals present in urine). He was also noted to have ascites and required large volume paracentesis with protein gradients consistent with portal hypertension-related ascites. A repeat liver biopsy was performed which showed stage 3-4 bridging fibrosis with focal nodule formation along with prominent bile ductular proliferation and focal ductular cholestasis. In light of his MELD score of 38, the patient underwent a successful liver and kidney transplant with resolution to normal of his hepatic and renal indices (Table 2).
| Date | February 22, 2016 |
| Creatinine (mg/dL) | 0.96 |
| T. Bili (mg/dL) | 0.5 |
Bile cast nephropathy is a rare and poorly understood entity characterized by progressive renal insufficiency in the setting of elevated serum bile salts and hyperbilirubinemia. Elevated total bilirubin levels, typically greater than 20 mg/dL, are reported in cases of bile cast nephropathy. Both direct and indirect bilirubin may be involved, suggesting a primary hepatocellular dysfunction. Renal biopsies in these patients typically show bile cast formation in the setting of elevated bilirubin and serum creatinine levels[5].
This entity was first described by Qunicke in 1899, when autopsies from patients with acute onset jaundice and renal failure showed deposition of bile pigments in the renal glomeruli. In 1922, Hessler showed that severe jaundice was associated with the presence of marked granulated cells and free bilirubin in the urine of dogs and humans, suggesting that the accumulation of bile in the renal cortex was nephrotoxic[9]. Subsequently, in 1937, Elsom[10] also observed that jaundice was associated with impairment of renal function that was reversible with the resolution of hyperbilirubinemia. In 2006, Betjes and Bajima used the term “jaundice-related nephropathy” for the historical term cholemic nephropathy for changes ranging from proximal tubular dysfunction to renal failure due to the deposition of bile and bile salts[4].
The exact etiology remains unknown, but any insult leading to profound bilirubinemia (whether hepatic or extrahepatic) may progress to this condition. Alcohol may exacerbate or be an etiologic factor with this entity, as all ten patients in van Slambrouck’s study with cirrhosis secondary to alcoholism had bile casts present on autopsy. These casts were absent, however, in all patients with cirrhosis secondary to hepatitis C (5 patients) or with indirect hyperbilirubinemia (2 patients)[1]. Other reported etiologies of bile cast nephropathy included patients with hyperbilirubinemia as a result of infectious mononucleosis and steroid use[3,5]. Yet another case involved a patient with colorectal cancer 3 wk after a wedge resection[8].
This disease entity likely represents a broad spectrum of disease, from mild reversible changes in those without underlying renal dysfunction and hyperbilirubinemia of short duration, to irreversible progressive disease in those with underlying renal insufficiency with prolonged and severe hyperbilirubinemia. The exact mechanism in which bile and bile salts cause acute tubular injury remains unknown, however, various mechanisms are worth considering.
During cholestasis, hepatocytes attempt to export bile acids to prevent intracellular damage by inducing basolateral bile acid pumps. The kidneys, similarly, undergo changes in the proximal tubule to excrete excess bile. This excess bilirubin is believed to cause oxidative damages of the cell membranes of the tubules and uncoupling of mitochondrial phosphorylation at the cellular level[11,12]. Furthermore, inhibition of the Na-H, Na-K, Na-Cl pumps by sulfated bile salts in proximal tubules and in the loop of Henle may result in pH changes which may enhance bile cast deposition, tubular toxicity and injury[2,4,13,14]. It has been hypothesized that there is a limit to bilirubin transport in the proximal tubules after which they become saturated, leading to cast formation and tubular obstruction[1,3]. These findings are supported by a study that demonstrated a direct correlation between the severity of hepatic dysfunction as measured by ALT, and the severity of renal dysfunction[15]. The 35 patients studied by Elini Bairtakari with obstructive jaundice had no underlying renal or chronic hepatic disease and had a baseline conjugated bilirubin of 10 mg/dL. They found changes consistent with proximal tubular damage in the form of glucosuria, phosphaturia, and microglobinuria. They also reported decreased serum uric acid and phosphate levels which were inversely proportional to the total and indirect bilirubin levels. The treatment of jaundice resulted in improvement of proximal tubule function[4,13].
Hemodynamic changes resulting in pre-renal azotemia may also be contributory. Elevated levels of bile salts have been demonstrated to have negative chronotropic and ionotropic effects, resulting in cardiovascular instability and decreased renal perfusion. This is further exacerbated by changes in endovascular reactivity believed to be due to the prevalence of endotoxemia, hypoalbuminemia, and nitric oxide-mediated mechanisms resulting in decreased peripheral vascular resistance and decreased renal blood flow, resulting in ischemia to the kidney. Studies in mice support this two-hit mechanism of renal dysfunction[2,16]. Aoyagi and Lowenstein observed that mice that were infused with bile acid followed by inducing renal ischemia for 30 min developed renal insufficiency. However, mice that were infused with either bile acid alone or underwent induced renal ischemia alone for 30 min did not develop renal insufficiency, supporting the concept of a two hit phenomenon[17]. While the dysfunction in this study was reversible with removal of the stressors, it is likely that those with chronic underlying disease or prolonged hyperbilirubinemia may result in irreversible changes.
Renal dysfunction is exhibited by elevated creatinine levels, pigmented bile crystals on urinalysis, natriuresis, and B2 microglobinuria[18]. Urine osmolality has been reported to fall significantly in rats as early as 24 h after bile duct ligation representing an underlying concentrating defect[19].
Sitprija et al[2] studied 15 patients with obstructive jaundice secondary to cholangiocarcinoma with no clinical evidence of hemolysis, renal dysfunction, or cardiac dysfunction at baseline. No changes in renal function were observed in patients with bilirubin levels less than 15 mg/dL. At bilirubin levels greater than 26 mg/dL, there was evidence of decreased free water clearance, creatinine clearance, and mean arterial pressure. At bilirubin levels greater than 40 mg/dL, renal perfusion was also decreased. The two patients described by Betjes and Bejemia with obstructive jaundice also had findings consistent with those of Sitprija et al[2]. These studies found pronounced GFR loss (mean creatinine 3.2 mg/dL) with natriuresis (mean urine sodium 57 mmol/L) in the presence of hyperbilirubinemia (mean T. bili 30 mg/dL) and low serum albumin (mean 34 g/L) concentration[4]. Glucosuria, phosphaturia, and microglobinuria have also been reported[8].
The primary findings in bile cast nephropathy include renal tubular hypertrophy, the presence of pigmented bile casts within the renal tubules and absence of glomerular pathology[20-22]. Other findings reported include tubular damage with evidence of dilatation of the lumen and cytoplasmic vacuolization, as well as the presence coarse granular brown casts[3]. Electron microscopy findings include dilated mitochondrial cristae and bile acid accumulation within lysosomes. Bile cast presence was more pronounced in the distal segments of nephrons, but was found in more proximal tubules with increasing severity of hyperbilirubinemia[5]. In this patient, bile cast deposition was seen in both proximal and distal tubules along with ischemic changes.
Historically, studies in patients with hyperbilirubinemia have shown histologic changes including glomerular congestion, nuclear extrusion with vacuolization, and necrosis in proximal convoluted tubules. Additionally, dilation with lymphocytic collection and necrosis in secondary convoluted tubules, interstitial edema, and bile cast deposition were also seen[21].
Van Slambrouck et al[1] studied the renal biopsies of 44 patients with severe liver dysfunction and bile casts were observed in 24 of these patients. Involvement of the distal nephron was found in mild cases (18 patients) and involvement of the proximal tubule was found in severe cases (6 patients). The mean serum total bilirubin was 26.2 mg/dL, direct bilirubin was 16.3 mg/dL, mean serum creatinine was 2.3 mg/dL, and mean albumin was 31 g/L in their patient population with renal casts. Sixty six percent of the patients in their study with bile casts had histological evidence of acute kidney injury giving the strongest evidence to date that bilirubin and bile salts are directly nephrotoxic.
Owing to the rarity of this condition, there are currently no accepted treatment guidelines. Interventions to reduce bilirubin burden, such as relief of biliary obstruction via ERCP with stent placement and hemodialysis, have been attempted to improve outcomes. While this appears to be an effective strategy early in the disease course, its efficacy in established disease is uncertain[4,6,7,23]. Plasmapheresis may additionally be of utility.
Other extracorporeal treatment options aimed at reduction of inflammatory cytokines and reduction of bilirubin have also emerged, including the molecular adsorbents recycling system (MARS), coupled plasma filtration adsorption (CPFA), and plasma filtration adsorption dialysis. These methods of blood and/or plasma filtration have served utility in patients with sepsis, acute liver failure and acute on chronic liver failure via the filtration of inflammatory cytokines, bilirubin and bile acids, among other compounds such as amino acids and free fatty acids. MARS has shown improved survival rates in patients with acute on chronic liver failure in the setting of sepsis[24,25]. CPFA has demonstrated efficacy with improved survival rates in early or middle stage liver failure secondary to viral hepatitis[26]. These newer extracorporeal treatment options may be utilized to allow liver regeneration after acute injury in an effort to circumvent transplantation or at the least, as a bridge until transplantation can occur[27,28].
Various medical therapies including the use of steroids, cholestyramine, ursodeoxycholic acid, and lactulose have been shown minimal benefit[4]. Interestingly, lactulose appeared to have a protective role on the kidney in experimental rats. This may have been a result of decreasing the endotoxemia associated with this condition[29-31]. In our patient, hemodialysis failed to successfully decrease bilirubin levels. Subsequently, daily plasmapheresis was initiated with effective reduction of bilirubin. However, cessation of plasmapheresis resulted in a rebound of hepatic and renal dysfunction.
In conclusion, cast nephropathy is a rare (or possibly underdiagnosed) entity which results from multiple concurrent insults to the kidney including direct toxicity from bile acids, obstructive physiology from bile casts, and systemic hypoperfusion from vasodilation. It likely represents a broad spectrum of clinical manifestations from initially reversible nephropathy to later irreversible, intractable disease requiring liver and kidney transplantation. The goals of therapy include reduction of bilirubin due to its various mechanisms of renal injury. In patients with cholestatic liver disease, such as patients with primary biliary cirrhosis, primary sclerosing cholangitis, or other liver disease with cholestatic predominance, bile cast nephropathy may be under reported and should be considered in the differential diagnosis. Obtaining a renal biopsy should be considered in patients suspected of this entity to aid in the diagnosis. Although not typical or always available, a trans-jugular approach for renal biopsy may be beneficial as a liver and kidney biopsy may be obtained during the same procedure. When trans-jugular route is not possible, the traditional percutaneous method of obtaining a renal biopsy is sufficient. Patients with hepatorenal dysfunction and the aforementioned characteristic findings on renal biopsy should be evaluated for liver and kidney transplant if more conservative measures are unsuccessful.
The authors would like to thank the University of Illinois College of Medicine, the departments of Graduate Medical Education, and the ROAPP fund for their assistance with the publication.
A 54-year-old male with past medical history of chronic kidney disease stage 3 and recently diagnosed chronic osteomyelitis of the left heel receiving treatment with Piperacillin-tazobactam presented with complaints of intractable pruritus, anorexia and jaundice.
He was found to have jaundice, scleral icterus, and a distended abdomen.
His differential diagnosis included acute liver failure, obstructive jaundice, and malignancy.
Laboratory evaluation was significant for hyperbilirubinemia, a bland urinalysis with the exception of trace protein and presence of bile, and acute on chronic kidney injury.
Abdominal ultrasound revealed the presence of mild splenomegaly and ascites, while a CT and MRCP were negative for evidence of biliary obstruction or pathology.
He was diagnosed with drug-induced liver injury secondary to Piperacillin-tazobactam therapy as well as acute on chronic kidney injury with liver and kidney biopsy indicating stage 1-2 liver fibrosis and bile cast nephropathy, respectively.
Initial treatment consisted of medical management consisting of steroids, ursodeoxycholic acid and N-acetylcysteine, followed by dialysis, plasmapheresis, and ultimately liver and kidney transplantation.
Bile cast nephropathy is a condition of renal insufficiency as a consequence of hepatic dysfunction that may go unrecognized as it is rarely reported and requires kidney biopsy for definitive diagnosis.
Bile cast nephropathy is a rare and poorly understood condition that occurs in the setting of concomitant renal and hepatic dysfunction largely as a result of hepatic dysfunction.
This entity is likely often under-recognized as it is rare and uncommonly described in the literature. A high index of suspicion for bile cast nephropathy in the setting of renal and hepatic dysfunction should be maintained, and prompt treatment with bilirubin reducing therapy should be initiated as reversal of liver dysfunction improves renal dysfunction.
This is a well-described case report on a rare condition involving concomitant kidney and liver dysfunction including a comprehensive and accurate review of the current literature on a topic which has not previously been well studied. It provides an in-depth explanation of the pathophysiology of bilirubin damage and information pertaining to acute renal injury from toxicity from bile acids with the need for dialysis, plasmapheresis, and ultimately dual organ transplant.
P- Reviewer: Bernieh B, Friedman EA, Scarpioni R S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | van Slambrouck CM, Salem F, Meehan SM, Chang A. Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int. 2013;84:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 2. | Sitprija V, Kashemsant U, Sriratanaban A, Arthachinta S, Poshyachinda V. Renal function in obstructive jaundice in man: cholangiocarcinoma model. Kidney Int. 1990;38:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 3. | Bredewold OW, de Fijter JW, Rabelink T. A case of mononucleosis infectiosa presenting with cholemic nephrosis. NDT Plus. 2011;4:170-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Betjes MG, Bajema I. The pathology of jaundice-related renal insufficiency: cholemic nephrosis revisited. J Nephrol. 2006;19:229-233. [PubMed] |
| 5. | Luciano RL, Castano E, Moeckel G, Perazella MA. Bile acid nephropathy in a bodybuilder abusing an anabolic androgenic steroid. Am J Kidney Dis. 2014;64:473-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | van der Wijngaart H, van Dam B, van den Berg JG, Krul-Poel YH, Klemt-Kropp M, Bax WA. A 73-year-old male with jaundice and acute kidney injury. Bile cast nephropathy. Neth J Med. 2014;72:95, 99. [PubMed] |
| 7. | Sequeira A, Gu X. Bile cast nephropathy: an often forgotten diagnosis. Hemodial Int. 2015;19:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Jain K, Gupta A, Singh HK, Nickeleit V, Kshirsagar AV. Bile cast nephropathy. Kidney Int. 2015;87:484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Haessler H, Rous P, Broun GO. The renal elimination of bilirubin. J Exp Med. 1922;35:533-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Elsom KA. Renal function in obstructive jaundice. Arch Internal Medicine. 1937;60:1028-1033. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Wu B, Gong D, Ji D, Xu B, Liu Z. Clearance of myoglobin by high cutoff continuous veno-venous hemodialysis in a patient with rhabdomyolysis: a case report. Hemodial Int. 2015;19:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Fickert P, Krones E, Pollheimer MJ, Thueringer A, Moustafa T, Silbert D, Halilbasic E, Yang M, Jaeschke H, Stokman G. Bile acids trigger cholemic nephropathy in common bile-duct-ligated mice. Hepatology. 2013;58:2056-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Bairaktari E, Liamis G, Tsolas O, Elisaf M. Partially reversible renal tubular damage in patients with obstructive jaundice. Hepatology. 2001;33:1365-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Sellinger M, Haag K, Burckhardt G, Gerok W, Knauf H. Sulfated bile acids inhibit Na(+)-H+ antiport in human kidney brush-border membrane vesicles. Am J Physiol. 1990;258:F986-F991. [PubMed] |
| 15. | Lee HT, Park SW, Kim M, D’Agati VD. Acute kidney injury after hepatic ischemia and reperfusion injury in mice. Lab Invest. 2009;89:196-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Green J, Better OS. Systemic hypotension and renal failure in obstructive jaundice-mechanistic and therapeutic aspects. J Am Soc Nephrol. 1995;5:1853-1871. [PubMed] |
| 17. | Aoyagi T, Lowenstein LM. The effect of bile acids and renal ischemia on renal function. J Lab Clin Med. 1968;71:686-692. [PubMed] |
| 18. | Rector WG, Kanel GC, Rakela J, Reynolds TB. Tubular dysfunction in the deeply jaundiced patient with hepatorenal syndrome. Hepatology. 1985;5:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Allison ME, Moss NG, Fraser MM, Dobbie JW, Ryan CJ, Kennedy AC, Blumgart LH. Renal function in chronic obstructive jaundice: a micropuncture study in rats. Clin Sci Mol Med. 1978;54:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | FAJERS CM. Experimental studies in the so-called hepato-renal syndrome. 3. On the effect of hepatic necrosis by itself and combined with ten minutes’ unilateral renal ischemia on the kidneys of hydrated rabbits-as judged by some renal function tests. Acta Pathol Microbiol Scand. 1958;44:5-20. [PubMed] |
| 21. | Sant SM, Purandare NM. Cholemic nephrosis--an autopsy and experimental study. J Postgrad Med. 1965;11:79-89. [PubMed] |
| 23. | Speer AG, Cotton PB, Russell RC, Mason RR, Hatfield AR, Leung JW, MacRae KD, Houghton J, Lennon CA. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987;2:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 451] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Davenport A. Extracorporeal support for patients with hepatic failure. Hemodial Int. 2003;7:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Stange J, Mitzner SR, Klammt S, Freytag J, Peszynski P, Loock J, Hickstein H, Korten G, Schmidt R, Hentschel J. Liver support by extracorporeal blood purification: a clinical observation. Liver Transpl. 2000;6:603-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Li L, Yang Q, Huang J, Xu X, Chen Y, Chen Y, Ma W, Chen Z, Fu S. Treatment of hepatic failure with artificial liver support system. Chin Med J (Engl). 2001;114:941-945. [PubMed] |
| 27. | Zhu XF, Chen GH, He XS, Lu MQ, Wang GD, Cai CJ, Yang Y, Huang JF. Liver transplantation and artificial liver support in fulminant hepatic failure. World J Gastroenterol. 2001;7:566-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Aron J, Agarwal B, Davenport A. Extracorporeal support for patients with acute and acute on chronic liver failure. Expert Rev Med Devices. 2016;13:367-380. [PubMed] |
| 29. | Pain JA, Cahill CJ, Gilbert JM, Johnson CD, Trapnell JE, Bailey ME. Prevention of postoperative renal dysfunction in patients with obstructive jaundice: a multicentre study of bile salts and lactulose. Br J Surg. 1991;78:467-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Pain JA, Bailey ME. Experimental and clinical study of lactulose in obstructive jaundice. Br J Surg. 1986;73:775-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Greve JW, Maessen JG, Tiebosch T, Buurman WA, Gouma DJ. Prevention of postoperative complications in jaundiced rats. Internal biliary drainage versus oral lactulose. Ann Surg. 1990;212:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |