INTRODUCTION
Acute lung injury (ALI) is the most common and serious extrapancreatic complication of acute severe pancreatitis (SAP) and also represents a dominant contribution to high morbidity and mortality rates[1]. However, the mechanism underlying the pathogenesis of SAP-induced ALI remains poorly understood. Current therapeutic approaches are limited, and predominantly aimed at symptomatic and supportive treatments. Studies have shown that SAP leads to the overproduction of several cytokines and inflammatory mediators, which initiates and amplifies systemic inflammatory response syndrome (SIRS), resulting in distant organ dysfunction and the development of ALI[2]. The unmet need for therapies against SAP-associated lung injury and paucity of immune response understanding in SAP urge us to explore the role of interleukin-22 (IL-22) and its possible signaling pathway.
IL-22 is a member of the IL-10 cytokine family with epithelial reparative and regenerative properties and is produced by T helper (Th) 22, Th1, and Th17 cells, γδ T cells, natural killer T (NKT) cells, and innate lymphoid cells (ILCs)[3]. Since its discovery in 2000[4], several research laboratories have made great progress in exploring the biology of IL-22 and the role of IL-22 has been identified in numerous tissues, such as the small intestine, liver, colon, lung, kidney, skin, thymus, and pancreas[3,5]. IL-22 exerts its functions by binding to a transmembrane receptor complex that is composed of two different subunits: IL-22 receptor subunit alpha-1 (IL-22RA1) and IL-10R2[6]. IL-22 receptor activation leads to signal transducer and activator of transcription (STAT) 3-mediated proliferative and anti-apoptotic pathway signaling, as well as antimicrobial induction that helps prevent damage and aid tissue repair[5,7]. Treatment with IL-22, via the activation of STAT3, alleviates tissue destruction, promotes intestinal epithelial cell proliferation and survival, and accelerates mucosal wound healing during dextran sodium sulfate (DSS)-induced colitis[8], and contributes to the recovery of goblet cell mucus and rapid amelioration of local intestinal inflammation in Th2-mediated colitis[9]. Similarly, IL-22 is a survival factor for hepatocytes in D-galactosamine (GalN)/lipopolysaccharide (LPS)-induced acute liver failure[10] and has a protective role against acute kidney injury induced by ischemia-reperfusion in mice[11]. In addition, IL-22 can also protect mice against acute pancreatitis induced by caerulein and by choline-deficient diet supplemented with DL-ethionine (CDE)[12]. However, no reports have described the effects of IL-22 on SAP-associated lung injury. The purpose of this study was to examine whether IL-22 could protect mice against SAP-associated lung injury induced by L-arginine and its possible signaling pathway.
MATERIALS AND METHODS
Experimental animals
Male Balb/c mice weighing 18-22 g were provided by the Experimental Animal Center of Shandong University (China). All animals were fed laboratory chow, given water ad libitum, and maintained in plastic cages at a constant temperature of 23 °C ± 2 °C and a relative humidity of 55% ± 2% under a 12 h/12 h light-dark cycle for one week prior to performing the experiments. All experiments were performed according to the guidelines of the Shandong University Institutional Animal Care and Use Committee (IACUC).
Animal model of SAP and treatments
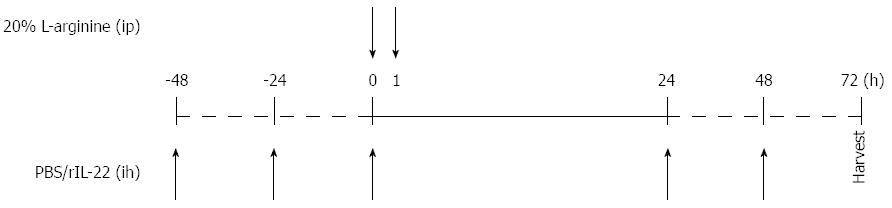
A total of 72 male Balb/c mice were deprived of food and received only water 12 h before the trial commenced. The mice were randomly assigned to four groups: normal control group (n = 12), SAP group (n = 36), treatment control group (phosphate-buffered saline (PBS) group, n = 12) and treatment group [recombinant IL-22 (rIL-22) group, n = 12]. Mice in the SAP, PBS and rIL-22 groups were injected intraperitoneally (ip) twice with 20% L-arginine hydrochloride (Sigma-Aldrich; pH = 7.0, 4 g/kg bodyweight), at an interval of 1 h. The normal control group received physiological saline injections. PBS or rIL-22 (Miltenyi Biotech) (200 ng/per, 5 times) was administered subcutaneously to mice in the PBS and rIL-22 groups (Figure 1). Mice in the SAP group were killed at 24 h, 48 h, and 72 h after the administration of L-arginine. The remaining mice were sacrificed 72 h after the L-arginine injection.
Figure 1 Time points of PBS or recombinant interleukin-22 injection.
PBS: Phosphate-buffered saline; rIL-22: Recombinant interleukin-22.
Serum amylase detection
Mice were thoroughly anesthetized with ether. Orbital blood was collected and stored at -80 °C until analysis. Serum amylase levels were measured using an automatic biochemical analyzer.
Determination of myeloperoxidase activity in the lung
The left upper lobe of the lung was harvested and stored at -80 °C until assessment. Cryopreserved tissue samples were homogenized, and myeloperoxidase (MPO) activities were measured with MPO detection assay kits following the manufacturer’s instructions (Jiancheng Company, Nanjing, China). MPO, a mark of neutrophil accumulation and activation, was expressed as activity units per gram of lung tissue.
Pathological examinations
The head of the pancreas and right upper lobe of the lung were harvested from each mouse and immediately fixed in 10% buffered formalin overnight. Samples were embedded in paraffin wax and cut into 4 μm sections. The sections were flattened, mounted, and heated on blank glass slides. After deparaffinization and dehydration, the sections were stained with hematoxylin and eosin (HE). Pathological examinations were performed by a blinded, unbiased pathologist. The severity of pancreas injury was evaluated based on pancreatic tissue edema, hemorrhage, necrosis and infiltration of inflammation cells. The severity of lung injury was measured according to alveolar congestion, necrosis, hemorrhage, leucocyte infiltration, and thickness of the alveolar membrane.
Real-time polymerase chain reaction
B cell lymphoma/leukemia-2 (Bcl-2), B cell lymphoma/leukemia-extra large (Bcl-xL) and IL-22RA1 mRNA expression levels in the lung tissue were detected by real-time polymerase chain reaction (PCR). Total RNA was isolated from the frozen lung tissue using Trizol reagent (Takara Bio Inc., Japan) following the manufacturer’s instructions. RNA purity was tested using a spectrophotometer (Nanodrop Technologies, Wilmington, DE). Reverse transcription-PCR amplification was performed according to the illustrations of the PrimeScriptTM RT Reagent Kit (Takara Bio Inc., Japan) with genomic deoxyribonucleic acid (gDNA) Eraser (Perfect Real Time). Real-time PCR was conducted using the Lightcycler480 (Roche). The primer sequences were as follows: Bcl-2: forward, 5’-TGAAGCGGTCCGGTGGATA-3’, reverse, 5’-CAGCATTTGCAGAAGTCCTGTGA-3’; Bcl-xL: forward, 5’-GAGGCAGGCGATGAGTTG-3’, reverse, 5’-ACGATGCGACCCCAGTTT-3’; IL-22RA1: forward, 5’-TCTGGGCTACAAATACATCACCAAG-3’, reverse, 5’-GGCCACTGAGGTCCAAGACA-3’; glyceraldehyde-3-phosphate dehydrogenase (GAPDH): forward, 5’-AAATGGTGAAGGTCGGTGTGAAC-3’, reverse, 5’-CAACAATCTCCACTTTGCCACTG-3’. The cycle conditions were as follows: cDNA denaturation at 95 °C for 2 min, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s. The specificity of the product was assessed from a melting curve analysis. Results were standardized using GAPDH, and relative amounts were then calculated according to a 2-ΔΔCT method.
Western blot analysis
Lung tissue (0.5 g) was ground rapidly in liquid nitrogen to provide 1 mL homogenate (including a protease inhibitor cocktail), which was diluted 20-fold with the radio-immunoprecipitation assay (RIPA) efficient cracking liquid (Beyotime Biotech, China) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore Biotechnology Inc., United States) after being separated on precast 10% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) (Beyotime Biotech, China). The membranes were blocked with 5% non-fat dry milk for 1 h. Primary rabbit monoclonal anti-STAT3 (1:2000) and phospho-STAT3Tyr705 (p-STAT3Tyr705) (1:2000) antibodies (Cell Signaling, Beverly, MA, United States) were then added and incubated overnight on a rotating wheel at 4 °C. The membranes were washed three times with tris-buffered saline with tween-20 (TBST) and incubated with a horseradish peroxidase-conjugated secondary antibody (1:2000) for 1 h at room temperature. Finally, the membranes were detected with an enhanced chemiluminescence reagent (ECL, Millipore Biotechnology Inc., United States). Band densities were measured using ImageJ Analysis Software. β-actin served as an internal control protein.
Statistical analysis
The data are presented as the mean ± SD and were statistically analyzed using SPSS version 20.0 software. The statistical differences between multiple groups were determined using one-way analysis of variance. Comparisons between the two groups were conducted using an unpaired t-test. A P-value < 0.05 was considered statistically significant. Statistical graphs were generated with GraphPad Prism 5 software.
RESULTS
Serum levels of amylase
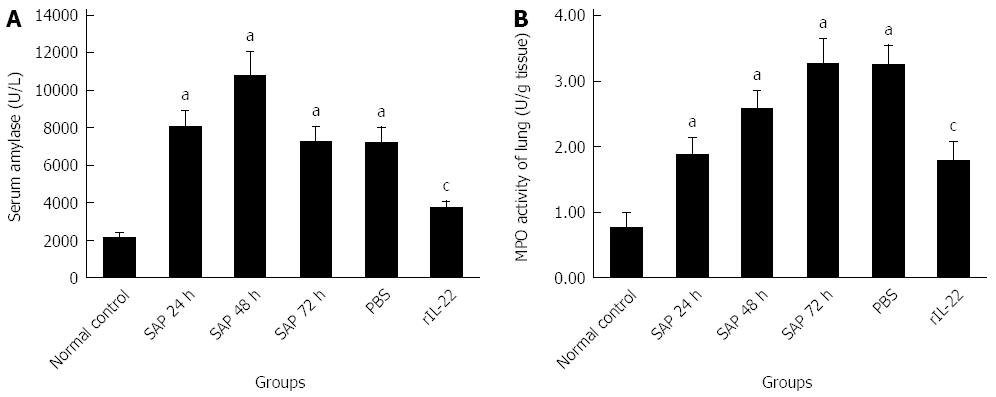
As indicated in Figure 2A, compared with the normal control group, the level of serum amylase in the SAP group was increased markedly (P < 0.05). The highest level of serum amylase was detected at 48 h after the injection of L-arginine. At 72 h after injection, the serum amylase level partially recovered. No significant decrease was found in the PBS group relative to the SAP group at 72 h after the injection (P > 0.05). However, the rIL-22 group was markedly lower than SAP group at 72 h after the injection (P < 0.05).
Figure 2 Serum amylase (A) and activity of lung myeloperoxidase (B).
Numbers of cases from each group statistically analyzed are 12 (Normal control), 9 (SAP 24 h), 8 (SAP 48 h), 8 (SAP 72 h), 8 (PBS) and 12 (rIL-22). Results are presented as mean ± SD. aP < 0.05 vs the normal control group, cP < 0.05 vs the SAP group at 72 h. SAP: Acute severe pancreatitis; MPO: Myeloperoxidase; PBS: Phosphate-buffered saline; rIL-22: Recombinant interleukin-22.
Myeloperoxidase activity analysis
As demonstrated in Figure 2B, a gradual increase over time (24, 48, and 72 h after the injection of L-arginine) was observed in the lung MPO activities relative to the normal control group (P < 0.05). Pretreatment with PBS did not significantly affect the MPO activity compared to the SAP group at 72 h after the injection (P > 0.05). In contrast, rIL-22 significantly decreased the MPO activity compared with the SAP group at 72 h after the injection.
Histomorphology of the pancreas
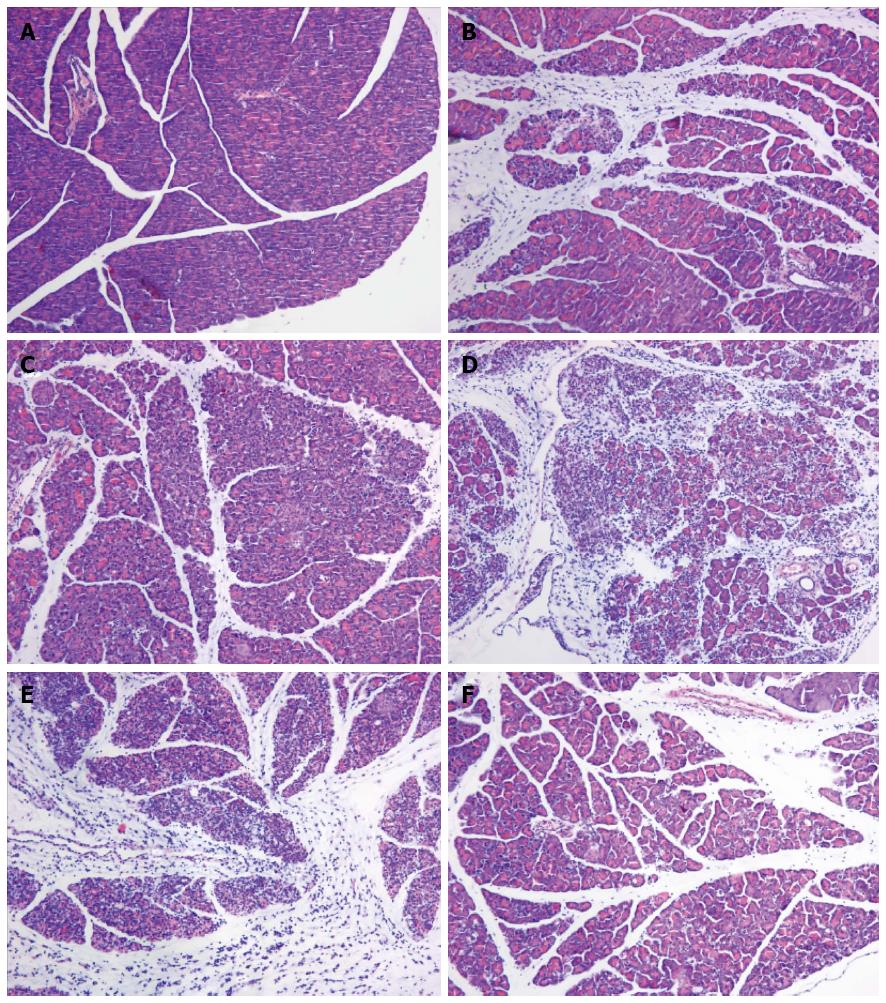
Macroscopically, the pancreas was edematous 24 and 48 h after the L-arginine administration. By contrast, the pancreas shrank, with many saponification spots on the omentum majus and mesentery, intestinal cavity expanding and bloody ascites, 72 h after the injection of L-arginine and pretreatment with PBS. Under the light microscope, mice in the SAP group 24 h after L-arginine injection exhibited interstitial edema and infiltration of a small number of neutrophils and mononuclear cells. The acinar architecture and integrity were partially destroyed with focal parenchyma necrosis and hemorrhage. The vascular and pancreatic ductal structures appeared undamaged (Figure 3B). At 48 h, interstitial edema, inflammatory cellular infiltration, parenchyma necrosis and hemorrhage were significantly aggravated (Figure 3C). The severity of pancreatic destruction became maximal 72 h after L-arginine administration. About 70%-80% of the pancreatic acinar cells had been destroyed and replaced by inflammatory and fibrotic cells. The pancreatic ducts were expanded and appeared more numerous because of a decrease in acini and shrinkage of the pancreatic tissue (Figure 3D). Compared with the SAP group, PBS did not significantly reduce the pancreatic injury (Figure 3E). However, the mice in the rIL-22 group showed milder interstitial edema, acinar cell necrosis and cellular infiltration (Figure 3F).
Figure 3 Hematoxylin and eosin staining of the pancreas.
Normal control group (A), SAP group (B: 24 h after L-arginine injection; C: 48 after L-arginine injection; D: 72 h after L-arginine injection), PBS group (E) and rIL-22 group (F). Original magnification × 200. SAP: Acute severe pancreatitis; PBS: Phosphate-buffered saline; rIL-22: Recombinant interleukin-22.
Histomorphology of the lung
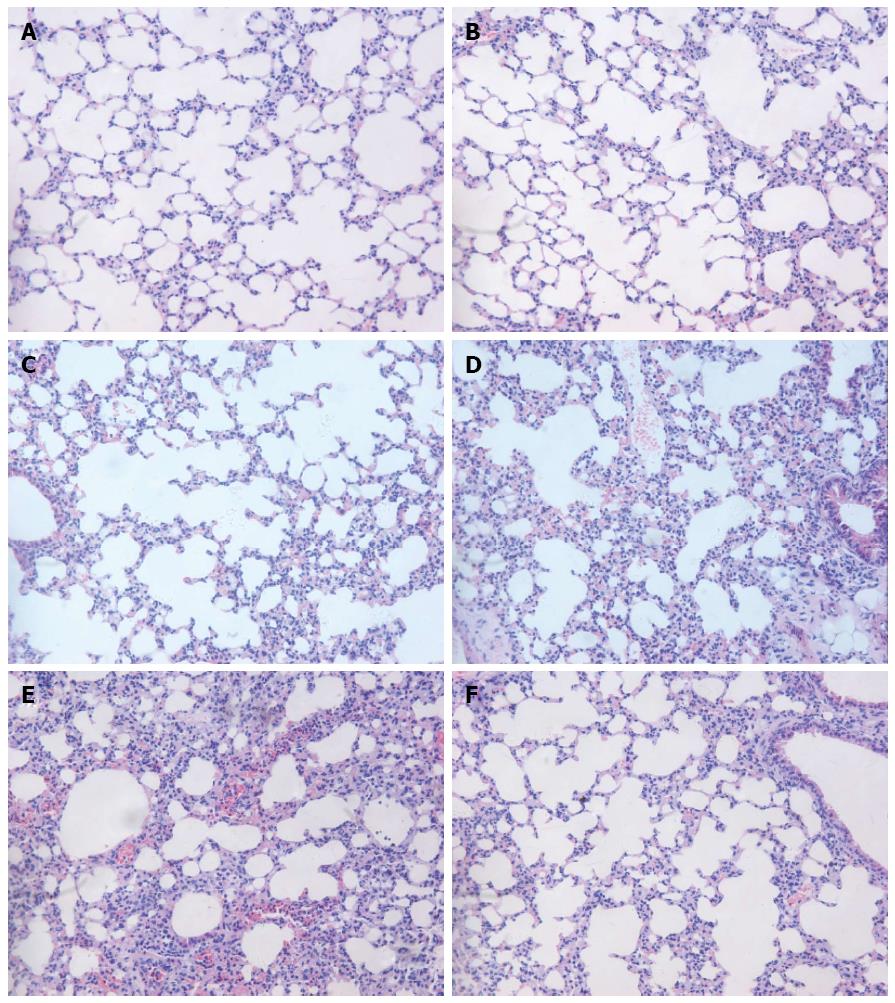
The macroscopic view of the lung showed significant edema and hemorrhage in the SAP and PBS groups. By contrast, pulmonary edema and hemorrhage in the rIL-22 group were not obvious. H&E staining showed no observable signs of lung damage in the normal control group (Figure 4A). The lungs of the mice showed no significant swelling, inflammation or necrosis 24 h and 48 h after the L-arginine injection (Figure 4B and D). However, interstitial edema, patchy hemorrhage, thickened alveolar interstitium and infiltration of inflammatory cells were markedly observed 72 h after the L-arginine injection (Figure 4D). No significant difference in the degree of lung injury was found between the PBS and SAP groups at 72 h (Figure 4E). In contrast, pretreatment with rIL-22 significantly reduced the degrees of edema, alveolar congestion and infiltration of inflammatory cells compared with the SAP group at 72 h (Figure 4F).
Figure 4 Hematoxylin-eosin staining of the lung.
Normal control group (A), SAP group (B: 24 h after L-arginine injection; C: 48 h after L-arginine injection; D: 72 h after L-arginine injection), PBS group (E) and rIL-22 group (F). Original magnification × 200. SAP: Acute severe pancreatitis; PBS: Phosphate-buffered saline; rIL-22: Recombinant interleukin-22.
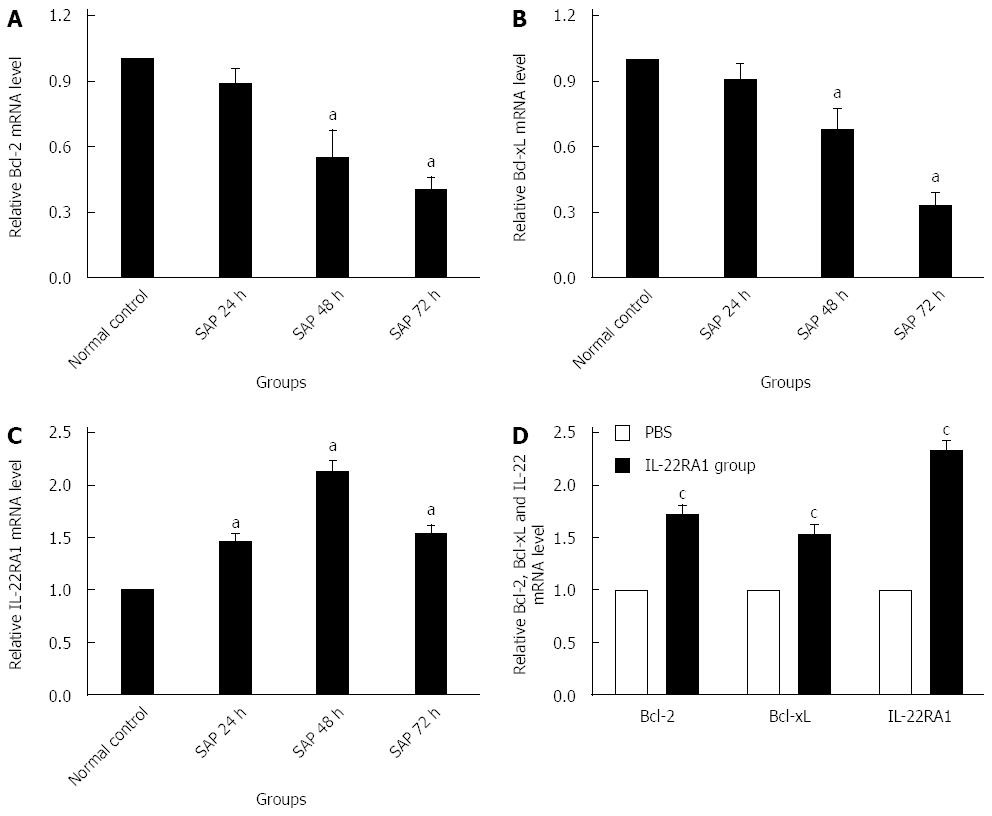
Bcl-2, Bcl-xL and IL-22R1 mRNA expression in lung tissue
Compared with the normal control group, the expression of Bcl-2 and Bcl-xL mRNAs in the lung tissue in the SAP group showed a significant decrease at 48 h and 72 h after the L-arginine injection (P < 0.05). However, no significant decrease was found at 24 h after the injection relative to the normal control group (P > 0.05, Figure 5A and B). IL-22RA1 mRNA expression significantly increased in the SAP group relative to that in the normal control group (P < 0.05). The lowest expression level was detected at 48 h after the L-arginine injection (Figure 5C).
Figure 5 Real-time PCR analysis of expression of Bcl-2(A and D), Bcl-xL (B and D) and IL-22RA1 mRNAs (C and D) in lung tissue.
Numbers of cases from each group statistically analyzed are 12 (Normal control), 9 (SAP 24 h), 8 (SAP 48 h), 8 (SAP 72 h), 8 (PBS) and 12 (rIL-22). Results are presented as mean ± SD. aP < 0.05 vs the normal control group, cP < 0.05 vs the PBS group. Bcl-2: B cell lymphoma/leukemia-2; Bcl-xL: B cell lymphoma/leukemia-extra large; IL-22RA1: Interleukin-22 receptor subunit alpha-1; PBS: Phosphate-buffered saline; rIL-22: Recombinant interleukin-22; STAT3: Signal transducer and activator of transcription 3.
Exogenous IL-22 promotes lung anti-apoptosis gene expression
As demonstrated in Figure 5D, rIL-22 stimulated the expression of Bcl-2 and Bcl-xL mRNAs, which play a key role in preventing cells from apoptosis involved in tissue regeneration (P < 0.05). In addition, IL-22RA1 expression in the rIL-22 group was also significantly higher than that in the PBS group (P < 0.05).
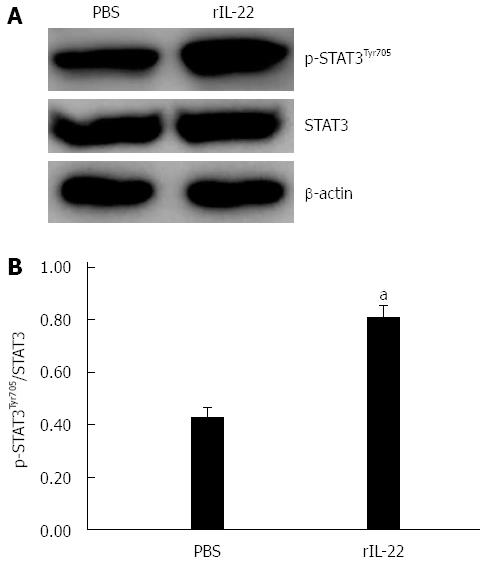
STAT3 is involved in the protective role of IL-22 in SAP-associated lung injury
STAT3 activation in the lung tissue in rIL-22 group was higher than that in the PBS group. However, there was no significant difference between the two groups as to the STAT3 expression (Figure 6A). The ratio of p-STAT3 to STAT3 protein in the rIL-22 group was significantly higher than that of the PBS group (P < 0.05, Figure 6B).
Figure 6 Western blot analysis of expression and activation (Tyr705 phosphorylation) of STAT3 protein in lung tissue (A) and the ratio of p-STAT3Tyr705 to STAT3 protein (B).
Numbers of cases from each group statistically analyzed were 8 (PBS) and 12 (rIL-22). Results are presented as mean ± SD. aP < 0.05 vs the PBS group. PBS: Phosphate-buffered saline; rIL-22: Recombinant interleukin-22; STAT3: Signal transducer and activator of transcription 3.
DISCUSSION
SAP is a life-threatening disease characterized by obvious inflammatory reactions and its morbidity has been reported to be increasing continually in recent years. Acinar necrosis-induced inflammation can lead to the occurrence of SIRS at an early stage which determines the severity of acute pancreatitis and can induce multiple organ dysfunction syndrome (MODS). ALI is the most prominent extra-pancreatic complication of SAP, and its severity ranges from mild hypoxemia to severe acute respiratory distress syndrome (ARDS) which is responsible for the high mortality rate[1,13]. Recent studies have indicated that the activation of numerous inflammatory cells, such as neutrophils and macrophages, leads to excessive production of cytokines and inflammatory mediators, regulating the severity of acute pancreatitis and SAP-associated ALI[2,14]. An ideal model for studying SAP and its associated multiple organ dysfunction should resemble the course in the human clinical setting, be easily reproducible and severe enough to induce MODS, yet still has a time window long enough for an intervention. In our experiment, the mouse SAP model induced by the L-arginine injection is compatible with the clinical manifestations of SAP, including lung damage. The serum amylase levels and lung tissue MPO activity were increased significantly after the L-arginine administration. In addition, the animals in the SAP group showed obvious pancreatic and lung injuries.
IL-22, formerly named IL-10-related T–cell-derived inducible factor (IL-TIF)[4], has recently gained considerable interest for its tissue protective effects in several murine models. At present, most studies support a well-established protective role for IL-22 in the prevention of hepatocellular damage. Specifically, application of recombinant IL-22 remarkedly attenuates murine liver injury induced by concanavalin A, alcohol, acetaminophen, hepatectomy or ischemia-reperfusion[15-19]. Furthermore, provision of IL-22 also ameliorates high fat diet-induced liver lipogenesis and hepatic steatosis[20] and decreases hepatic fibrosis in mice[21]. In addition to the protective effects of IL-22 on the liver, the benefits of IL-22 application on other targets are also well testified. Administration of IL-22 contributes to the tissue protection and regeneration in murine models of mucocutaneous infection[22-25], ventilator-induced lung injury[26], renal ischemia-reperfusion injury[27] and inflammatory bowel disease[28]. However, it was unknown whether IL-22 also plays a protective role in SAP-induced ALI. In the present study, we found that the rIL-22-treated mice had significantly lower MPO activity in the lungs than in the untreated SAP mice. Moreover, when compared to the untreated SAP mice, the rIL-22-treated mice showed significantly reduced degrees of edema, alveolar congestion, thickness of the alveolar wall and infiltration of inflammatory cells in the lung injury. Our results are in agreement with the previous findings and demonstrate that rIL-22 may be beneficial to the recovery of SAP-associated lung injury induced by L-arginine.
IL-22 is an important cytokine allowing for cross-talk between leukocytes and epithelial cells because IL-22 production and receptor expression are restricted to leukocytes and epithelial cells, respectively[3,29]. Ligation of the IL-22-IL-22R1-IL-10R2 complex leads to the activation of the JAK1/Tyk2/STAT3 pathway which is known as the principal and dominant mechanism of cellular activation by IL-22[7,8]. Of the cellular tasks associated with STAT3, anti-apoptosis, proliferation and regeneration are the major biological properties of IL-22[30,31]. Apoptosis is recognized as “programmed cell death”. Excessive apoptosis probably leads to lung dysfunction in pathological situations. There are increasing data indicating that increased epithelial/endothelial cell apoptosis is involved in the pathogenesis of ALI[32-34]. The signal transduction mechanisms of apoptosis are very complex, and the anti-apoptosis pathway is associated with genes whose functions include “death receptors” and apoptotic regulation. Apoptosis-inducing genes include p53, factor associated suicide (Fas), interlukin-1β-converting enzyme (ICE), reaper (rpr), Bcl-xs, Bcl-2 associated X protein (Bax), Bcl-2 homologous antagonist killer (Bak), Bcl-2/ Bcl-2-XL-associated death promoter (Bad), Bcl-2 inhibitory BH3 domain-containing protein (bid), and Bcl-2 interacting killer (bik). Genes that inhibit apoptosis include Bcl-2, the inhibitor of apoptosis protein (IAP) family, Bcl-xl, Bcl-2 related protein A1 (A1)/Bfl1, Bcl-w, myeloid cell leukemia (Mcl), and Bcl-2-associated athanogene 1 (BAG-1)[35,36]. Bcl-2 is involved in the transduction of the intrinsic mitochondria pathway[37]. IL-22 up-regulates STAT3-inducible proteins, such as Bcl-2, Bcl-xL, cyclin-dependent kinase 4 (CDK4), cyclin D1, cellular-myelocytomatosis viral oncogene (c-myc), and p21[15,19,21,38-40], and is associated with tissue protection and regeneration from injury. Along with the activation of extracellular regulated protein kinases (ERK) 1/2[41] and Akt/protein kinase B (PKB)[42], these pro-survival proteins are likely to form the cellular basis for tissue protective characteristics of IL-22. In this study, we examined the expression of IL-22RA1 in the lung of mice with SAP. Interestingly, the level of IL-22R1 expression increased at first and decreased later after the L-arginine administration. This finding indicates that IL-22RA1 expression is stress inducible. Based on the high expression of IL-22RA1, we hypothesized that the lung would respond strongly to an IL-22 administration. To test this hypothesis, we administered rIL-22 systemically to mice and assessed the IL-22 downstream signaling pathway. Consistent with our hypothesis, the lung expression of Bcl-2 and Bcl-xL, downstream targets of STAT3 activation, was decreased significantly in mice with SAP, but was interestingly elevated after rIL-22 treatment. Similarly, IL-22RA1 was also increased after rIL-22 administration. In addition, significant STAT3 activation was observed in the lung after the exogenous IL-22 administration. These data indicate that the mice with SAP-associated lung injury were responsive to rIL-22 administration by enhancing the expression of anti-apoptosis genes through the STAT3 signaling pathway.
Taken together, these findings indicate that systemic administration of rIL-22 can alleviate the SAP-associated ALI in mice by enhancing the expression of anti-apoptosis genes, such as Bcl-2 and Bcl-xL. While the underlying mechanism of IL-22 in protection against SAP-associated lung injury is not fully understood, our findings demonstrate that the STAT3 signaling pathway may be associated with this process. Therefore, IL-22 and the components of STAT3 signaling pathway may be promising targets in the treatment of SAP-associated lung injury.
COMMENTS
Background
Interleukin-22 (IL-22) is recognized today as a key player in the antimicrobial defense, regeneration, and protection against damage. However, no reports have described the effects of IL-22 on acute severe pancreatitis associated lung injury. In this article, the authors sought to investigate the potential protective effect of exogenous rIL-22 on SAP associated lung injury induced by L-arginine and its possible signaling pathway.
Research frontiers
IL-22 is recognized today as a key player in the antimicrobial defense, regeneration, and protection against damage. However, no reports have described the effects of IL-22 on acute severe pancreatitis associated lung injury.
Innovations and breakthroughs
In this article, the authors investigated the potential protective effect of exogenous rIL-22 on SAP associated lung injury induced by L-arginine and its possible signaling pathway. IL-22 was demonstrated to alleviate acute severe pancreatitis-associated acute lung injury in mice by enhancing the expression of anti-apoptosis genes such as Bcl-2 and Bcl-xL through the STAT3 signaling pathway.
Applications
IL-22 and the components of STAT3 signaling pathway may be promising targets in the treatment of acute severe pancreatitis associated lung injury.
Terminology
IL-22, a member of the IL-10 family, is a cytokine secreted by several types of immune cells such as T helper (Th) 22, Th1, and Th17 cells, γδ T cells, natural killer T cells, and innate lymphoid cells. It is a principal component in mucosal barrier defense, tissue repair, epithelial cell survival, and proliferation.
Peer-review
The authors show that recombinant IL-22 protected the mice against L-arginine-induced SAP and associated lung injury by enhancing the expression of anti-apoptosis genes. The work is very innovative and is an indication for further research on the problem of complications of acute pancreatitis.