Published online Feb 21, 2015. doi: 10.3748/wjg.v21.i7.2067
Peer-review started: April 23, 2014
First decision: May 29, 2014
Revised: June 20, 2014
Accepted: July 29, 2014
Article in press: July 30, 2014
Published online: February 21, 2015
Processing time: 294 Days and 18.5 Hours
AIM: To investigate crural diaphragm (CD) function in systemic sclerosis (SSc) using high-resolution manometry and standardized inspiratory maneuvers.
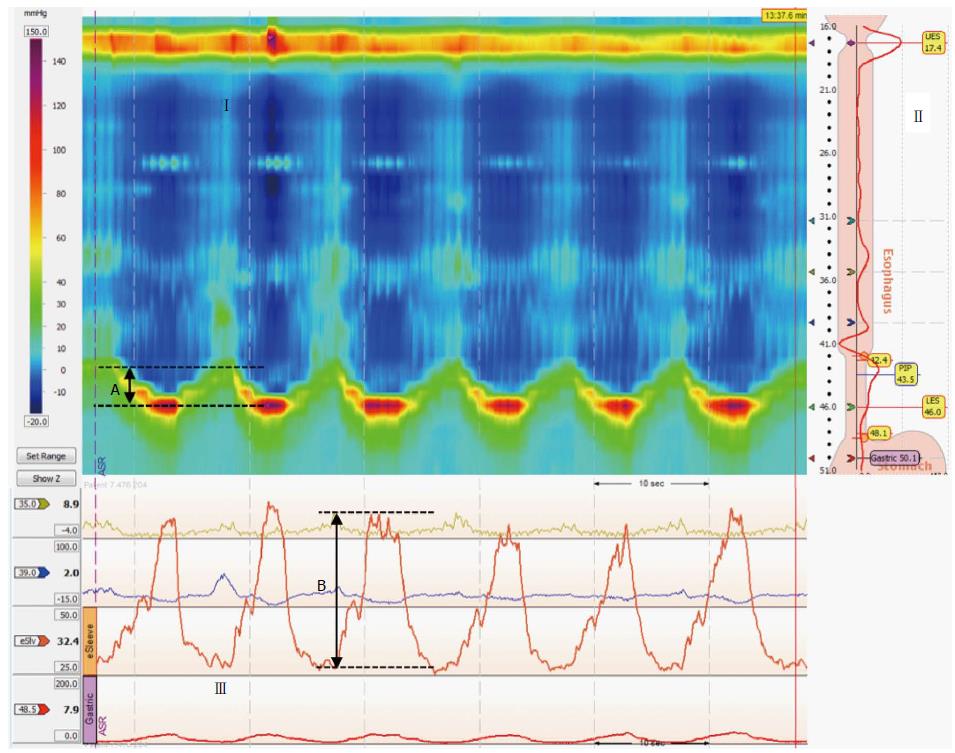
METHODS: Eight SSc volunteers (average age, 40.1 years; one male) and 13 controls (average age, 32.2 years; six males) participated in the study. A high-resolution manometry/impedance system measured the esophagus and esophagogastric junction (EGJ) pressure profile during swallows and two respiratory maneuvers: sinus arrhythmia maneuver (SAM; the average of six EGJ peak pressures during 5-s deep inhalations) and threshold maneuver (TM; the EGJ peak pressures during forced inhalation under 12 and 24 cmH2O loads). Inspiratory diaphragm lowering (IDL) was taken as the displacement of the EGJ high-pressure zone during the SAM.
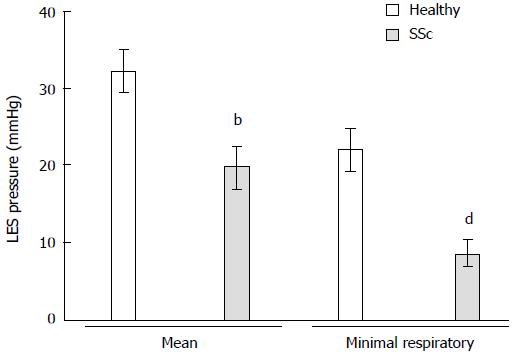
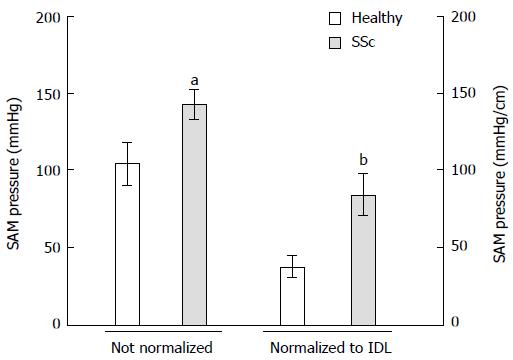
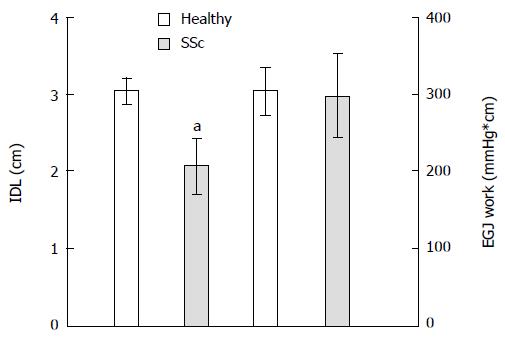
RESULTS: SSc patients had lower mean lower esophageal sphincter pressure than controls during normal breathing (19.7 ± 2.8 mmHg vs 32.2 ± 2.7 mmHg, P = 0.007). Sinus arrhythmia maneuver pressure was higher in SSc patients than in controls (142.6 ± 9.4 mmHg vs 104.6 ± 13.8 mmHg, P = 0.019). Sinus arrhythmia maneuver pressure normalized to IDL was also higher in SSc patients than in controls (83.8 ± 13.4 mmHg vs 37.5 ± 6.9 mmHg, P = 0.005). Threshold maneuver pressures normalized to IDL were also greater in SSc patients than in controls (TM 12 cmH2O: 85.1 ± 16.4 mmHg vs 43.9 ± 6.3 mmHg, P = 0.039; TM 24 cmH2O: 85.2 ± 16.4 mmHg vs 46.2 ± 6.6 mmHg, P = 0.065). Inspiratory diaphragm lowering in SSc patients was less than in controls (2.1 ± 0.3 cm vs 3 ± 0.2 cm, P = 0.011).
CONCLUSION: SSc patients had increased inspiratory EGJ pressure. This is an add-on to EGJ pressure and indicates that the antireflux barrier can be trained.
Core tip: Crural diaphragm adaptation in systemic sclerosis may be an add-on to the antireflux barrier. This is an indication that the antireflux barrier can be trained. Our aim was to evaluate crural diaphragm function in systemic sclerosis using high-resolution manometry and standardized inspiratory maneuvers. Systemic sclerosis patients with severe esophageal disease have increased inspiratory esophagogastric junction pressure, despite a low normal-breathing lower esophageal sphincter pressure.
- Citation: Nobre e Souza MÂ, Bezerra PC, Nobre RA, Holanda ESDF, Santos AAD. Increased inspiratory esophagogastric junction pressure in systemic sclerosis: An add-on to antireflux barrier. World J Gastroenterol 2015; 21(7): 2067-2072
- URL: https://www.wjgnet.com/1007-9327/full/v21/i7/2067.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i7.2067
Systemic sclerosis (SSc) is a clinically heterogeneous and generalized disease that affects the connective tissue of the skin and internal organs, such as the gastrointestinal (GI) tract, lungs, kidneys, and heart. SSc is characterized by severe and often progressive cutaneous and visceral fibrosis, marked alterations in the microvasculature, and frequent cellular and humoral immunity abnormalities[1].
GI is frequent in SSc and is the presenting feature of the disease in 10% of cases. Severe GI manifestations appear to be mostly related to fibrosis, but early neural disease can also lead to clinical manifestations. The result is altered peristaltic activity with multiple secondary problems, including gastroesophageal reflux disease (GERD), early satiety, nausea, vomiting, pseudo-obstruction, pneumatosis cystoid intestinalis, small-intestine bacterial overgrowth, malabsorption, and ultimately malnutrition[2].
Esophageal involvement occurs in up to 90% of SSc cases, affecting the smooth muscle segment of the organ. It leads to substantially reduced amplitudes of distal esophageal contractions and diminished pressure of the lower esophageal sphincter (LES)[2]. GERD is common in SSc and adds significantly to the morbidity of the disease. Endoscopic reflux esophagitis occurs in 77% of patients[3]. Therefore, antireflux mechanisms are always a concern in SSc patients.
The esophagogastric junction (EGJ) acts as a gatekeeper that controls both normal bolus transit and GER. After inspiratory muscle training[4] and the endoscopic placement of polymer prosthesis at the EGJ[5], the number of proximal refluxes decreased, possibly secondary to a lower refluxate volume caused by a tighter EGJ. The crural diaphragm (CD) is an essential part of the EGJ and contributes to EGJ pressure[6]. However, despite marked LES dysfunction in SSc, unknown is whether the CD is involved in SSc. The goal of the present study was to evaluate inspiratory EGJ pressure in SSc patients. Herein, we considered controlled inspiratory LES pressure a surrogate for CD strength.
Eight SSc volunteers (average age, 40.1 years; one male) were selected to participate in this study. The diagnosis of SSc was based on the criteria of the American College of Rheumatology[7]. Patients with other connective tissue disorders were excluded. All of the SSc volunteers attended the Gastroenterology outpatient facility at Walter Cantídio University Hospital (Federal University of Ceará, Brazil). A gastroenterologist interviewed SSc volunteers to obtain a complete clinical history and scored heartburn and regurgitation according to frequency using a standardized questionnaire: 0 (none), 1 (less than once per week), 2 (once per week), 3 (two to four times per week), 4 (more than five times per week). The study protocol was publicly announced at the hospital. Additionally, 13 healthy volunteers (average age, 32.2 years; six males) without any symptoms served as controls. All of the controls had a normal physical examination and no antecedents of abdominal surgery. Written informed consent was obtained from each participant. The Research Ethics Committee of the University Hospital approved the study protocol prior to the experiments (No. 044.06.09).
Manometric studies were performed with the volunteers in the supine position after at least a 6-h fast. The catheters were 4.2 mm-outer-diameter solid-state assemblies with 36 circumferential sensors at 1-cm intervals and 18 impedance segments at 2-cm intervals (Given Imaging, Yoqneam, Israel). Pressure transducers were calibrated at 0 and 300 mmHg and zeroed to atmospheric pressure. The catheter was placed transnasally and positioned to record from the upper esophageal sphincter to the stomach with at least five sensors distal to the diaphragm. The manometric protocol included a basal period for EGJ pressure measurements, ten 5-mL saline swallows, and two respiratory maneuvers in the supine position.
The respiratory maneuvers have been previously described[8]. The volunteers remained in the supine position while EGJ pressure was measured. The volunteers did not swallow 20 s before or during the maneuvers. First, six cycles of 5-s deep inhalation and 5-s exhalation [sinus arrhythmia maneuver (SAM)][9] produced six EGJ inspiratory peak pressures. Second, the volunteers made a quick and forced inhalation through a device that incorporated a flow-independent one-way valve that ensured consistent resistance[10]. When the volunteers inhaled through the device, a spring-loaded valve provided an adjustable resistance (in cmH2O; Threshold IMT, Philips Respironics, Andover, MA, United States). Each subject continued fast inhalations under 12- and 24-cmH2O loads [threshold maneuvers (TM12 and TM24)]. The TM procedures were performed twice. The volunteers practiced both the SAM and TM before the study.
For LES pressure and the esophageal body, the horizontal cursors for gastric and EGJ landmarks were manually set in the pressure topography display mode of the ManoView Analysis 3.0 software workspace (Yoqneam, Israel). The mean LES pressure and minimal respiratory LES pressure were then measured. Individual swallows were reviewed and analyzed according to the Chicago classification[11]. Lower esophageal sphincter pressures were referenced to intragastric pressure.
The CD vertical position was determined using the smart mouse tool of ManoView before and during each inhalation of the SAM maneuver. Inspiratory diaphragmatic lowering (IDL; in centimeters) was defined as how far the CD moved downward relative to its resting position before the maneuver (Figure 1). Maneuver pressure acquisition was referenced to atmospheric pressure and measured as follows. The LES cursor was repositioned on the downward-shifted CD during the inspiratory maneuvers (topographic display mode), and the inspiratory pressures were measured with the e-sleeve. The e-sleeve length was set to the length of the EGJ high-pressure zone. Inspiratory peak values were then determined in the tracing display mode. The average of the six peak pressures during the SAM (SAM pressure) and highest peak pressure of the two loaded forced inhalations at each load (TM12 and TM24 pressures) were calculated relative to the mean EGJ pressure during a stable 30-s period before the maneuvers. The data were corrected for the thermal sensitivity of the pressure sensors. Maneuver pressures are presented as raw data (mmHg) and data normalized to IDL (mmHg/cm). Work in physics is defined as force plus displacement. EGJ pressure was substituted for force. Thus, EGJ work was defined by the EGJ pressure that developed during the SAM plus IDL (mmHg*cm). The data are expressed as mean and SEM.
The normalities of the variables’ distributions were tested using the D’Agostino test. The only non-normal variable was SAM pressure; thus, the Mann-Whitney test was used to compare the distribution of that variable across SSc patients and controls. Student’s unpaired-samples t-test was used to compare the averages of the other quantitative and continuous variables. The level of statistical significance was set at 0.05 for differences in mean values and distributions. JMP Statistical Discovery, version 7.0.1 (SAS Institute, Cary, NC, United States), and Prism (GraphPad Software, La Jolla, CA, United States) were used for the statistical analyses.
The eight SSc volunteers presented severe esophageal motility disorders. Six had 100% distal contraction failure. The other two had 30% and 70% peristalsis failure; they also had an exceedingly low Distal Contractility Integral (DCI; 187.5 and 147 mmHg•cm•s, respectively). The mean Integrated Relaxation Pressure was 8.3 mmHg. All of the SSc patients showed incomplete bolus transit after a single 5-mL liquid swallow. All of the SSc volunteers had interstitial lung disease. Six had lung fibrosis, indicated by computed tomography. Two suffered from exertion dyspnea.
All of the SSc patients complained of heartburn at least two times per week. Six of the SSc patients presented regurgitation, and five had intermittent esophageal dysphagia. Mean LES pressure was lower in SSc patients than in controls (19.7 ± 2.8 mmHg vs 32.2 ± 2.7 mmHg, P = 0.007). Minimal respiratory LES pressure was even lower in SSc patients than in healthy volunteers (8.5 ± 1.7 mmHg vs 21.9 ± 2.8 mmHg, P = 0.002; Figure 2). Sinus arrhythmia maneuver pressure was significantly higher in SSc patients than in healthy volunteers (142.6 ± 9.4 mmHg vs 104.6 ± 13.8 mmHg, P = 0.019; Figure 3). Sinus arrhythmia maneuver pressure normalized to IDL was also significantly higher in SSc patients than in healthy controls (83.8 ± 13.4 mmHg vs 37.5 ± 6.9 mmHg, P = 0.005). Threshold maneuver pressures in SSc patients were similar to controls (TM12: 140.8 ± 13.6 mmHg vs 124.4 ± 11.5 mmHg, P = 0.158; TM24: 141.2 ± 15.1 mmHg vs 130.9 ± 12.2 mmHg, P = 0.491). At 12 cmH2O, TM pressure normalized to IDL was greater in SSc patients than in controls (TM12: 85.1 ± 16.4 mmHg vs 43.9 ± 6.3 mmHg, P = 0.039) and tended to be greater at 24 cmH2O (TM24: 85.2 ± 16.4 mmHg vs 46.2 ± 6.6 mmHg, P = 0.065). Inspiratory diaphragm lowering was less in SSc patients than in healthy volunteers (2.1 ± 0.3 cm vs 3 ± 0.2 cm, P = 0.011). Therefore, SSc patients developed the same EGJ work as healthy volunteers during inhalation (296.7 ± 53.7 mmHg•cm vs 303.5 ± 30.5 mmHg•cm, P = 0.906; Figure 4).
The present study showed that standardized inspiratory pressure of the EGJ was higher in SSc patients than in healthy volunteers. This occurred despite marked esophageal involvement in the patients.
The intraluminal EGJ pressures were measured using a state-of-the-art method. Two respiratory maneuvers were adapted to be performed during EGJ manometry. The first maneuver, the SAM, was based on a long-accepted maneuver that was designed to evaluate cardiac sinus arrhythmia[9]. The second maneuver, the TM, took advantage of a device that is often used by physical therapists to improve diaphragmatic function[12]. Both maneuvers have recently been used to study EGJ pressure in GERD[8]. The SAM was quite easy for the volunteers to execute. The TM was more difficult and required the volunteers to undergo some training beforehand. Moreover, the TM pressures had a larger standard error than the SAM pressures. The TM pressures in SSc patients at 24 cmH2O were not significantly higher than controls possibly because of a type 2 inference error. The healthy volunteers were younger than the SSc patients. However, this fact cannot explain why they had smaller inspiratory EGJ pressures than the older SSc patients. Indeed, older individuals usually have weaker striated muscles. As early as around the fourth decade of life, both muscle mass and strength begin to decline[13]. Muscle loss appears to be unavoidable and accelerates with advancing age[14]. Therefore, age differences may not have played an essential role in the results of this study.
All of the SSc volunteers had clinically significant lung disease. A reasonable consideration is that such patients have adapted the diaphragm, which is the principal inspiratory muscle, to allow for adequate lung ventilation. Substantial evidence indicates respiratory muscle adaptation in disease states[15]. The contractile apparatus of the diaphragm may change as a result of interstitial lung disease to shift its length-tension relationship. As a result, the CD may be able to deliver stronger inspiratory tension and greater inspiratory EGJ pressures. In fact, augmenting inspiratory EGJ pressure is possible after inspiratory muscle training[8]. Interestingly, in addition to improving GERD symptoms and inspiratory EGJ pressure, inspiratory muscle training also diminishes proximal reflux progression[8]. SSc patients with lung fibrosis have more distal and proximal GER relative to SSc patients without lung fibrosis[16]. The present study found possible pathophysiological diaphragm adaptation in SSc patients that may confer some degree of protection against proximal gastroesophageal reflux. The higher inspiratory pressures in SSc are likely attributable to the lung disease itself because GERD is associated with crural insufficiency[8]. If SSc patients with lung fibrosis do not improve their inspiratory EGJ pressure during disease progression, would they have more lung fibrosis?
If indeed the microaspiration of gastric contents triggers pulmonary parenchymal lesions[17], that proximal reflux is necessary for microaspiration, and that increasing inspiratory EGJ pressure diminishes proximal reflux, then the naturally occurring high inspiratory pressures in SSc with lung disease may be useful to partially control GERD in SSc. Furthermore, increases in inspiratory EGJ pressure (e.g., after inspiratory muscle training) may be an add-on to GERD treatment in SSc patients and GERD in general.
This study was funded in full by Conselho Nacional de Desenvolvimento Científico e Tecnológico and Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP).
Diaphragm function changes in different conditions. In respiratory diseases, diaphragm dynamics may adapt to a new pathophysiological steady state. In gastroesophageal reflux disease (GERD), symptoms and the antireflux barrier may improve after inspiratory training. The authors evaluated esophagogastric junction (EGJ) pressures in systemic sclerosis (SSc) that may be associated with both lung fibrosis and GERD.
The authors showed that inspiratory intraluminal EGJ pressures are higher in SSc patients than in controls. They measured inspiratory pressures during new maneuvers that unveiled these differences in pressures. Inspiratory EGJ pressures are produced by crural diaphragm contraction that may undergo changes in its dynamics by lung fibrosis.
This is believed to be the first study to show a pathophysiological adaptation of the antireflux barrier to a disease.
This supports the idea that the crural diaphragm and antireflux barrier can be trained by inspiratory exercises that may prove useful in GERD treatment.
The crural diaphragm (CD) is an important antireflux barrier component. High-resolution manometry of the esophagus and a topographic display of pressures allow the study of CD function. Standardized inspiratory maneuvers (sinus arrhythmia and threshold) were used to measure CD strength and work.
The authors reported an interesting phenomenon that increased inspiratory EGJ pressure in SSc could contribute to pathophysiological diaphragm adaptation in patients with lung fibrosis. The increased EGJ pressure may also serve as a protective factor in GERD and be enforced through training.
P- Reviewer: Caviglia RD, Gong JS, Guo YM, Luo HS S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 792] [Cited by in RCA: 865] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 2. | Gyger G, Baron M. Gastrointestinal manifestations of scleroderma: recent progress in evaluation, pathogenesis, and management. Curr Rheumatol Rep. 2012;14:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Thonhofer R, Siegel C, Trummer M, Graninger W. Early endoscopy in systemic sclerosis without gastrointestinal symptoms. Rheumatol Int. 2012;32:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Souza M, Lima MJV, Gomes TN, Souza MH, Santos AA. Inspiratory diaphragm workout increases heart rate variability and improves GERD symptoms. Gastroenterology. 2011;140:S-304. |
| 5. | Cicala M, Gabbrielli A, Emerenziani S, Guarino MP, Ribolsi M, Caviglia R, Costamagna G. Effect of endoscopic augmentation of the lower oesophageal sphincter (Gatekeeper reflux repair system) on intraoesophageal dynamic characteristics of acid reflux. Gut. 2005;54:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Mittal RK. The crural diaphragm, an external lower esophageal sphincter: a definitive study. Gastroenterology. 1993;105:1565-1567. [PubMed] |
| 7. | Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581-590. [PubMed] |
| 8. | Nobre e Souza MÂ, Lima MJ, Martins GB, Nobre RA, Souza MH, de Oliveira RB, dos Santos AA. Inspiratory muscle training improves antireflux barrier in GERD patients. Am J Physiol Gastrointest Liver Physiol. 2013;305:G862-G867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8:491-498. [PubMed] |
| 10. | Bailey SJ, Romer LM, Kelly J, Wilkerson DP, DiMenna FJ, Jones AM. Inspiratory muscle training enhances pulmonary O(2) uptake kinetics and high-intensity exercise tolerance in humans. J Appl Physiol (1985). 2010;109:457-468. [PubMed] |
| 11. | Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012;24 Suppl 1:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 604] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 12. | Enright S, Chatham K, Ionescu AA, Unnithan VB, Shale DJ. Inspiratory muscle training improves lung function and exercise capacity in adults with cystic fibrosis. Chest. 2004;126:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Peterson CM, Johannsen DL, Ravussin E. Skeletal muscle mitochondria and aging: a review. J Aging Res. 2012;2012:194821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 14. | Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:B209-B217. [PubMed] |
| 15. | Clanton TL, Levine S. Respiratory muscle fiber remodeling in chronic hyperinflation: dysfunction or adaptation? J Appl Physiol (1985). 2009;107:324-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Savarino E, Bazzica M, Zentilin P, Pohl D, Parodi A, Cittadini G, Negrini S, Indiveri F, Tutuian R, Savarino V. Gastroesophageal reflux and pulmonary fibrosis in scleroderma: a study using pH-impedance monitoring. Am J Respir Crit Care Med. 2009;179:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Raghu G, Freudenberger TD, Yang S, Curtis JR, Spada C, Hayes J, Sillery JK, Pope CE, Pellegrini CA. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 419] [Article Influence: 22.1] [Reference Citation Analysis (0)] |