INTRODUCTION
For a long time, cardiac dysfunction in liver cirrhosis, termed “cirrhotic cardiomyopathy”, was thought to be a common occurrence in patients suffering from alcoholic cirrhosis[1,2]. During the last decade, however, non-alcoholic cirrhotic patients have also been reported to demonstrate these cardiac abnormalities[3]. Cardiovascular dysfunction is observed in cirrhosis, but the underlying mechanisms are not still well understood. Despite the hyperdynamic systemic circulation and the absence of coronary artery or valvular disease and hypertension, cardiac hypertrophy and cardiomyocyte edema are observed in cirrhotic patients[3-7]. Furthermore, there is evidence for a concomitant decrease of inotropic effect along with impaired myocardial contractility[6]. Previous studies have shown that both portal hypertension and cirrhosis contribute to cardiomyopathy[1,8]. Cardiomyopathy is characterized by latent heart failure with impaired contractile responsiveness to pharmacological or physiological stress and/or altered diastolic relaxation with electrophysiological abnormalities, without any diagnosed cardiac disease and causes of cirrhosis[4,6].
A variety of mechanisms are responsible for the pathogenesis of cirrhotic cardiomyopathy. The major predisposing factors of cardiac contractility include alteration in ventricular receptor signal transduction (i.e., β-adrenergic, muscarinic, and cannabinoid receptors)[9-12] and ionic channel function (i.e., K+ and L-type voltage-gated Ca2+)[13-15], cardiomyocyte plasma membrane fluidity changes[5,6], and complex alterations of carbon monoxide and nitric oxide (NO)[16,17]. Moreover, a rise in pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) is observed in this condition, resulting in stimulation of inducible nitric oxide synthase (iNOS) and NO overproduction[18].
Mammalian target of rapamycin (mTOR), a serine/threonine kinase component downstream of phosphotidylinositide 3-kinase (PI3K)/Akt signaling pathway[19,20], is a key regulator of mRNA translation and cell growth in cardiomyocytes[21,22]. Protein synthesis, a major factor for cardiac hypertrophic growth, is regulated by the PI3K/Akt/mTOR signaling pathway through inactivation of eukaryotic translation initiation factor 4E-binding proteins (4E-BPs)[23], leading to stimulation of polymerase I and III transcription[24], control of ribosome biogenesis and mitochondrial metabolism[25], and suppression of autophagy[26-28]. Zhang et al[29] found that mTOR knockout mice had improved baseline cardiomyocyte survival, decreased dilated cardiac hypertrophy, and less heart failure than control mice. Moreover, it was shown that activation of PI3K/Akt/mTOR signaling may lead to the development of cardiac hypertrophy[30]. Indeed, the mTOR inhibitor rapamycin appeared to block the development of cardiomyocyte hypertrophy[29], and cohort studies have shown that rapamycin has cardioprotective effects in patients after liver transplantation[31,32].
Although our current knowledge of the predisposing factors of cirrhotic cardiomyopathy is somewhat understood, the role of other pathophysiological mechanisms underlying cardiac dysfunction induced by cirrhosis remains to be clarified. To this purpose, we examined the hypothesis that tetrachloride carbon (CCl4)-induced cardiac inotropic dysfunction in response to adrenergic stimulation is associated with altered expression of cardiac p-mTOR in a rat model of cirrhotic cardiomyopathy. In this study, we demonstrate for the first time the positive inotropic effect of mTOR suppression by rapamycin and its ability to normalize cardiac levels of p-mTOR and the pro-inflammatory factor TNF-α in cirrhotic cardiomyopathy.
MATERIALS AND METHODS
Chemicals and reagents
The following compounds and reagents were applied in this investigation: rapamycin (Wyeth, Kildare, United Kingdom/Ireland), isoproterenol hydrochloride (Sigma, St. Louis, MO, United States), carbon tetrachloride (Merck, Darmstadt, Germany); TNF-α assay kit, polyclonal p-mTOR antibody (pSer2448), and horseradish peroxidase (HRP)-conjugated rabbit anti-rat Immunoglobulin G antibody (Biorbyt Co. Ltd., Cambridge, United Kingdom).
Animal model of cirrhosis
Male albino Wistar rats weighing 100-120 g were used with housing facilities (environment temperature at 21 °C-23 °C, 12-h regular light/dark cycle). Animals had unlimited access to food and water except for a brief time during injection and during the surgical procedure. The rats were divided into four main groups: control/drinking water, control/rapamycin, cirrhotic/drinking water, and cirrhotic/rapamycin. All experiments and manipulations were conducted in Prof. Dehpour’s Hepatological Research Laboratory in accordance with the institutional animal care and use committee (Department of Pharmacology, School of Medicine, Tehran University of Medical Sciences) guidelines. This study was approved by the Ethics Committee of Tehran University of Medical Sciences.
To induce cirrhosis, CCl4 (0.4 g/kg; a solution of 1:6 in mineral oil) was intraperitoneally injected to the animals three times a week for 8 wks until the appearance of ascites[33]. Rapamycin (2 mg/kg per day) was freshly dissolved in normal saline and daily administered in drinking water in a constant volume of 14 mL/100 g body weight during the 8-wk period[34,35]. Twenty-four hours after cessation of CCl4, animals were sacrificed by guillotine decapitation. The liver was removed, sectioned, and stained with hematoxylin-eosin (HE). Light microscopy of stained liver sections confirmed the induction of cirrhosis in rats[4].
Twenty-four hours after the last administration of either CCl4 or N/S, a lead II electrocardiogram (ECG) was recorded for 15 min using three stainless steel subcutaneous electrodes attached to a bioamplifier (ADInstrument, Sydney, Australia) from the anesthetized rats. The signals were digitized at a sampling rate of 10 kHz by a Powerlab system and were displayed using Lab Chart 7 software (ADInstrument). The Q-T intervals, presented as corrected Q-T (QTc), were calculated in a 5-min ECG. The QTc was presented using Bazett’s formula (QTc = QT/√R-R)[36].
Preparation of isolated papillary muscle
Briefly, animals’ hearts were excised following decapitation and left ventricular papillary muscles were dissected in cold oxygenated physiological salt solution (PSS) containing (in mmol/L) NaCl, 112; KCl, 5; CaCl2, 1.8; MgCl2, 1; NaH2PO4, 0.5; KH2PO4, 0.5; NaHCO3, 25; glucose, 10; and EDTA, 0.004[37,38]. The isolated papillary muscles were suspended in a 25-mL organ bath chamber containing PSS buffer solution bubbled with a gas mixture of 95% O2: 5% CO2 at 37 °C for 90 min to reach equilibrium. The contractility was induced by electrical field stimulation (Grass 88 Stimulator; Grass Instruments, West Warwick, RI, United States) at 1 Hz and 30 V, 20% higher than the threshold. After achievement of baseline contractile force, the muscle contraction was stimulated by addition of cumulative concentrations of isoproterenol (10-10 to 10-5 mol/L). The contractile force induced by the highest concentration of isoproterenol (10-5 mol/L) was considered as maximal contractility[16]. The resulted contractile forces were expressed as a percentage of the baseline papillary muscle contractility.
Immunohistochemistry
The ventricle samples were immediately fixed in freshly prepared 10% formalin and paraffin-embedded blocks. After deparaffinizing in xylene and rehydrating in decreasing concentrations of ethanol, 3% hydrogen peroxidase was added for 5 min to block dual endogenous peroxidase activity. Then, the immunohistochemical staining was performed based on the Avidin-Biotin peroxidase method. Polyclonal p-mTOR antibody (pSer2448) (1:50 dilution) was reacted for 1 h at room temperature followed by secondary HRP-conjugated rabbit anti-rat Immunoglobulin G antibody (1:50 dilution) for 30 min at room temperature. The sections were washed three times with Tris (pH 7.4), incubated with diaminobenzidine (DAB) solution for 10 min, and then incubated with 5% CuSO4 for 5 min. Ultimately, the slides were washed and counterstained with H&E to obtain brown-colored precipitation for examination under light microscopy.
Ventricular TNF-α quantification
To measure tissue TNF-α, the left ventricles were excised, rinsed in PSS, snap-frozen in liquid nitrogen, and stored at -80 °C for further analysis. The samples were then homogenized in ice-cold phosphate-buffered saline (PBS) and centrifuged at 14200 g for 30 min. Fifty microliters of the samples and standards were pipetted into a 96-well plate precoated with rat TNF-α specific antibody. Following addition of 50 μL of biotinylated anti-TNF-α solution, the plate of the enzyme linked immunosorbent assay kit was incubated for 90 min at room temperature. The wells were washed, exposed to 100 μL of streptavidin-peroxidase, incubated for 45 min at room temperature, and washed four times with PBS. Finally, 100 μL of both stabilized chromogen and stop solution were respectively added in two stages and incubated for 20 min for spectrophotometrically analysis at λ = 450 nm[16].
Western blot analysis
The dissection and snap-freezing of left ventricles were performed as described in the above section. Briefly, left ventricles were homogenized in buffer (20 mmol/L Tris-HCl (pH 7.2), 0.2 mmol/L phenylmethylsulfonyl fluoride, and 1 mmol/L dithiothreitol), centrifuged at 40000 g, and resuspended in Tris buffer containing proteinase inhibitor. Thirty micrograms of protein samples were loaded and separated on sodium dodecyl sulfate-10% polyacrylamide gel (SDS-PAGE) by electrophoresis and were wet electroblotted onto nitrocellulose membrane at 4 °C for 12 h[39,40]. The blots were blocked for 1 h at room temperature with 2% bovine serum albumin in 0.1% Tween Tris-buffered saline (TBS-T) (pH 7.5). Then, the membranes were washed and incubated overnight at 4 °C with polyclonal p-mTOR primary antibody (pSer2448) (1:100 dilution). After washing, these blots were exposed to HRP-conjugated anti-rat secondary antibody (1:1000 dilution). Detection of blots was performed using enhanced chemiluminescence (ECL kit, Amersham, Chalfont St. Giles, United Kingdom) method. The levels of p-mTOR in cirrhotic, control, and rapamycin-treated animals were semi-quantified using ImageJ software (National Institutes of Health, Bethesda, MD, United States), which was defined as the p-mTOR/glyceraldehyde 3 phosphate dehydrogenase (GAPDH) densitometric ratio (%).
Statistical analysis
All data are expressed as mean ± SD and analyzed using GraphPad Prism software (version 5.0, GraphPad Software, Inc., La Jolla, CA, United States). To examine the differences between three or more experimental groups, one-way analysis of variance (ANOVA) followed by a Tukey’s post test was used. For two-group comparisons, Student’s t-test was applied. Evaluation of the effects of two variables (cirrhosis vs control and type of treatment) was performed using two-way ANOVA followed by a Bonferroni post test. A value of P < 0.05 was considered to be statistically significant.
RESULTS
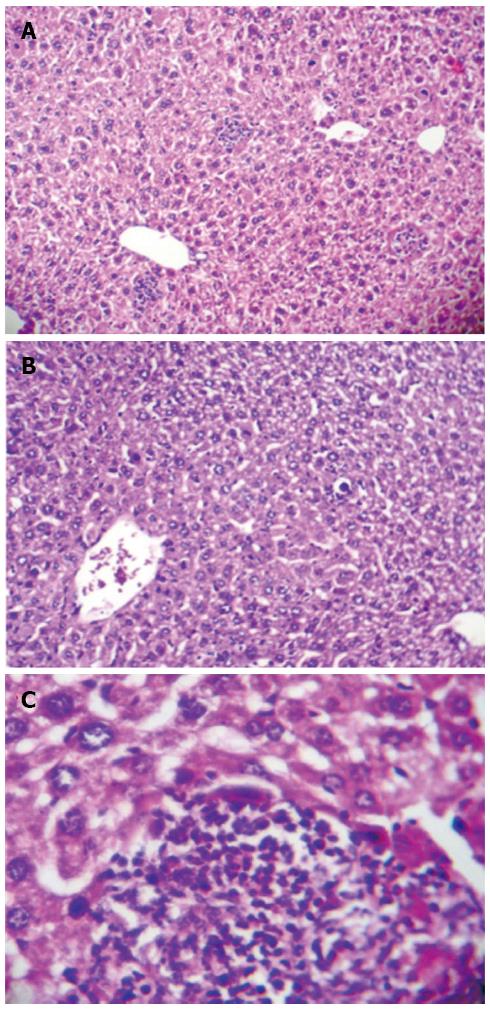
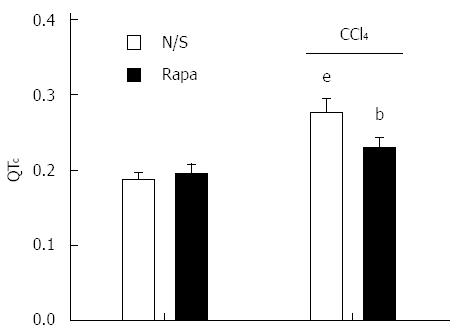
Presence of CCl4-induced cirrhosis was confirmed by visual observation of lethargy, weight loss, jaundice, brown urine, and ascites along with liver stiffness and a significant increase in spleen weight (1.52 ± 0.13 g vs 2.74 ± 0.41 g in control vs cirrhotic rats, P < 0.001), which contributed to the development of portal hypertension. H&E staining of liver tissues sampled from cirrhotic rats demonstrated focal hepatocellular necrosis and apoptotic cells as well as enhanced inflammatory cell infiltration into the portal tract. Fatty degeneration areas and central vein dilation were also seen histologically (Figure 1). Moreover, cirrhosis model animals had significantly prolonged QTc intervals compared to controls (P < 0.001; Figure 2). The prolonged QTc interval in cirrhotic rats was decreased by rapamycin (2 mg/kg) (P < 0.01; Figure 2).
Figure 1 Histological change in liver tissue of CCl4-induced cirrhotic rats (hematoxylin and eosin; magnification × 100 and × 400).
A: Focal hepatocellular necrosis, apoptotic cells, and patchy inflammatory cell infiltration along with central vein dilation are observed; B: Fatty degeneration areas are clearly seen; C: Inflammatory cell infiltration into the portal tract.
Figure 2 QT interval in control and CCl4-induced cirrhotic rats treated with normal saline or rapamycin (2 mg/kg).
QT intervals were defined as corrected QT (QTc) using Bazett’s formula. The data are expressed as the mean ± SD. eP < 0.001 vs control/normal saline group; bP < 0.01 vs control/rapamycin and cirrhotic/saline group.
Effect of rapamycin on papillary muscle contractility
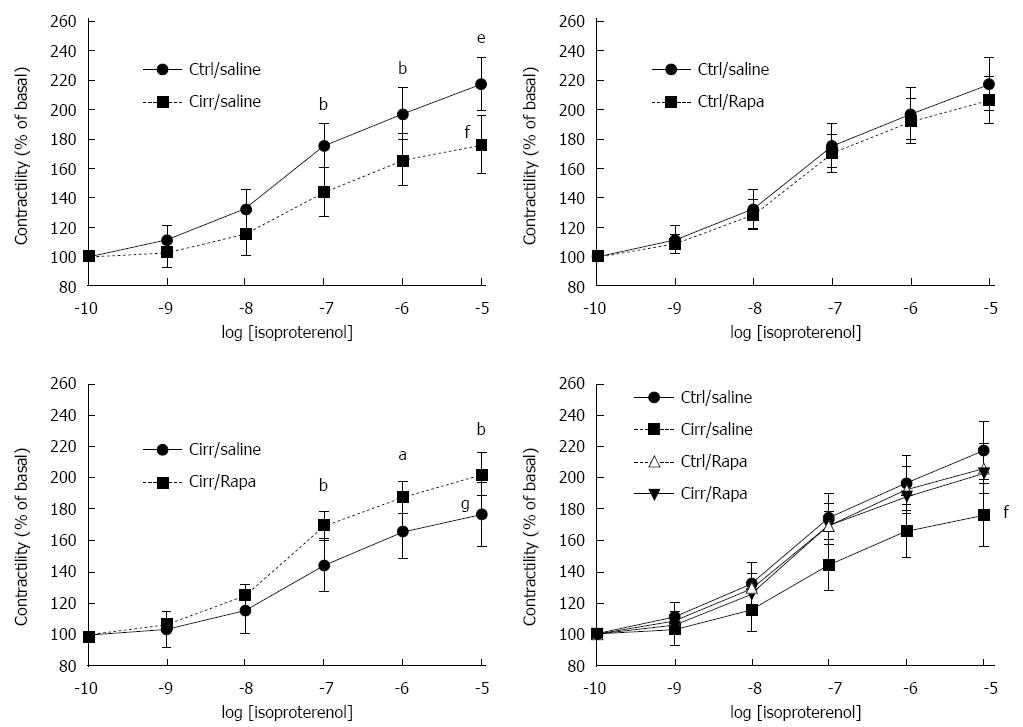
As shown in Figure 3A, baseline papillary muscle inotropic responses to isoproterenol stimulation in cirrhotic rats were significantly decreased compared to controls (P < 0.001). The order was in agreement with the maximum response (Rmax) to isoproterenol (76.46% ± 10.08% vs 117.36% ± 8.25%, P < 0.001; Figure 3A). Rapamycin did not significantly alter Rmax in control rats. Likewise there was no significant difference in the EC50 of isoproterenol (4.08 ± 1.35 × 10-8 and 6.59 ± 1.29 × 10-8 in N/S- and rapamycin-treated non-cirrhotic control groups, respectively; P > 0.05; Figure 3B). In cirrhotic rats, there was a significant rise in papillary muscle contractility and a significant enhancement of Rmax following chronic treatment with rapamycin (2 mg/kg) compared to cirrhotic rats treated with N/S (P < 0.001; Figure 3C). There were no significant differences in the EC50 of isoproterenol among all four studied groups (P > 0.05; Figure 3D).
Figure 3 Contractile force in response to β-adrenergic stimulation in cirrhotic and control rats treated with normal saline or rapamycin (2 mg/kg).
Inotropic responsiveness to β-adrenergic stimulation with isoproterenol in the isolated papillary muscle from cirrhotic and control rats treated with normal saline or rapamycin (2 mg/kg) was analyzed to determine the contractile force (% of basal). The data are expressed as the mean ± SD. Maximal response (Rmax) in the CCl4-induced cirrhotic rats was significantly lower than the other groups. There were no significant differences in EC50 values among the four studied groups. fP < 0.001 vs the control group receiving normal saline; gP < 0.001 vs the cirrhosis group receiving normal saline; aP < 0.05, bP < 0.01, eP < 0.001 vs the cirrhotic group receiving normal saline in that concentration.
Effect of rapamycin treatment on ventricular TNF-α concentration
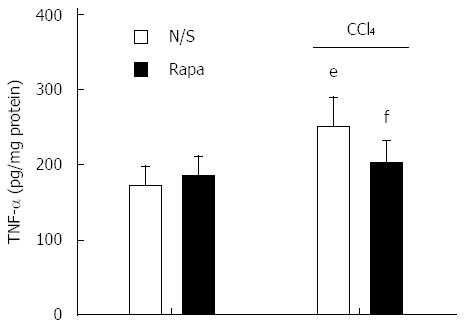
As shown in Figure 4, there was a significant increase in ventricular levels of TNF-α in cirrhotic rats compared to controls (P < 0.001). Treatment with rapamycin (2 mg/kg) for 8 wk caused no marked enhancement in tissue TNF-α concentration in the control group (P > 0.05). In addition, rapamycin significantly decreased the elevation in tissue TNF-α concentration in animals with cirrhosis (P < 0.05).
Figure 4 Left ventricular tumor necrosis factor-α levels in control and CCl4-induced cirrhotic rats treated with normal saline or rapamycin (2 mg/kg).
The data are expressed as the mean ± SD. eP < 0.001 vs control/normal saline group; fP < 0.001 vs cirrhosis/normal saline group. TNF-α: Tumor necrosis factor-α.
Ventricular p-mTOR expression
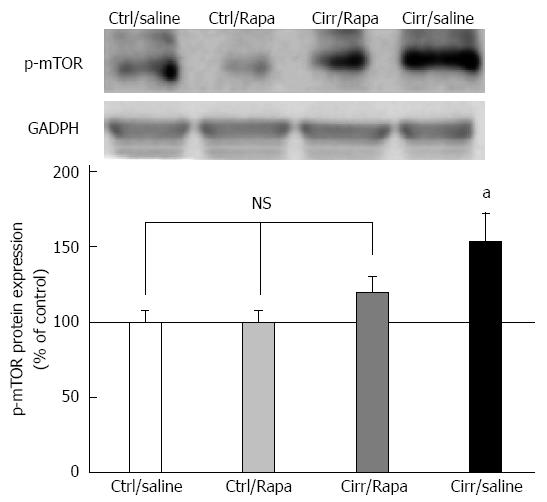
As shown in Figure 5, expression of p-mTOR in the left ventricles of cirrhotic rats was increased compared to controls (P < 0.001). Rapamycin treatment reversed this increase in p-mTOR level in animals with cirrhosis (P < 0.001). Moreover, treatment of cirrhotic rats with rapamycin decreased p-mTOR protein expression to the level of control animals (P > 0.05).
Figure 5 Western blot analysis of p-mammalian target of rapamycin protein in the left ventricles of control and CCl4-induced cirrhotic rats treated with normal saline or rapamycin (2 mg/kg).
The upper panels demonstrate the representative immunoblots of p-mTOR and glyceraldehyde 3 phosphate dehydrogenase (GAPDH) proteins in the control, control + rapamycin, cirrhotic and cirrhotic + rapamycin. The lower panel shows the densitometric analysis after normalization with GAPDH. Values are expressed as p-mTOR/GAPDH ratio (%) and normalized to the control group receiving normal saline (mean ± SD). aP < 0.05 vs the other three groups; NS: Non-significant.
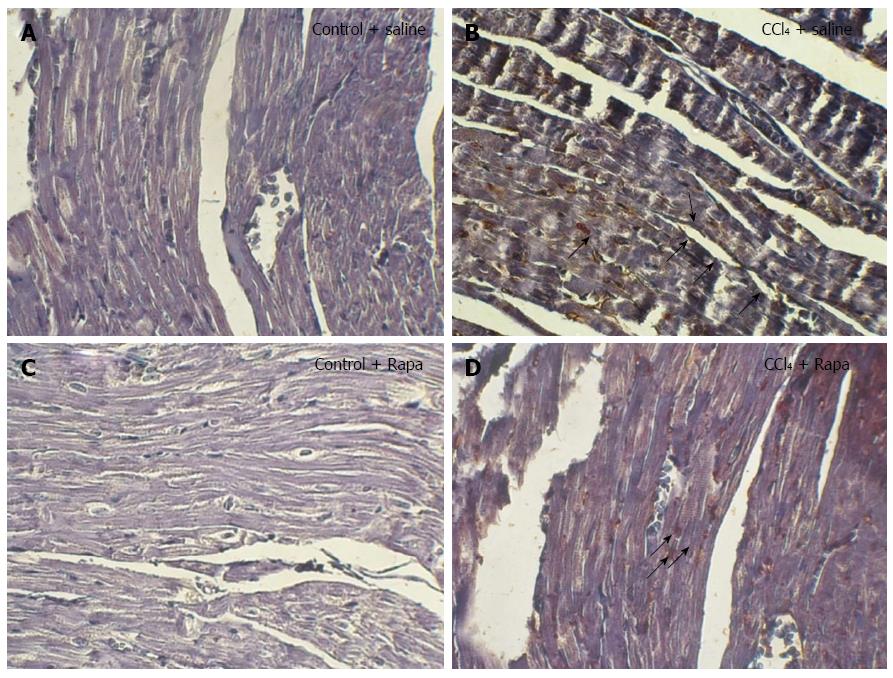
To explore which cells express p-mTOR, immunohistochemical analysis was performed. Although almost no immunostaining was observed in the ventricular myocytes and endothelial cells in the control group (Figure 6A), p-mTOR immunostaining was markedly stronger in endothelial cells, but not in myocardial layer, of cirrhotic rats (Figure 6B). In the cirrhosis group, rapamycin could decrease p-mTOR immunostaining and induce mTOR phosphorylation in ventricular myocytes, as shown in Figure 6D.
Figure 6 Immunohistochemical staining for p-mTOR in the ventricles of the rats in the following groups: control, cirrhotic, control + rapamycin and cirrhotic + rapamycin (× 400 magnification).
Human gastric tissue was used as the positive control. Note the increased immunostaining of p-mTOR in the myocytes of the rats with cirrhosis. No significant immunostaining was localized to the cardiomyocytes of the untreated cirrhotic rats. In contrast, treatment with rapamycin caused significant immunostaining in the cardiomyocytes of the cirrhotic rats. The black arrows indicate to the p-mTOR immunoblots in rat ventricles.
DISCUSSION
The main finding of the present study is the demonstration that cardiac mTOR expression and protein levels are increased in rats with cirrhotic cardiomyopathy. For the first time we showed that altered expression of p-mTOR in cirrhotic heart contributed to cardiac contractile suppression. This effect was confirmed by immunohistochemical assay, which showed a strong p-mTOR signal in cirrhotic left ventricles, especially in endothelial cells. Interestingly, the data from an in vitro papillary muscle study suggested that the enhanced expression of p-mTOR caused cardiac dysfunction. Consistent with that finding, we found a relationship between changes of mTOR activity and hypertrophic cardiomyopathy and heart failure[20,30,40-44]. Moreover, an increase in cardiac tissue TNF-α was observed in cirrhotic animals, which was accompanied by cardiomyocyte contractile dysfunction. Recently, several studies have investigated the role of TNF-α in the pathogenesis of heart failure and impaired cardiac contractility and have demonstrated that increased NO synthesis, an underlying mechanism for cirrhosis, in cardiac tissues of cirrhotic mice is attributed to elevated TNF-α level[4].
We also showed that repeated treatment with rapamycin normalized the cardiac contractile force defect in cirrhotic rats. To our knowledge, this is the first investigation to examine the hypothesis that rapamycin, via mTOR suppression, improves cardiac inotropic responsiveness to isoproterenol β-adrenergic stimulation and shortens the prolonged QTc in rats with cirrhosis. Since mTOR phosphorylation was not obviously detectable in ventricular cardiomyocytes taken from CCl4-induced cirrhotic rats, rapamycin caused significantly greater increases in p-mTOR protein level in cardiomyocytes than endothelial cells. Interestingly, despite the abundant expression of p-mTOR in cardiomyocytes, but not in endothelial cells, of rapamycin-treated rats with cirrhosis, total cardiac p-mTOR protein was reduced in comparison with cirrhotic rats receiving N/S. This finding was correlated with the positive inotropic effects of rapamycin in this paradigm. Decreased tissue levels of TNF-α after treatment with rapamycin confirmed the hypothesis that reduction in overproduced cytokines, such as TNF-α and interleukin-1β, from hepatic and systemic reticuloendothelial cells can reverse their negative inotropic effects[16,45,46]. Evidence has shown that rapamycin acts as an effective agent, like isoproterenol, to raise intracellular cyclic adenosine monophosphate by reducing the expression and release of the pro-inflammatory cytokine TNF-α from human heart tissue[47]. Also, rapamycin may inhibit nuclear factor-kappa B (NFκB) activation and TNF-α, a potent inducer of in vascular smooth muscles[48].
During the last two decades, many investigations have been performed to explore the possible manifestations and potential mechanisms underlying cirrhotic cardiomyopathy. For instance, a decrease in isolated papillary muscle contractile force was observed in response to adrenergic stimulation in bile duct-ligated rats[12,36,49-51]. These results were similar to our observation that negative inotropic responsiveness to adrenergic stimulation is a result of CCl4-induced cirrhosis. Although most of the studies are based on the hypothesis that defects of cardiac contractile force may result from downregulation of β-adrenergic receptors[10,37] as well as increased cardiac NO synthesis[16], we tried to investigate the role of mTOR inhibition in a rat model of cirrhosis to attenuate the impairment in cardiac contractile performance. Previous studies have reported the protective effects of rapamycin on the development of left ventricle hypertrophy and ischemia/reperfusion injury after myocardial infarction[21,22,42-44,52]. Blockade of NFκB and PI3K/Akt/mTOR signaling pathway may play an essential role in ameliorating myocardial hypertrophy induced by p70S6K, a main component downstream of mTOR, activation in the infarcted hearts[21,22,30,43]. In addition to the role of mTOR in cardiomyocyte hypertrophy, p-mTOR played a role in the impairment of cardiac survival and structure and also myocardial contractile dysfunction[53]. Inhibition of mTOR activated 4E-BP1, another downstream target of mTOR, resulting in inhibition of protein synthesis, pathogenesis of cardiomyopathy, and subsequent complications of cardiac hypertrophy[29,43].
Moreover, increment of autophagy and autophagosome formation upon mTOR inhibition with rapamycin is considered to be other protective mechanisms in heart failure[43,54]. Regarding the requirement of the ubiquitin proteasome system for activation of NFκB, rapamycin can restrict the myocardial infarction size and remodeling by inhibiting the ubiquitin proteasome and subsequent NFκB activity[43,55,56].
In addition to the observed positive effect of rapamycin on electrophysiological and mechanical cardiac function in cirrhosis, it is noteworthy that rapamycin has protective effects on human liver fibrosis and inhibits the progression of fibrosis, especially at early stages[35,57,58]. Rapamycin exerts this effect by inhibiting cell proliferation, deposition of extracellular matrix, and the profibrogenic pathway and factors[59-62]. Additionally, cohort studies have reported that patients receiving rapamycin after liver transplantation had no cardiovascular problems. They showed that rapamycin not only did not increase the risk of congestive heart failure and myocardial infarction but plays a role as a cardioprotective agent[31,32]. In our study, the positive role of rapamycin on cirrhotic cardiomyopathy was attributed to a direct effect on cirrhotic heart, and it was assumed that a part of this phenomenon was associated with the anti-fibrogenic effect of this drug. This assumption is strongly amplified since cardiac and liver diseases share a common etiology[6]. Although experimental and clinical investigations on cirrhotic patients revealed latent heart failure with impaired response to provocations and subsequent mortality, no effective treatment has been found for improving cardiac contractility in patients with cirrhotic cardiomyopathy and evident ventricular failure[6]. As the prolongation in QT interval is considered an important life-threatening element in patients with cirrhotic cardiomyopathy, early identification and treatment of patients are necessary. Therefore, due to the anti-cytokine and beneficial role of rapamycin in correcting the abnormal cardiac contractile force and QT interval, rapamycin is expected to be the subject for further clinical investigations in patients with cirrhotic cardiomyopathy.
The present study has provided evidence that an increase in p-mTOR is responsible for the impaired cardiac contractility in animals with CCl4-induced cirrhosis. Moreover, mTOR blockade corrected the cardiac contractile dysfunction in liver cirrhosis, highlighting the possible therapeutic potential for the mTOR antagonist rapamycin in this condition. This treatment may increase survival in cirrhosis-associated heart failure until a transplant becomes available. In addition, our utilization of an experimental model of cirrhotic cardiomyopathy and its translation to clinical benefits may guide future research studies.
COMMENTS
Background
“Cirrhotic cardiomyopathy” has been recognized as cardiac dysfunction in liver cirrhosis, which commonly occurs in patients suffering from cirrhosis. Unfortunately, the responsible mechanisms underlying the pathophysiology of cirrhotic cardiomyopathy are not well understood. Therefore, understanding these mechanisms may help to develop possible treatments for this disease.
Research frontiers
To date, a variety of mechanisms have been described that are responsible for the pathogenesis of cirrhotic cardiomyopathy. The major predisposing factors of cardiac contractility include alterations in ventricular receptor signal transduction and ionic function, cardiomyocyte plasma membrane fluidity changes, and complex alterations in carbon monoxide and nitric oxide.
Innovations and breakthroughs
Although the current knowledge of the mechanisms underlying cirrhotic cardiomyopathy is somewhat understood, the role of other pathophysiological mechanisms remains to be clarified. To this purpose, the authors examined the hypothesis that CCl4-induced cardiac inotropic dysfunction in response to adrenergic stimulation is associated with altered expression of cardiac phosphorylated-mammalian target of rapamycin (mTOR) in a rat model of cirrhotic cardiomyopathy. Therefore, this study is the first to demonstrate the positive inotropic effect of mTOR suppression by rapamycin and its ability to normalize cardiac levels of phosphorylated-mTOR as well as the pro-inflammatory factor TNF-α in cirrhotic cardiomyopathy.
Applications
mTOR blockade corrected the cardiac contractile dysfunction in liver cirrhosis, highlighting the therapeutic potential of the mTOR antagonist rapamycin in this condition. Treatment with rapamycin may increase survival in those with cirrhosis-associated heart failure until a transplant becomes available. This study may guide researches to utilize the experimental model of cirrhotic cardiomyopathy translating to clinical benefits.
Peer-review
This is an interesting study about the role of mTOR in the pathogenesis of cirrhotic cardiomyopathy and the potential use of rapamycin for improving cardiac dysfunction.