Published online Jul 21, 2016. doi: 10.3748/wjg.v22.i27.6201
Peer-review started: March 28, 2016
First decision: May 12, 2016
Revised: May 26, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: July 21, 2016
Processing time: 109 Days and 19 Hours
Statins are a class of molecules that inhibit HMG CoA reductase. They are usually prescribed as a lipid lowering medication. However, there is accumulating evidence that statins have multiple secondary effects both related and unrelated to their lipid-lowering effect. This narrative review of the literature aims to provide the reader with information from clinical studies related to the effect of statin and statins’ potential use in patients with liver diseases. In patients with advanced liver disease due to any etiology, statins exhibit an antifibrotic effect possibly through the prevention of hepatic sinusoidal microthrombosis. Two randomized controlled trials confirmed that statins decrease hepatic vein pressure gradient in patients with portal hypertension and improve the survival of patients after variceal bleeding. Lower rates of infections were observed in patients with cirrhosis who received statin treatment. Statins decrease the risk of hepatocellular carcinoma (HCC) in patients with advanced liver disease in general but particularly in patients with chronic hepatitis B and C. Statins in patients with chronic hepatitis C likely increase the virological response to the treatment with pegylated interferon and ribavirin and have the potential to decrease the rate of fibrosis. Finally, data from randomized controlled trials also confirmed that the addition of statin prolongs the survival of patients with advanced HCC even more than sorafenib. Statins are a very promising group of drugs especially in patients with liver disease, where therapeutic options can often be limited. Some indications, such as the prevention of re-bleeding from esophageal varices and the palliative treatment of HCC have been proven through randomized controlled trials, while additional indications still need to be confirmed through prospective studies.
Core tip: The greatest benefit of statins seems to be in patients with advanced liver disease. Observational studies suggest that statins have an antifibrotic effect possibly through the prevention of hepatic sinusoidal microthrombosis, reduce the rate of infections and decrease the risk of hepatocellular carcinoma in all cirrhotics, but particularly in patients with chronic hepatitis B and C. Data from randomized controlled trials confirmed that statins decrease hepatic vein pressure gradient, prevent re-bleeding, and improve the survival of patients after variceal bleeding. Statins also seem to prolong the survival of patients with advanced hepatocellular carcinoma even more than those treated with Sorafenib, which is the current standard of care for these patients.
- Citation: Janicko M, Drazilova S, Pella D, Fedacko J, Jarcuska P. Pleiotropic effects of statins in the diseases of the liver. World J Gastroenterol 2016; 22(27): 6201-6213
- URL: https://www.wjgnet.com/1007-9327/full/v22/i27/6201.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i27.6201
Statins are an inhomogeneous group of molecules that inhibit the activity of hydroxymetylglutaryl-coenzyme A reductase (HMG CoA reductase), a key enzyme in the synthesis of cholesterol. Statins were discovered as a byproduct in the search for new antimicrobial agents. The first statin (mevastatin) was the product of Penicillinum citrinium, but its clinical use was abandoned due to hepatotoxicity[1]. Lovastatin was the first clinically successful statin to be used effectively[2]. Scandinavian simvastatin survival study (4S) confirmed that statins reduce cardiovascular as well as general mortality in patients with atherosclerosis[3].
Individual molecules from the statin group differ in several important attributes. Lovastatin, simvastatin, fluvastatin and atorvastatin are lipophilic, whereas pravastatin and rosuvastatin are hydrophilic. Pravastatin is metabolized in the liver by sulfation, while lovastatin, simvastatin and atorvastatin are metabolized by cytochrome P-450 3A4. Fluvastatin and rosuvastatin are metabolized partially by cytochrome P-450 2C9[4].
Besides lipid lowering properties statins also exhibit multiple pleiotropic effects, which could be detrimental (i.e., adverse effects) or beneficial. It is unknown whether the pleiotropic effects are directly related to the primary effect of the drug. Statins exhibit various antiatherogenic effects, such as the improvement of endothelial function, antioxidative, antiproliferative and antiinflammatory properties as well as neoangiogenesis[4,5]. Additionally, statins reduce the risk of sudden cardiac death, deep vein thrombosis and fibrosclerotic aortic stenosis, while treatment with statins could influence the regression of left ventricular hypertrophy[4]. Statins also exhibit multiple non-cardiovascular effects. For example, a cross-sectional analysis of three hospital databases showed that patients using statin had a 60% lower prevalence of Alzheimer’s disease[6]. Besides Alzheimer’s, statins also reduce the risk of other types of dementia[7] and have a lower prevalence of vitiligo, osteoporosis, rheumatoid arthritis and sclerosis multiplex[4]. Increased risk of type 2 diabetes mellitus could be counted among the negative pleiotropic (adverse) effects of statin therapy[8].
Non-alcoholic steatosis (NAFLD) of the liver is considered a hepatic manifestation of metabolic syndrome[9]. While the exact causality is unknown, the significant increase of both subcutaneous and visceral fat along with dyslipoproteinemia in the majority of these patients is closely associated with the accumulation of fat in the liver. In some patients the hepatic fat stimulates an inflammatory response that causes non-alcoholic steatohepatitis that in turn could progress to liver cirrhosis. Besides liver related morbidity and mortality, the presence of NAFLD is a significant and independent risk factor for cardiovascular events[10]. Statins are prescribed in these patients to positively influence lipoprotein metabolism. Moreover, increasing evidence suggests that statins improve all aspects of NAFLD. Statins decrease the elevated plasmatic activity of liver enzymes[11], while statins in monotherapy and in combination with antioxidants decrease hepatic fat accumulation[12,13]. Prolonged administration of statins could also reduce liver fibrosis[12]. Interestingly one study showed that statin therapy longer than two years in obese patients reduces the prevalence of liver steatosis[14]. Statin treatment also reduces the risk of cardiovascular mortality, and the risk reduction is significantly greater in patients with elevated liver enzymes[11].
Another well-established indication for statins are cholestatic liver diseases, particularly primary biliary cholangitis, which is commonly associated with elevated total and LDL-cholesterol levels, and statins could partially reverse this negative effect[15].
Chronic viral hepatitis B and C could progress to liver cirrhosis, which increases the risk of developing hepatocellular cancer (HCC). Chronic viral hepatitis, particularly hepatitis B, could lead to hepatocellular cancer even without cirrhosis[16,17].
The aim of the treatment of chronic hepatitis C is the elimination of the virus. An undetectable virus 24 wk after the end of treatment is termed “sustained viral response (SVR)”. Interferon based therapy was the standard of care of chronic hepatitis C patients before direct-acting antivirals became available. The rate of SVR for interferon based therapy depends on multiple factors (IL28B gene polymorphisms, pre-treatment hepatitis C virus (HCV) viral load, HCV reduction dynamics, the degree of fibrosis, etc.)[18].
Multiple authors havereported the effect of statin treatment on HCV viral load. An in-vitro study conducted by Ikeda et al[19] showed that fluvastatin, lovastatin, simvastatin and atorvastatin prevent the replication of HCV RNA, and that this effect is significantly stronger in fluvastatin compared to other statins. In vivo studies showed varied results. Forde et al[20] compared three groups of patients with chronic hepatitis C. Group A consisted of patients with dyslipidemia on statin treatment (without specification) for at least 60 d prior to the HCV RNA quantification, group B included dyslipidemic patients without statin, and group C included patients without dyslipidemia and not on statin treatment. The authors did not report significant differences in HCV RNA levels among these three groups of patients. Fluvastatin dosed 80 mg daily led to the reduction of HCV RNA in 50% of patients, with the highest weekly reduction by 1.75 decadic logarithm. The reduction of HCV RNA occurred in the first four weeks of treatment in 82% patients with viral response. However, after the reduction of the dose the HCV RNA increased in 22% of responders in the following 2-5 wk[21]. Another observational study from Romania showed a significant decrease of HCV RNA after treatment with either 40 mg of fluvastatin or 20 mg of lovastatin (mean levels of HCV RNA before treatment 2376074 ± 3427596 IU/mL, and 1321136 ± 1343570 IU/mL after treatment, P = 0.001).The administration of both statins was associated with significant reduction of proinflammatory signaling by IL6 and TNF-α, while the fluvastatin group also had lower IL-8 levels[22]. On the other hand, a study by Sheridan et al[23] did not find significant differences in HCV RNA levels between patients treated with 40-80 mg of fluvastatin (+/- ω-3-polyunsaturated fatty acids) and controls after 12 wk of treatment. The main limitation of this study is, that it included 35% of patients that had already been diagnosed with cirrhosis and 45% that were non-responders to PEG IFN treatment. Fluvastatin treatment also had a surprisingly negative effect in HCV/HIV coinfected patients, where it led to a mild increase of HCV RNA (HCV RNA before treatment 5.63 ± 0.5 log10 IU/mL vs 5.84 ± 0.6 log10 IU/mL after treatment, P = 0.001), compared to no change in HCV RNA in the control group[24].
The effect of other statins on HCV RNA has not been proven in any studies. Simvastatin treatment for three months did not affect HCV RNA levels significantly[25] and neither did the combination of simvastatin with sertralin[26]. Twelve weeks treatment with rosuvastatin titrated to 40 mg daily led to the decrease of HCV RNA higher than one decadic logarithm only in one out of eleven patients[27]. A meta-analysis showed a relatively small but significant decrease of HCV RNA (0.2 decadic logarithm decrease, 95%CI: 0.09-0.31, P < 0.001) in patients treated with fluvastatin,but lovastatin, simvastatin, atorvastatin and rosuvastatin had no effect on HCV RNA levels[28]. These results suggest that standard statin therapy does not have a significant effect on the dynamics of HCV RNA viral load, with the possible exception of fluvastatin.
Despite the dubious effects of statins on HCV viral load, there is a distinctive antifibrotic effect of this treatment in HCV infected patients. The data comes from a large observational study from Taiwan, performed in 1997-2010 included 226856 patients with chronic hepatitis C. Cirrhosis was present in 34273 patients. The incidence of cirrhosis during the follow-up was significantly higher in patients not taking statins (1311.2 vs 445.5 cases per 100000 person-years) Hazard ratios were 0.33 (95%CI: 0.31-0.36), 0.24 (95%CI: 0.22-0.25), and 0.13 (95%CI: 0.12-0.15) when statin users were compared with non-statin users with cumulative defined daily doses (cDDD) of 28-83, 84-365, and greater than 365 respectively[29].
The possibility of improving the treatment efficacy of standard antiviral treatment with the addition of statins has been evaluated with interferon based treatment, which was the standard of care before the development of direct acting antivirals. The efficacy of pegylated interferon with ribavirin was about 50%[30,31]. The addition of a statin effectively enhanced the antiviral effect of this treatment, particularly fluvastatin, exhibiting synergistic inhibitory effect on HCV RNA replication[19]. Several studies have explored this synergy in studies In vivo. Japanese authors reported the SVR rate in patients treated with PEG IFN and ribavirin with the addition of 20 mg fluvastatin to be as high as 67%, however it is important to note that this observational study did not have any control group[32]. Another study explored the addition of 20 mg fluvastatin to PEG IFN + ribavirin treatment (46 patients) and compared them to a control group (48 patients). The duration of treatment was 48 wk in patients with complete early viral response (cEVR) and 72 wk in patients without cEVR, but with HCV RNA negativity in 13-36 wk of treatment. There was no difference in cEVR between the statin and control group (50% vs 54.2%), but patients with cEVR achieved SVR in the fluvastatin group more frequently than the control group (91.3% vs 65.4%, P = 0.042). Furthermore, patients in the control group relapsed significantly more often than in the fluvastatin group (39.4% vs 14.7% respectively, P = 0.027)[33]. There is also anecdotal evidence that the addition of pitavastatin and eicosapentaenoic acid to the PEG IFN + ribavirin treatment could increase the rate of achieving SVR[34]. These results were confirmed in a meta-analysis of five different studies that included fluvastatin, simvastatin, rosuvastatin and pitavastatin. The addition of statin doubled the chance of SVR (OR = 2.02, 95%CI: 1.38-2.94) as well as rapid and early viral responses[35].
The addition of statin to the PEG IFN + ribavirin treatment could influence not only the SVR, but also the risk of complications, such as progressive fibrosis or HCC. The HALT-C study included non-responders to the previous interferon based treatment with advanced fibrosis (Ishak score ≥ 3). Patients that did not achieve virological response (HCV RNA negativity in week 20 were treated with PEG IFN + ribavirin for the next 3.5 years. Patients who were concomitantly treated with statin displayed a decrease of liver fibrosis (-0.34 ± 0.94 points) compared to non-statin users, where fibrosis progressed (0.42 ± 1.42), P = 0.006. Overall, fibrosis progression was found in 10% of statin users and 29% of non-users (adjusted HR = 0.31, 95%CI: 0.10-0.97). Statin treatment did not significantly influence the histology activity index or the plasmatic activity of ALT[36]. Similar data was reported from a registry-based study (ERCHIVES Registry) of 9135 HCV infected veterans treated with interferon based therapy in the years 2001-2014. Liver cirrhosis occurred in 1649 patients and HCC in 239 patients. The risk of cirrhosis in statin users was 44% lower (adjusted HR = 0.6, 95%CI: 0.53-0.68) and the risk of HCC was lower by 49% (adjusted HR = 0.51, 95%CI: 0.36-0.72) compared to non-users. The strongest antifibrotic effect was attributed to atorvastatin and fluvastatin[37].
The addition of statin to the interferon-based therapy has the potential to decrease the degree of fibrosis and the risk of HCC; however, patients with chronic hepatitis C receive statins less frequently compared to patients without HCV infection[38]. The introduction of direct acting antivirals, with SVR rates up to 100% also in cirrhotics and nonresponders to previous IFN based treatment, limits the benefit of statin treatment in HCV infected patients[39-41]. However, statins may potentially play a role in other aspects of chronic HCV infection[42].
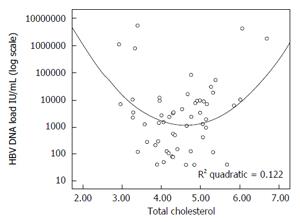
There is no relevant information about the statin influence on hepatitis B virus both in terms of hepatitis B virus (HBV) DNA dynamics or fibrogenesis. However, in an earlier study we reported that cholesterol has a significant quadratic relationship with HBV DNA. Thus, patients with cholesterol levels above and below the normal range had higher levels of HBV DNA (Figure 1)[43]. It has been well documented that HBV DNA level is the strongest predictor of fibrosis progression. According to Iloeje et al[44] “the cumulative incidence of cirrhosis is 4.5% in patients with HBV DNA < 300 copies/mL compared to 36.2% in patients with HBV DNA ≥ 106 copies/mL (P < 0.001)”. However, it is unclear if statin treatment in hypercholesterolemic patients would in any way influence HBV DNA levels. There is some in vitro data that simvastatin might increase the antiviral activity of nucleot(s)ide analogues[45], but it is unlikely that this information will have any clinical meaning, because of the high efficacy of currently available tenofovir and entecavir.
Statins in general positively influence endothelial dysfunction and this effect is also present in intrahepatic sinusoids. They show an anti-inflammatory effect in the inflammatory response caused by endotoxin, angiotensin II or hypovolemia, and the diminished activation of hepatic stellatae cells (HSC)[46]. Statins inhibit non-canonical hedgehog signaling and cirrhotic portal hypertension[47], resulting in a protective effect in ischemic hepatitis[48] and have protective effects against the thrombosis of hepatic sinusoids and portal vein[49].
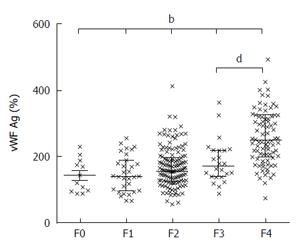
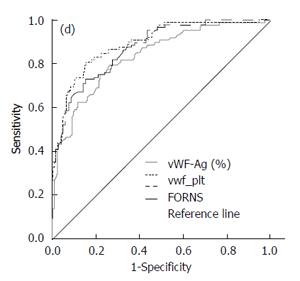
Parenchymal extinction theory of fibrogenesis proposes the microthrombosis of liver sinusoids as the driving force of inflammation and fibrosis. This is supported by the frequent finding of factor V Leiden mutations, protein C deficiency and increased factor VIII expression in cirrhotics[50]. Thrombin, generated as the result of coagulation cascade activation, might activate HSC through protease activated receptors 1 and 4[51]. The administration of statin in these circumstances increases protein C activity[52] and decreases thrombin generation in plasma[53]. One of the key prothrombotic factors in liver cirrhosis is von Willebrand factor antigen (vWF:Ag). This is released by endothelial cells and megacaryocytes and promotes the endothelial adhesion of thrombocytes, the transport and binding of factor VIII and thrombus formation. The level of vWF:Ag directly correlates with the degree of liver fibrosis (Figure 2). Maieron et al[54] developed a novel scoring system, that included vWF:Ag divided by thrombocytes (VITRO) for the prediction of liver cirrhosis with AUC 0.893 compared to Forns score AUC 0.874, P = NS (Figure 3). The VITRO score is also more accurate for the noninvasive diagnosis clinically significant portal hypertension than the ELF or APRI score[55]. Simvastatin and pravastatin significantly decrease vWF:Ag levels [SMD: -0.54, 95%CI: -0.87-(-0.21), P = 0.001], in contrast to fluvastatin, atorvastatin and rosuvastatin. The greatest decrease of vWF:Ag level was observed after 12 wk of statin administration[56]. Statins in animal models upregulate Kruppel-like factor 2 (KLF2) signaling pathways that leads to the decrease of circulating vWF:Ag, decreased activation of HSC, and the regression of liver fibrosis[46,57,58].
Increasing evidence from interventional studies provides support for the microthrombotic theory of fibrosis. The Italian authors evaluated 48 wk of enoxaparin treatment in cirrhotic patients (Child Pugh 7-10 points) with parent portal vein. Enoxaparin decreased not only the incidence of portal vein thrombosis, but also the incidence of decompensation and mortality[49]. It is unclear if statin treatment alone could decrease the incidence of portal vein thrombosis in cirrhotics; however, in patients with diagnosed malignancy, this treatment significantly decreases the cumulative incidence of deep vein thrombosis. Six months after the start of the statin the incidence of deep vein thrombosis was only sporadic[59]. This data indicates the need to further study statins in compensated and decompensated cirrhotics aimed at the prevention of portal vein thrombosis.
Two retrospective observational studies evaluated the effect of statins on clinical outcomes of cirrhotic patients. The first study included 81 cirrhotics on statin treatment and 162 controls. The median follow-up was 36 mo in the statin group and 30 mo in the control group. There was no difference in etiology, age, Child-Pugh, MELD, HCC prevalence, beta blockers use, esophageal varices or selected biochemical parameters at inclusion. Patients in the statin group had a significantly higher prevalence of coronary heart disease and a lower prevalence of diabetes mellitus. The mean survival time of patients in the statin group was 10.8 years compared to 6.3 years in the control group (P = 0.06). The mean survival time of Child Pugh A patients was 14.4 years in the statin group and 7 years in the control group (P = 0.01). The adjusted hazard ratio for overall mortality was 0.53, P = 0.01 in statin users. The authors also reported lower risk of cirrhosis decompensation in statin users[60]. Another study performed by Mohanty et al[61] was registry-based and included patients with cirrhosis caused by hepatitis C infection between 1996-2009. The study cohort included 40 512 patients, 98% of which were male with an average age of 56 years, 2802 patients were using statins. The authors compared the propensity matched cohorts of statin users and non-users and found that patients using statin had a lower risk of decompensation (HR = 0.55, 95%CI: 0.39-0.77) and death (HR = 0.56, 95%CI: 0.46-0.69). These observational studies provide a solid foundation to consider a randomized controlled trial with statin in liver cirrhosis, despite the already decreased level of cholesterol in cirrhotics that correlates with the prognosis[62].
Changes in intrahepatic microcirculation, increased intrahepatic vascular resistance and splanchnic vasodilation are the main factors leading to portal hypertension[63]. Nitric oxide (NO) is the main modulator of the vascular tonus both in the liver and in the splanchnic region. The physiological production of NO is associated with anti-fibrotic, anti-inflammatory and anti-thrombotic effects. Decreased NO production in the sinusoidal endothelial cells has a proinflammatory and profibrotic effect in the liver[48,64]. Simvastatin increases NO production in hepatosplanchnic region, decreases vascular resistance, and ameliorates the postprandial increase of portal pressure in cirrhotic patients without a substantial effect on systemic circulation[65]. Abraldes et al[66] performed a randomized controlled trial in patients with portal hypertension that evaluated the efficacy of 20 mg simvastatin, later titrated to 40 mg on the hepatic vein pressure gradient (HVPG). The decrease of HVPG was greater in the statin group (8.3% ± 12.2% vs 1.6% ± 12.3%, P = 0.041). Statin treatment led to the decrease of HVPG both in patients treated (-11%, P = 0.033) and not treated (-5.9%, P = 0.013) with beta-blocker. Statin treatment did not affect systemic circulation and the incidence of adverse effects was the same in the treatment and control group. Another prospective study by Pollo-Flores et al[67] included 34 patients with portal hypertension. Fourteen patients received 40 mg of simvastatin and 20 patients received placebo for 3 mo. Three patients in the statin group were excluded because of a contrast medium reaction and newly diagnosed HCC, while seven patients were excluded from the control group. In the per-protocol analysis the authors reported the decrease of HVPG in the statin group compared to no change in the control group (2 ± 2.2 Torr vs 0 ± 1.1 Torr, P = 0.02). Primary endpoint (the decrease HVPG of at least 20% from the baseline or under 12 mmHg) was achieved in 55% of patients in the statin group and 0% of patients in the control group (P = 0.036). Clinical outcomes related to portal hypertension, particularly variceal re-bleeding, have been evaluated in the BLEPS study which was a multicenter double-blind randomized controlled trial. It included 69 patients in the active group that received 20 mg of simvastatin titrated to 40 mg after 15 d and 78 patients in the control group. Patients were followed up for 24 mo. The primary endpoint was re-bleeding or death. Nine percent of patients in the statin group and 22% of patients in the control group died during the study (HR = 0.39, 95%CI: 0.15-0.99, P = 0.030). Simvastatin treatment reduced the relative risk of death compared to the placebo by 61%. The rate of re-bleeding did not differ significantly between the two groups. Two patients from the statin group developed rhabdomyolysis during the statin treatment[68]. As practically all of the studies used simvastatin it is not clear if this effect is a class effect of all statins or is limited to simvastatin.
Infections are common in cirrhotic patients and increase mortality by approximately four-fold. Thirty percent of patients die in the first month after infection diagnosis and another 30% in the following year[69]. Motzkus-Feagans et al[70] evaluated the effect of statin treatment on the incidence of infections. The study included 19379 patients with compensated cirrhosis from United States Veterans Health Administration database, with a mean follow-up of 1194 (365-3103) d. 2468 patients were receiving statin, the most common was simvastatin. Infection was diagnosed in 12.4% of patients during follow-up, with a mean time to infection of 608 d. The most common infections were pneumonia and skin infections. Statin treatment was associated with reduced infection rate and mortality rate in the whole cohort (aHR = 0.42, 95%CI: 0.36-0.48), as well as in the propensity score matched sample that included 503 statin users and 1760 statin non-users (aHR = 0.67, 95%CI: 0.47-0.95)[70]. The question remains if statins improve the outcome of patients with severe infection or sepsis. Although no data exists about this particular issue in cirrhotic patients, there are many studies about this topic in the general population. Meta-analysis showed that patients with severe infections or sepsis, who were given statin had lower mortality for sepsis [aOR = 0.40 (95%CI: 0.23-0.57)], pneumonia [aOR = 0.33 (95%CI: 0.09-0.75)] and mixed infection-related mortality [aOR = 0.50 (95%CI: 0.18-0.83)] compared to statin non-users[71]. These findings, however, were not confirmed in the randomized placebo-controlled trial with 40 mg of atorvastatin. Although statin treatment reduced the conversion rate from sepsis to severe sepsis (4% vs 24%, P = 0.007), no significant differences were found in mortality, length of hospital stay, or the number of re-hospitalizations[72]. Therefore, more studies are needed to evaluate the clinical benefit of statins in cirrhotics with infections or sepsis.
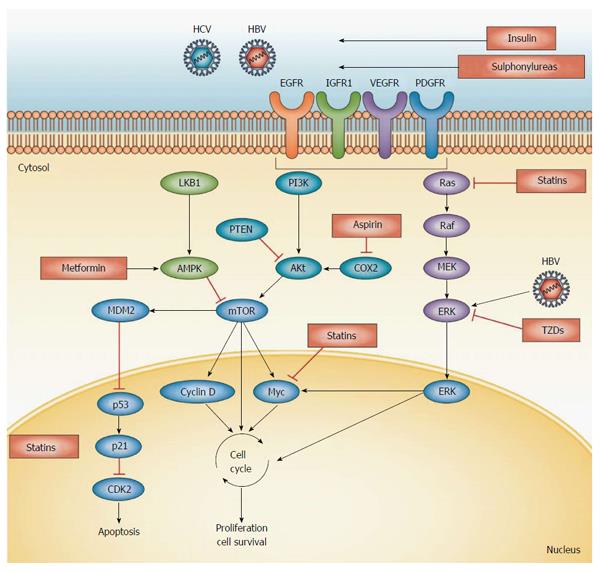
Statin treatment does not generally affect the incidence of cancer or cancer related mortality[73]. Hepatocellular carcinoma (HCC) occurs mostly in cirrhotic liver, with less than 20% of HCC occurring in non-cirrhotic liver[74]. Therefore, statin therapy may indirectly influence the risk of HCC with its anti-fibrotic effect. Accumulated evidence from mostly observational studies suggest, that statins could also decrease the incidence of HCC by direct chemopreventive effect. The carcinogenesis of HCC along with potential targets for prophylaxis or treatment is depicted on Figure 4. Multiple target sites of statins include the inhibition of post-translational prenylation of Ras/Raf proteins, inhibition of the proteasome pathway activation, limitation of the cyclin-dependent kinase inhibitors p21 and p27 degradation, and the blocking of Myc phosphorylation and activation, suppressing tumor initiation and growth[75].
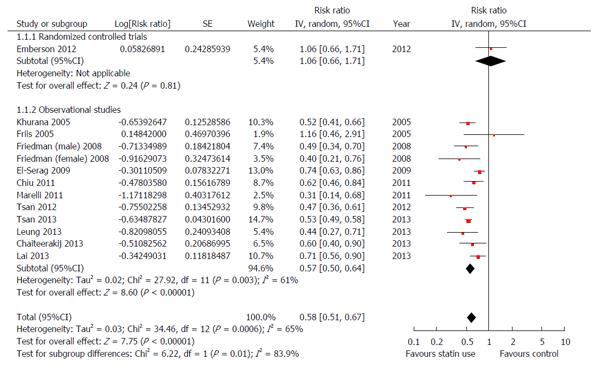
Multiple clinical studies evaluated the effect of statin treatment on the incidence of HCC. Singh et al[76] included 10 of the studies in the meta-analysis published in 2013. Seven studies were observational (3 case-control and 4 cohort studies) and three were RCTs, six studies included Western population and four studies Asian population[73,77-85]. A total of 1459417 patients were included. The chemopreventive effect of statin administration was reported in half of the studies. Overall, statin administration was associated with lower risk of HCC (aOR = 0.63, 95%CI: 0.52- 0.76). The risk of HCC was lower in statin users in both the Western (aOR = 0.67, 95%CI: 0.53- 0.85) and Asian population (aOR = 0.52, 95%CI: 0.42- 0.64). These findings were confirmed in an updated meta-analysis by Shi et al[86] that included 12 studies (6 case-control studies, 5 cohort studies and 1 randomised controlled study)[73,77-82,85,87-90]. The relative risk of HCC in statin users was 0.58, (95%CI: 0.51-0.6), Figure 5. A smaller meta-analysis by Zhou et al[91] that included five observational studies[78,85,87,92,93], also showed a significant risk reduction of HCC in statin users. Odds ratios were 0.63, 95%CI: 0.45-0.89 for atorvastatin and OR = 0.58, 95%CI: 0.40-0.85 for fluvastatin. The chemopreventive effect of statins was also described in patients with chronic hepatitis C without cirrhosis. A registry-based study from Taiwan included 35023 statin users and 225841 non-statin users. The authors reported a significant dose response relationship between statin use and the risk of HCC with an aHR of 0.66, 95%CI: 0.59-0.74, aHR = 0.47, 95%CI: 0.40-0.56 and aHR = 0.33, 95%CI: 0.25-0.42) in groups with cDDD of 28-89, 90-180 and more than 180 respectively[90].
The incidence of HCC in patients with chronic hepatitis B partially depends on the viral load. Levels of HBV DNA ≥ 10000 copies/mL are a significant predictor of HCC independent of ALT levels, HBeAg or the presence of liver cirrhosis[94]. Statin use significantly decreased the risk of HCC in patients with hepatitis B in a registry-based observation from Taiwan with dose-dependent relationship. Adjusted hazard ratios were 0.66, 95%CI: 0.44-0.99, 0.41, 95%CI: 0.27-0.61 and 0.34, 95%CI: 0.18-0.67 for cDDD of 28-90, 91-365 and greater than 365 respectively[85]. Another study from Hong Kong also reported that statin use was associated with 32% risk reduction of HCC development [weighted sub-hazard ratio (SHR) = 0.68; 95%CI: 0.48-0.97], however statins did not reduce the risk of mortality (weighted HR = 0.92, 95%CI: 0.76-1.11). The addition of statin to the standard nucleot(s)ide analogue treatment reduced the risk of HCC by 59% (weighted SHR 0.41, 95%CI: 0.19-0.89) compared to patients with only nucleot(s)ide analogue treatment[95]. This corresponds with the presumed synergy between nucleot(s)ide analogue and statins for HCC risk reduction[45].
It has been shown that statins do not influence the incidence of cancer in the general population[73]. Interestingly, the risk reduction seems to be significant in patients with chronic hepatitis B. An observational study by Chen et al[96] included 71847 patients with chronic hepatitis B. Statin users from this study had significantly lower risk of not only liver cancer but also all malignancies in general. The concomitant use of statin and metformin reduced the risk of malignancies even further in patients with chronic hepatitis B (Table 1).
| All group (n = 71824) | No. of patients | Nonuser(n = 53037) | Only-metformin(n = 4774) | Only-statin(n = 8861) | M + S(n = 5152) |
| Adjusted HR (95%CI) | Adjusted HR(95%CI) | Adjusted HR(95%CI) | Adjusted HR(95%CI) | ||
| Total cancer | 5434 | 1 | 1.03 (0.94-1.14) | 0.60 (0.55-0.66)d | 0.46 (0.40-0.52)d |
| Liver cancer | 1735 | 1 | 1.25 (1.06-1.47)b | 0.34 (0.27-0.42)d | 0.35 (0.27-0.45)d |
| Nonliver cancer | 3699 | 1 | 0.94 (0.83-1.06) | 0.72 (0.65-0.80)d | 0.50 (0.44-0.58)d |
| Lung cancer | 439 | 1 | 0.91 (0.66-1.26) | 0.51 (0.37-0.70)d | 0.49 (0.34-0.71)d |
| Stomach cancer | 144 | 1 | 0.77 (0.42-1.42) | 0.59 (0.35-1.00)a | 0.31 (0.14-0.69)b |
| Colorectal cancer | 572 | 1 | 1.14 (0.85-1.53) | 0.84 (0.65-1.09) | 0.51 (0.35-0.75)d |
| Esophagus cancer | 93 | 1 | 1.19 (0.61-2.31) | 0.38 (0.17-0.86)a | 0.30 (0.11-0.87)a |
| Pancreatic cancer | 127 | 1 | 1.33 (0.74-2.41) | 0.73 (0.40-1.31) | 0.70 (0.34-1.43) |
| Prostate cancer | 225 | 1 | 0.94 (0.59-1.50) | 0.77 (0.51-1.15) | 0.63 (0.37-1.05) |
| Breast cancer | 288 | 1 | 0.80 (0.47-1.32) | 0.91 (0.63-1.33) | 0.56 (0.33-0.95)a |
| Cervical cancer | 105 | 1 | 0.70 (0.31-1.58) | 0.67 (0.35-1.25) | 0.28 (0.10-0.79)a |
| Other cancers | 1706 | 1 | 0.91 (0.76-1.09) | 0.51 (0.42-0.64)d | 0.75 (0.65-0.88)d |
Statins have also been tried as a concomitant therapy in patients with confirmed HCC. Two randomized controlled trials evaluated the role of statins in the treatment of advanced hepatocellular carcinoma. Japanese authors randomized 83 patients with non-resectable HCC undergoing transarterial chemoembolisation into 40 mg pravastatin and control group. The mean survival rate was significantly longer in the statin group (18 mo vs 9 mo)[97]. These results were confirmed in a similarly designed German RCT that included 131 patients. Survival in the statin group was 20.9 mo, 95%CI: 15.5-26.3 compared to 12.0 mo, 95%CI: 10.3-13.7, P = 0.003 in the control group[98]. Similar data was reported from observational studies in Taiwan and United States. The Taiwanese authors observed 20200 patients who received palliative treatment for HCC with median follow-up of 1.66 years. Statin treatment in this group was associated with lower HCC-related deaths in all stages of HCC. The risk of HCC-related death was reduced in 50% during 18 mo´ follow-up in patients with stage II and III[99]. The American authors observed 1036 with early HCC (stage I or II) undergoing standard treatment for HCC. Patients who used statin lived significantly longer (23.9 vs 18.9 years, P = 0.047). However, after adjustment for confounders and immortal time bias, statin use did not confer lower risk of death (HR = 0.98, 95%CI: 0.80-1.20)[100]. The reviewed studies suggest that the addition of statin to the treatment of patients with advanced HCC could extend survival by 5-9 mo. Surprisingly, the results of two RCTs are more favorable than the results of the SHARP study, where sorafenib treatment extended the survival of patients with non-resectable HCC by only 2.8 mo[101].
The conclusions that can be drawn from this review are limited by the mostly observational nature of the reviewed studies. However, the risk of bias seems to be relatively low because the control groups come from the same population as treated patients[102]. Moreover, the evidence from RCTs is accumulating as well. There is not enough data to conclude if the various benefits of statins are related to the class effect, or if they are limited to particular a molecule (fluvastatin in chronic hepatitis B, simvastatin in portal hypertension, atorvastatin or fluvastatin in HCC risk reduction and pravastatin in palliative treatment of HCC).
A second concern about statins in liver disease is the potential hepatotoxicity. There are two possible reactions to the the statin treatment. The most common is an asymptomatic, dose-dependent increase of plasma transaminase activity. This is present in as much as 2.7% of all high dose statin users, and is also dependent on the particular statin molecule. For example, rosuvastatin has the least chance of causing the elevation of liver enzymes[103]. The second possibility is a drug induced liver injury (DILI) that is a result of idiosyncratic reaction, dose independent, and has potentially serious consequences. The diagnostic criteria for DILI increased of ALT ≥ 5-times above the upper limit of norm, or the increase of ALP ≥ 2-times above the upper limit of norm. According to data from the Swedish Adverse Drug Reactions Advisory Committee, only 73 cases of DILI, two deaths and one liver transplant occurred over 23 years (1988-2010) of statin use in Sweden. That represents about 1.2 cases of DILI per 100000 users. The lowest rate of DILI was reported for pravastatin and highest for fluvastatin[104]. This rate is at the lower end of the range reported for general DILI incidence (from 1:10 000 to 1:100 000)[105]. Despite this information, almost 50% of academic physicians hesitate to prescribe statin if ALT is greater than 1.5 times the upper limit of norm[106].
Finally, the most common complication of statin treatment that leads to the statin treatment being stopped is drug-induced myopathy. This condition is associated with single nucleotide polymorphism in SLCO1B1[48]. The risk of statin-induced myopathy can be lowered by the administration of coenzyme Q10 alone, or in combination with selenium[107].
This review summarized the potential uses of statins in patients with various liver disease states. In patients with chronic hepatitis C the addition of statin improves SVR rates of PEG IFN treatment and slows down fibrogenesis,. While it is not clear if statins influence HCV RNA levels, the main benefit is in patients with advanced fibrosis or cirrhosis. Statins have the potential to decrease the rate of fibrosis possibly through the prevention of hepatic sinusoidal microthrombosis. Statins decrease HVPG in patients with portal hypertension, and improve the survival of patients after variceal bleeding. Lower rates of infections were observed in patients with cirrhosis who received statin treatment. Statins decrease the risk of HCC in patients with advanced liver disease in general but particularly in patients with chronic hepatitis B and C. The addition of statin could prolong the survival of patients with advanced HCC. Most of the presented information comes from observational studies, randomized controlled trials are warranted to confirm these effects and allow the routine clinical use of statins in new indications.
Manuscript source: Invited manuscript
P- Reviewer: Gong ZJ, Liu ZW, Mumtaz K S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Endo A, Kuroda M, Tsujita Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J Antibiot (Tokyo). 1976;29:1346-1348. [PubMed] |
| 2. | Alberts AW, Chen J, Kuron G, Hunt V, Huff J, Hoffman C, Rothrock J, Lopez M, Joshua H, Harris E. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci USA. 1980;77:3957-3961. [PubMed] |
| 3. | Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383-1389. [PubMed] |
| 4. | Pella D, Rybar R, Mechirova V. Pleiotropic effcts of statins. Acta Cardiologica Sinica. 2005;21:190-198. |
| 5. | Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1200] [Cited by in RCA: 1323] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 6. | Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439-1443. [PubMed] |
| 7. | Vaughan CJ. Prevention of stroke and dementia with statins: Effects beyond lipid lowering. Am J Cardiol. 2003;91:23B-29B. [PubMed] |
| 8. | Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1657] [Cited by in RCA: 1735] [Article Influence: 115.7] [Reference Citation Analysis (0)] |
| 9. | Kim CH, Younossi ZM. Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve Clin J Med. 2008;75:721-728. [PubMed] |
| 10. | Kunutsor SK, Apekey TA, Khan H. Liver enzymes and risk of cardiovascular disease in the general population: a meta-analysis of prospective cohort studies. Atherosclerosis. 2014;236:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 11. | Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 501] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 12. | Ekstedt M, Franzén LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol. 2007;47:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol. 2011;106:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 14. | de Keyser CE, Koehler EM, Schouten JN, Visser LE, Hofman A, Janssen HL, Stricker BH. Statin therapy is associated with a reduced risk of non-alcoholic fatty liver in overweight individuals. Dig Liver Dis. 2014;46:720-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Cash WJ, O’Neill S, O’Donnell ME, McCance DR, Young IS, McEneny J, McDougall NI, Callender ME. Randomized controlled trial assessing the effect of simvastatin in primary biliary cirrhosis. Liver Int. 2013;33:1166-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 557] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 17. | Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58-S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 615] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 18. | Andriulli A, Di Marco V, Margaglione M, Ippolito AM, Fattovich G, Smedile A, Valvano MR, Calvaruso V, Gioffreda D, Milella M. Identification of naïve HCV-1 patients with chronic hepatitis who may benefit from dual therapy with peg-interferon and ribavirin. J Hepatol. 2014;60:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Ikeda M, Abe K, Yamada M, Dansako H, Naka K, Kato N. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 20. | Forde KA, Law C, O’Flynn R, Kaplan DE. Do statins reduce hepatitis C RNA titers during routine clinical use? World J Gastroenterol. 2009;15:5020-5027. [PubMed] |
| 21. | Bader T, Fazili J, Madhoun M, Aston C, Hughes D, Rizvi S, Seres K, Hasan M. Fluvastatin inhibits hepatitis C replication in humans. Am J Gastroenterol. 2008;103:1383-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Mihăilă R, Nedelcu L, Frăţilă O, Rezi EC, Domnariu C, Ciucă R, Zaharie AV, Olteanu A, Bera L, Deac M. Lovastatin and fluvastatin reduce viremia and the pro-inflammatory cytokines in the patients with chronic hepatitis C. Hepatogastroenterology. 2009;56:1704-1709. [PubMed] |
| 23. | Sheridan DA, Bridge SH, Crossey MM, Felmlee DJ, Fenwick FI, Thomas HC, Neely RD, Taylor-Robinson SD, Bassendine MF. Omega-3 fatty acids and/or fluvastatin in hepatitis C prior non-responders to combination antiviral therapy - a pilot randomised clinical trial. Liver Int. 2014;34:737-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Milazzo L, Meroni L, Galazzi M, Cesari M, Caramma I, Marchetti G, Galli M, Antinori S. Does fluvastatin favour HCV replication in vivo? A pilot study on HIV-HCV coinfected patients. J Viral Hepat. 2009;16:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Mihaila RG, Nedelcu L, Fratila O, Retzler L, Domnariu C, Cipaian RC, Rezi EC, Beca C, Deac M. Effects of simvastatin in patients with viral chronic hepatitis C. Hepatogastroenterology. 2011;58:1296-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Patel K, Lim SG, Cheng CW, Lawitz E, Tillmann HL, Chopra N, Altmeyer R, Randle JC, McHutchison JG. Open-label phase 1b pilot study to assess the antiviral efficacy of simvastatin combined with sertraline in chronic hepatitis C patients. Antivir Ther. 2011;16:1341-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Patel K, Jhaveri R, George J, Qiang G, Kenedi C, Brown K, Cates C, Zekry A, Tillmann HL, Elliott L. Open-label, ascending dose, prospective cohort study evaluating the antiviral efficacy of Rosuvastatin therapy in serum and lipid fractions in patients with chronic hepatitis C. J Viral Hepat. 2011;18:331-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Grammatikos G, Farnik H, Bon D, Böhlig A, Bader T, Berg T, Zeuzem S, Herrmann E. The impact of antihyperlipidemic drugs on the viral load of patients with chronic hepatitis C infection: a meta-analysis. J Viral Hepat. 2014;21:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Yang YH, Chen WC, Tsan YT, Chen MJ, Shih WT, Tsai YH, Chen PC. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J Hepatol. 2015;63:1111-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [PubMed] |
| 31. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4748] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 32. | Sezaki H, Suzuki F, Akuta N, Yatsuji H, Hosaka T, Kobayashi M, Suzuki Y, Arase Y, Ikeda K, Miyakawa Y. An open pilot study exploring the efficacy of fluvastatin, pegylated interferon and ribavirin in patients with hepatitis C virus genotype 1b in high viral loads. Intervirology. 2009;52:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Atsukawa M, Tsubota A, Kondo C, Itokawa N, Narahara Y, Nakatsuka K, Hashimoto S, Fukuda T, Matsushita Y, Kidokoro H. Combination of fluvastatin with pegylated interferon/ribavirin therapy reduces viral relapse in chronic hepatitis C infected with HCV genotype 1b. J Gastroenterol Hepatol. 2013;28:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Kohjima M, Enjoji M, Yoshimoto T, Yada R, Fujino T, Aoyagi Y, Fukushima N, Fukuizumi K, Harada N, Yada M. Add-on therapy of pitavastatin and eicosapentaenoic acid improves outcome of peginterferon plus ribavirin treatment for chronic hepatitis C. J Med Virol. 2013;85:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Zhu Q, Li N, Han Q, Zhang P, Yang C, Zeng X, Chen Y, Lv Y, Liu X, Liu Z. Statin therapy improves response to interferon alfa and ribavirin in chronic hepatitis C: a systematic review and meta-analysis. Antiviral Res. 2013;98:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Simon TG, King LY, Zheng H, Chung RT. Statin use is associated with a reduced risk of fibrosis progression in chronic hepatitis C. J Hepatol. 2015;62:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 37. | Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: Results from ERCHIVES. Hepatology. 2016;64:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 38. | Chandra R, Dolder NM, Dolder CR, O’Neill LW, Robinette C. Treatment of dyslipidemia with statins by primary care providers in Veterans with and without chronic Hepatitis C. Am J Health Syst Pharm. 2016;73:S30-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1064] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 40. | Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida EM. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 683] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 41. | Lawitz E, Gane E, Pearlman B, Tam E, Ghesquiere W, Guyader D, Alric L, Bronowicki JP, Lester L, Sievert W. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385:1075-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 42. | Zhu Q, Han Q, Liu Z. Potential role for statins in the treatment of chronic HCV infection. Future Virology. 2013;8:727-729. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Jarčuška P, Janičko M, Kružliak P, Novák M, Veselíny E, Fedačko J, Senajová G, Dražilová S, Madarasová-Gecková A, Mareková M. Hepatitis B virus infection in patients with metabolic syndrome: a complicated relationship. Results of a population based study. Eur J Intern Med. 2014;25:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1174] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 45. | Bader T, Korba B. Simvastatin potentiates the anti-hepatitis B virus activity of FDA-approved nucleoside analogue inhibitors in vitro. Antiviral Res. 2010;86:241-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Marrone G, Maeso-Díaz R, García-Cardena G, Abraldes JG, García-Pagán JC, Bosch J, Gracia-Sancho J. KLF2 exerts antifibrotic and vasoprotective effects in cirrhotic rat livers: behind the molecular mechanisms of statins. Gut. 2015;64:1434-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 47. | Uschner FE, Ranabhat G, Choi SS, Granzow M, Klein S, Schierwagen R, Raskopf E, Gautsch S, van der Ven PF, Fürst DO. Statins activate the canonical hedgehog-signaling and aggravate non-cirrhotic portal hypertension, but inhibit the non-canonical hedgehog signaling and cirrhotic portal hypertension. Sci Rep. 2015;5:14573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 48. | Cabrera L, Abraldes JG. Statins: the Panacea of Cirrhosis? Current Hepatology Reports. 2016;15:1-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 49. | Villa E, Cammà C, Marietta M, Luongo M, Critelli R, Colopi S, Tata C, Zecchini R, Gitto S, Petta S. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253-1260.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 534] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 50. | Anstee QM, Wright M, Goldin R, Thursz MR. Parenchymal extinction: coagulation and hepatic fibrogenesis. Clin Liver Dis. 2009;13:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Bitto N, Salerno F, Tripodi A, La Mura V. Coagulation and fibrosis: A potential non-negligible target of statins in chronic hepatitis. J Hepatol. 2015;63:277-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Undas A, Brummel-Ziedins KE, Mann KG. Anticoagulant effects of statins and their clinical implications. Thromb Haemost. 2014;111:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 53. | Tripodi A, Pellegatta F, Chantarangkul V, Grigore L, Garlaschelli K, Baragetti A, Lemma L, Catapano A. Statins decrease thrombin generation in patients with hypercholesterolemia. Eur J Intern Med. 2014;25:449-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Maieron A, Salzl P, Peck-Radosavljevic M, Trauner M, Hametner S, Schöfl R, Ferenci P, Ferlitsch M. Von Willebrand Factor as a new marker for non-invasive assessment of liver fibrosis and cirrhosis in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2014;39:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Hametner S, Ferlitsch A, Ferlitsch M, Etschmaier A, Schöfl R, Ziachehabi A, Maieron A. The VITRO Score (Von Willebrand Factor Antigen/Thrombocyte Ratio) as a New Marker for Clinically Significant Portal Hypertension in Comparison to Other Non-Invasive Parameters of Fibrosis Including ELF Test. PLoS One. 2016;11:e0149230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 56. | Sahebkar A, Serban C, Ursoniu S, Mikhailidis DP, Undas A, Lip GY, Bittner V, Ray K, Watts GF, Hovingh GK. The impact of statin therapy on plasma levels of von Willebrand factor antigen. Systematic review and meta-analysis of randomised placebo-controlled trials. Thromb Haemost. 2016;115:520-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 57. | Simon TG, King LY, Chung RT. Reply to: “Coagulation and fibrosis: A potential non-negligible target of statins in chronic hepatitis”. J Hepatol. 2015;63:279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 58. | Ray K. Liver: Sussing out statins in cirrhosis--KLF2 is the key. Nat Rev Gastroenterol Hepatol. 2015;12:64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Lötsch F, Königsbrügge O, Posch F, Zielinski C, Pabinger I, Ay C. Statins are associated with low risk of venous thromboembolism in patients with cancer: a prospective and observational cohort study. Thromb Res. 2014;134:1008-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Kumar S, Grace ND, Qamar AA. Statin use in patients with cirrhosis: a retrospective cohort study. Dig Dis Sci. 2014;59:1958-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 61. | Mohanty A, Tate JP, Garcia-Tsao G. Statins Are Associated With a Decreased Risk of Decompensation and Death in Veterans With Hepatitis C-Related Compensated Cirrhosis. Gastroenterology. 2016;150:430-440.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 62. | Janičko M, Veselíny E, Leško D, Jarčuška P. Serum cholesterol is a significant and independent mortality predictor in liver cirrhosis patients. Ann Hepatol. 2013;12:581-587. [PubMed] |
| 63. | Sikuler E, Groszmann RJ. Interaction of flow and resistance in maintenance of portal hypertension in a rat model. Am J Physiol. 1986;250:G205-G212. [PubMed] |
| 64. | Groszmann RJ, Abraldes JG. Portal hypertension: from bedside to bench. J Clin Gastroenterol. 2005;39:S125-S130. [PubMed] |
| 65. | Zafra C, Abraldes JG, Turnes J, Berzigotti A, Fernández M, Garca-Pagán JC, Rodés J, Bosch J. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126:749-755. [PubMed] |
| 66. | Abraldes JG, Albillos A, Bañares R, Turnes J, González R, García-Pagán JC, Bosch J. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 324] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 67. | Pollo-Flores P, Soldan M, Santos UC, Kunz DG, Mattos DE, da Silva AC, Marchiori RC, Rezende GF. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: A randomized controlled trial. Dig Liver Dis. 2015;47:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 68. | Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, Rodriguez M, Castellote J, García-Pagán JC, Torres F. Addition of Simvastatin to Standard Therapy for the Prevention of Variceal Rebleeding Does Not Reduce Rebleeding but Increases Survival in Patients With Cirrhosis. Gastroenterology. 2016;150:1160-1170.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 69. | Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-156, 1246-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 837] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 70. | Motzkus-Feagans C, Pakyz AL, Ratliff SM, Bajaj JS, Lapane KL. Statin use and infections in Veterans with cirrhosis. Aliment Pharmacol Ther. 2013;38:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 71. | Janda S, Young A, Fitzgerald JM, Etminan M, Swiston J. The effect of statins on mortality from severe infections and sepsis: a systematic review and meta-analysis. J Crit Care. 2010;25:656.e7-656.22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 72. | Patel JM, Snaith C, Thickett DR, Linhartova L, Melody T, Hawkey P, Barnett AH, Jones A, Hong T, Cooke MW. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS Trial). Crit Care. 2012;16:R231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 73. | Emberson JR, Kearney PM, Blackwell L, Newman C, Reith C, Bhala N, Holland L, Peto R, Keech A, Collins R. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 74. | Alkofer B, Lepennec V, Chiche L. Hepatocellular cancer in the non-cirrhotic liver. J Visc Surg. 2011;148:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 75. | Singh S, Singh PP, Roberts LR, Sanchez W. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2014;11:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 76. | Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 352] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 77. | Chiu HF, Ho SC, Chen CC, Yang CY. Statin use and the risk of liver cancer: a population-based case-control study. Am J Gastroenterol. 2011;106:894-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 78. | El-Serag HB, Johnson ML, Hachem C, Morgana RO. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology. 2009;136:1601-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 79. | Friedman GD, Flick ED, Udaltsova N, Chan J, Quesenberry CP, Habel LA. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol Drug Saf. 2008;17:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 80. | Friis S, Poulsen AH, Johnsen SP, McLaughlin JK, Fryzek JP, Dalton SO, Sørensen HT, Olsen JH. Cancer risk among statin users: a population-based cohort study. Int J Cancer. 2005;114:643-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 232] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 81. | Khurana V, Saluja A, Caldito G, Fort C, Schiff E. Statins are protective against hepatocellular cancer in patients with hepatitis C virus infection: Half a million US veterans’ study. Gastroenterology. 2005;128:A714. |
| 82. | Marelli C, Gunnarsson C, Ross S, Haas S, Stroup DF, Cload P, Clopton P, DeMaria AN. Statins and risk of cancer: a retrospective cohort analysis of 45,857 matched pairs from an electronic medical records database of 11 million adult Americans. J Am Coll Cardiol. 2011;58:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 83. | Matsushita Y, Sugihara M, Kaburagi J, Ozawa M, Iwashita M, Yoshida S, Saito H, Hattori Y. Pravastatin use and cancer risk: a meta-analysis of individual patient data from long-term prospective controlled trials in Japan. Pharmacoepidemiol Drug Saf. 2010;19:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Sato S, Ajiki W, Kobayashi T, Awata N. Pravastatin use and the five-year incidence of cancer in coronary heart disease patients: from the prevention of coronary sclerosis study. J Epidemiol. 2006;16:201-206. [PubMed] |
| 85. | Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 86. | Shi M, Zheng H, Nie B, Gong W, Cui X. Statin use and risk of liver cancer: an update meta-analysis. BMJ Open. 2014;4:e005399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 87. | Chaiteerakij R, Yang JD, Harmsen WS, Slettedahl SW, Mettler TA, Fredericksen ZS, Kim WR, Gores GJ, Roberts RO, Olson JE. Risk factors for intrahepatic cholangiocarcinoma: association between metformin use and reduced cancer risk. Hepatology. 2013;57:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 88. | Lai SW, Liao KF, Lai HC, Muo CH, Sung FC, Chen PC. Statin use and risk of hepatocellular carcinoma. Eur J Epidemiol. 2013;28:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 89. | Leung HW, Chan AL, Lo D, Leung JH, Chen HL. Common cancer risk and statins: a population-based case-control study in a Chinese population. Expert Opin Drug Saf. 2013;12:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 90. | Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol. 2013;31:1514-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 91. | Zhou YY, Zhu GQ, Wang Y, Zheng JN, Ruan LY, Cheng Z, Hu B, Fu SW, Zheng MH. Systematic review with network meta-analysis: statins and risk of hepatocellular carcinoma. Oncotarget. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 92. | Björkhem-Bergman L, Backheden M, Söderberg Löfdal K. Statin treatment reduces the risk of hepatocellular carcinoma but not colon cancer-results from a nationwide case-control study in Sweden. Pharmacoepidemiol Drug Saf. 2014;23:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | McGlynn KA, Hagberg K, Chen J, Graubard BI, London WT, Jick S, Sahasrabuddhe VV. Statin use and risk of primary liver cancer in the Clinical Practice Research Datalink. J Natl Cancer Inst. 2015;107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 94. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2365] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 95. | Hsiang JC, Wong GL, Tse YK, Wong VW, Yip TC, Chan HL. Statin and the risk of hepatocellular carcinoma and death in a hospital-based hepatitis B-infected population: A propensity score landmark analysis. J Hepatol. 2015;63:1190-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 96. | Chen CI, Kuan CF, Fang YA, Liu SH, Liu JC, Wu LL, Chang CJ, Yang HC, Hwang J, Miser JS. Cancer risk in HBV patients with statin and metformin use: a population-based cohort study. Medicine (Baltimore). 2015;94:e462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 97. | Kawata S, Yamasaki E, Nagase T, Inui Y, Ito N, Matsuda Y, Inada M, Tamura S, Noda S, Imai Y. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br J Cancer. 2001;84:886-891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 264] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 98. | Graf H, Jüngst C, Straub G, Dogan S, Hoffmann RT, Jakobs T, Reiser M, Waggershauser T, Helmberger T, Walter A. Chemoembolization combined with pravastatin improves survival in patients with hepatocellular carcinoma. Digestion. 2008;78:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 99. | Shao JY, Lee FP, Chang CL, Wu SY. Statin-Based Palliative Therapy for Hepatocellular Carcinoma. Medicine (Baltimore). 2015;94:e1801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 100. | Jeon CY, Goodman MT, Cook-Wiens G, Sundaram V. Statin Use and Survival with Early-Stage Hepatocellular Carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 101. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10265] [Article Influence: 603.8] [Reference Citation Analysis (2)] |
| 102. | Abraldes JG, Burak KW. STAT order: Should patients with chronic liver disease be prescribed statins to prevent fibrosis progression and hepatocellular carcinoma? Hepatology. 2016;64:13-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 103. | Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 104. | Björnsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol. 2012;56:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 216] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 105. | Bell LN, Chalasani N. Epidemiology of idiosyncratic drug-induced liver injury. Semin Liver Dis. 2009;29:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 106. | Bader T. Liver tests are irrelevant when prescribing statins. Lancet. 2010;376:1882-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Fedacko J, Pella D, Fedackova P, Hänninen O, Tuomainen P, Jarcuska P, Lopuchovsky T, Jedlickova L, Merkovska L, Littarru GP. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |