Published online Dec 28, 2015. doi: 10.3748/wjg.v21.i48.13457
Peer-review started: June 16, 2015
First decision: July 19, 2015
Revised: August 9, 2015
Accepted: September 28, 2015
Article in press: September 30, 2015
Published online: December 28, 2015
Processing time: 192 Days and 16 Hours
AIM: To investigate the effect of fenugreek lactone (FL) on palmitate (PA)-induced apoptosis and dysfunction in insulin secretion in pancreatic NIT-1 β-cells.
METHODS: Cells were cultured in the presence or absence of FL and PA (0.25 mmol/L) for 48 h. Then, lipid droplets in NIT-1 cells were observed by oil red O staining, and the intracellular triglyceride content was measured by colorimetric assay. The insulin content in the supernatant was determined using an insulin radio-immunoassay. Oxidative stress-associated parameters, including total superoxide dismutase, glutathione peroxidase and catalase activity and malondialdehyde levels in the suspensions were also examined. The expression of upstream regulators of oxidative stress, such as protein kinase C-α (PKC-α), phospho-PKC-α and P47phox, were determined by Western blot analysis and real-time PCR. In addition, apoptosis was evaluated in NIT-1 cells by flow cytometry assays and caspase-3 viability assays.
RESULTS: Our results indicated that compared to the control group, PA induced an increase in lipid accumulation and apoptosis and a decrease in insulin secretion in NIT-1 cells. Oxidative stress in NIT-1 cells was activated after 48 h of exposure to PA. However, FL reversed the above changes. These effects were accompanied by the inhibition of PKC-α, phospho-PKC-α and P47phox expression and the activation of caspase-3.
CONCLUSION: FL attenuates PA-induced apoptosis and insulin secretion dysfunction in NIT-1 pancreatic β-cells. The mechanism for this action may be associated with improvements in levels of oxidative stress.
Core tip: Fenugreek is a widely used traditional Chinese medicine that can improve hyperglycemia. The hypoglycemic active ingredients and mechanisms of fenugreek remain unclear. We studied an ingredient of fenugreek, fenugreek lactone, and our results suggest that fenugreek lactone attenuates palmitate-induced apoptosis and insulin secretion dysfunction in NIT-1 pancreatic β-cells by improving oxidative stress.
- Citation: Gong J, Dong H, Jiang SJ, Wang DK, Fang K, Yang DS, Zou X, Xu LJ, Wang KF, Lu FE. Fenugreek lactone attenuates palmitate-induced apoptosis and dysfunction in pancreatic β-cells. World J Gastroenterol 2015; 21(48): 13457-13465
- URL: https://www.wjgnet.com/1007-9327/full/v21/i48/13457.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i48.13457
Diabetes mellitus (DM), a metabolic disease, is characterized mainly by elevated blood glucose. Chronic hyperglycemia may produce organ damage and cause serious harm to human health. Because the worldwide prevalence of DM continues to increase, much attention has been paid to the pathophysiology and treatment of DM. On the basis of the latest reports, the incidence of diabetes among Chinese adults has reached 11.6% and the incidence of prediabetes is 50.1%[1]. Patients who develop type 2 diabetes (T2DM) account for more than 90% of these individuals. The development of T2DM is pertinent to insulin resistance and dysfunctional insulin secretion. Recent research has shown that increased pancreatic β-cell apoptosis and decreased islet β-cell mass contribute to the insulin secretion impairment observed in T2DM[2]. However, the reason for this increased β-cell apoptosis remains unclear. However, an increasing amount of evidence suggests that hyperglycemia (glucotoxicity), elevated fatty acids (lipotoxicity), and oxidative stress are closely associated with enhanced apoptosis in β-cells[3].
The mechanism by which free fatty acids (FFA) induce β-cell impairment is not fully clear. However, FFA-related oxidative stress injury has attracted extensive attention. When β-cells are exposed to FFA, they first convert FFA into triglycerides or cholesterol esters and then store them as lipid droplets inside the cytoplasm. When lipid influx exceeds the cell’s storage threshold, excessive FFA may impair insulin secretion and induce apoptosis. It has been demonstrated that diglycerol (DAG), a metabolite of palmitic acid (PA), can induce the activation of the protein kinase C (PKC)/NADPH oxidase pathway, leading to the intracellular accumulation of reactive oxygen species (ROS) products[4]. Because pancreatic β-cells produce few antioxidant substances, they are more susceptible to oxidative stress[5]. Sustained production of ROS inside the cells can trigger apoptosis via caspase-dependent pathways and decrease the secretion of insulin, thereby impairing the mass and function of β-cells[6,7]. Therefore, antioxidants may exert a beneficial effect that protects β-cells and thereby play an important role in treating diabetes.
Fenugreek (Trigonella foenum-graecum L.) is a widely used traditional Chinese medicine that can improve hyperglycemia. Our previous studies also showed that fenugreek reduced oxidative stress and improved glucose and lipid metabolism by inhibiting the PKC-α/NADPH oxidase pathway in diabetic rats[8]. The hypoglycemic active ingredients and mechanisms of fenugreek remain unclear. Some studies have suggested that trigonelline and 4-hydroxy isoleucine in fenugreek improve hyperglycemia[9-11], but this evidence does not deny that other compounds also display hypoglycemic activity. Fenugreek lactone (FL), a flavor component of many foods[12], exists naturally in fenugreek, wine, Virginia tobacco, and other foods[13]. This spice is widely used in condiments, baked products, wine, caramel and coffee[14]. Some studies have revealed that FL has antioxidant activities[12]; however, its medical effect has been less studied. In the current research, we studied the impact of FL on PA-induced apoptosis and insulin secretion dysfunction in NIT-1 islet β-cells.
Fetal bovine serum (FBS) was purchased from Gibco. Sigma-Aldrich Co. provided the reagents 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), FL (purity ≥ 97%) and PA (purity ≥ 99%). RPMI-1640 was obtained from Thermo Fisher Scientific Co., Ltd. Trypsin was obtained from Boster Biological Technology Co., Ltd. Penicillin and streptomycin were acquired from HyClone Laboratories. Insulin radio-immunoassay reagents were obtained from HTA Co. Total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) activity assay kits and malondialdehyde (MDA) level assay kits were bought from Jiancheng Bio-engineering Co., Ltd. The triglyceride detection kit was provided by Mind Bioengineering Co., Ltd. Rabbit anti-mouse p47phox, phosphorylated PKC-α, PKC-α and β-actin antibodies were provided by Abcam Company. The Prime Script RT reaction Kit, SYBR Premix Ex Taq and Trizol reagent were obtained from TaKaRa Bio Inc. The fluorescein isothiocyanate (FITC)-labeled annexin V/propidium iodide (PI) apoptosis detection kit was provided by BestBio Co.
Mouse insulinoma NIT-1 cells were acquired from Tongji Medical College. NIT-1 islet cells were cultured with 1640 medium (containing 11.1 mmol glucose) replenishing 100 &mgr;g/mL streptomycin, 100 units/mL penicillin and 10% FBS in a humid environment with 5% CO2/95% air at 37 °C. At approximately 70% confluence, the NIT-1 cells were further incubated with or without FL and 0.25 mmol/L palmitate for 48 h.
NIT-1 cells were seeded at 8 × 103 cells per well in 96-well culture plates and incubated for 24 h. After cells had adhered, the medium was replaced by medium containing the experimental drugs at the indicated concentrations for 24 h. The viability of NIT-1 cells in wells containing different concentrations of FL or PA was assessed by MTT assay. After the treatments, 10 μL MTT solution was added to every well and cells were then cultured for 4 h at 37 °C. The crystals in each well were dissolved in DMSO and the absorbance was measured at 570 nm using a Synergr2 Almighty Microplate Reader. When the survival rate was between 60%-70%, the corresponding PA concentration was chosen for modeling.
At a density of 4 × 105 cells/well, NIT-1 pancreatic cells were seeded in 6-well plates and cultured for 48 h exposed to 0.25 mmol/L PA in the model group. In the treatment group, the medium also contained FL (1 μmol/L). When the culture period was over, the cells were washed twice with PBS and immobilizated with 4% paraformaldehyde for 30 min. After two rinses with double distilled water, the cells were dyed with oil red O at 37 °C for 30 min and soon afterwards decolorized in 60% isopropanol for 5 s. Cells were then stained with hematoxylin for 1 min. After washing and the observation of the lipid droplets in NIT-1 cells, pictures were taken using a light microscope. The triglyceride (TG) contents were determined as previously described[15].
When the treatment period ended, cells were collected following trypsin digestion. Cells were washed twice in PBS, and 2 × 107 cells were suspended in 400 μL PBS. An ultrasonic cell disrupter was then used to break the cells. MDA levels, T-SOD, CAT activity and GSH-Px activity in the suspensions were tested according to the manufacturer’s protocols in commercial kits. The protein concentrations in the suspension were measured using BCA assays (Kerui Institute of Biotechnology, Wuhan, China).
Cells were seeded into 6-well plates at 6 × 105 cells per well. After treatment, the cells were pre-incubated with glucose-free medium for 30 min. Then, high-glucose (25 mmol/L) DMEM medium was substituted for the glucose-free medium and cells were incubated for another 2 h. The supernatant was collected for further measurements. Insulin was determined with an insulin radio-immunoassay kit. Each experiment was repeated five times. The insulin concentration was corrected according to the number of NIT-1 pancreatic cells.
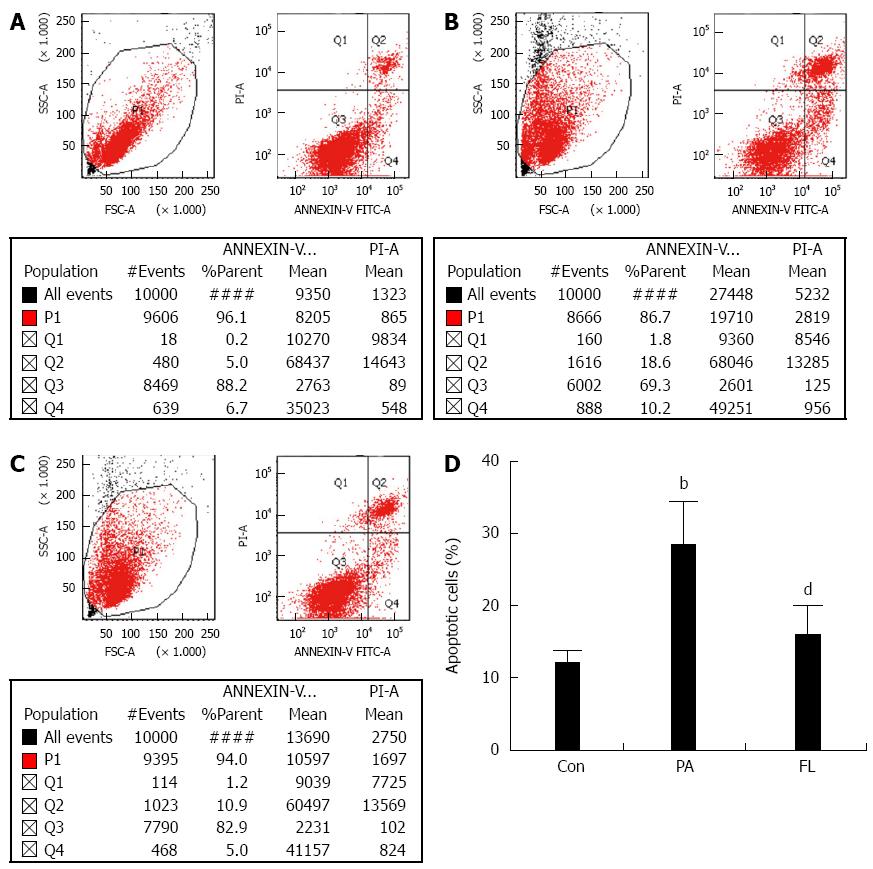
As previously described[16], a fluorescein isothiocyanate (FITC)-labeled annexin V/propidium iodide (PI) apoptosis detection kit was used to analyze apoptosis in cells in accordance with the instructions. The cells were collected after EDTA-free trypsin digestion. After two washes in cold PBS, the cell pellet was collected via centrifugation at 1000 g for 10 min. The number of cells was adjusted to 1 × 106 cells/mL. Then, the NIT-1 cells were resuspended in binding buffer, stained lucifugally with PI for 15 min at 4 °C and stained with FITC-labeled annexin V at 4 °C for 5 min away from light. FACSCalibur flow cytometer (BD LSR II, San Jose, CA, United States) was used to perform the Flow cytometric analysis. The percentages of apoptotic cells were calculated using CellQuest software (Becton, Dickinson and Co.). The quadrant containing annexin V-positive and PI-negative cells represented the early phase of apoptosis, while the quadrant containing cells positive for both annexin V and PI represented the late phase. The total ratio of apoptotic cells was calculated by the sum of cells in the early and late stages of apoptosis. A caspase-3 viability kit was also used to measure apoptosis in NIT-1 cells, as previously described[17].
After centrifugation and determination of total protein concentrations, NIT-1 cell supernatant extracts in RIPA lysis buffer were combined with sample buffer and heated in boiled water for approximately ten minutes. Then, 80 μg of the protein extraction was separated on a 10% SDS-PAGE gel (120 V, 1.5 h), and the proteins on the gel were thereafter transferred onto nitrocellulose (NC) membranes. The NC membranes were blocked using 5% non-fat milk powder in distilled water for 1h at normal temperature and then incubated at 4 °C overnight with primary antibodies (p47phox, phosphorylated PKC-α, PKC-α, and β-actin). After washing the membranes using TBST three times for 10 min each, the membranes were lucifugally incubated with a fluorescent secondary antibody at normal temperature for 1h. Thereafter the membranes were washed with TBST lucifugally four times. Finally, protein immunoreactivity was measured using Odyssey near-infrared laser imaging apparatus. The ratio between the OD value of target band and that of β-actin was used to quantify the band densities.
In the light of the manufacturer’s directions, total RNA in NIT-1 cells was extracted using Trizol reagent. A Nucleic Acid/Protein Analyzer was used to determine the purity and concentration of the RNA. The reverse transcription of extracted total RNA (1 μg) was performed in a total reaction volume of 20 μL on a Mastercycler gradient PCR instrument using a PrimeScript RT reaction Kit. Before PCR amplification, the cDNA was stored at -80 °C. Real-time fluorescence quantitative PCR reactions were manipulated with SYBR Premix Ex Taq enzyme and StepOne Real-Time PCR System. The 2-ΔΔCT method was employed to analyze the results. Primer sequences are all listed in Table 1.
| Gene | Forward | Reverse |
| β-actin | 5'-CATCCGTAAAGACCTCTATGCCAAC-3' | 5'-ATGGAGCCACCGATCCACA-3' |
| p47phox | 5'-ATAACGGGCGTAGGGAGTCT-3' | 5'-AGCAAAGTCTCCGCATCACT-3' |
| PKC-α | 5'-GTGCAAGGAACACATGATGG -3' | 5'-ACGCCCACCAATCTACAGAC-3' |
All outcomes represent at least three independent experiments. All of the results are displayed as the mean ± SD and were analysed with SPSS 20.0 software. The statistical significance was determined by one-way analysis of variance (ANOVA). A LSD test was also employed when analyzing the data, and P < 0.05 suggested that a statistical difference exists.
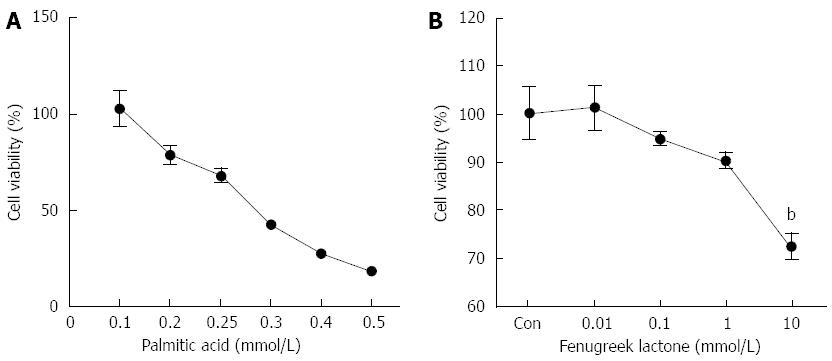
In this study, PA was used to induce oxidative stress in NIT-1 β-cells. After 24 h of treatment with PA, cell viability decreased in a dose-dependent manner. Compared to the controls (untreated cells), the cells treated with 0.25 mmol/L PA for 24 h showed a 34.68% decrease in cell viability (Figure 1A).
To determine the concentration of FL that could be used without affecting the viability of the NIT-1 pancreatic β-cells, we also performed an MTT assay. The cells were seeded in 96-well plates and therein cultured for 24 h. Different concentrations of FL were then added to the cells followed by incubation for 24 h. FL was well-tolerated up to a concentration of 1 μmol/L. At concentrations higher than this, FL decreased cell viability by 27.49% (Figure 1B). Thus, we concluded that the optimal concentration of FL for use in further experiments was up to 1 μmol/L.
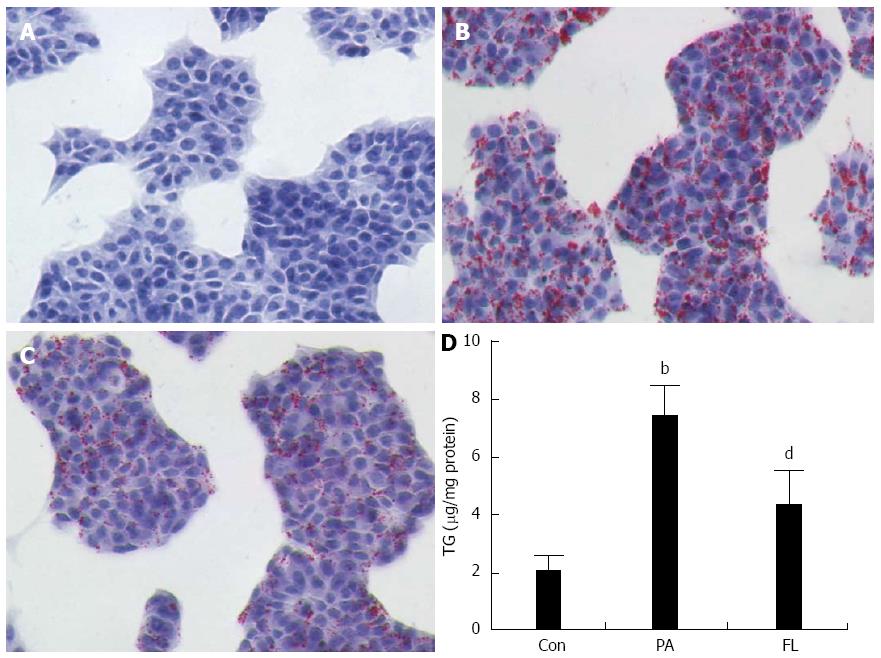
To measure the influence of FL on lipid accumulation in NIT-1 islet cells, oil Red O dyeing and colorimetric assays were applied. As shown in Figure 2, cellular lipid droplets in the FL group were diminished significantly compared to the PA group. In addition, intracellular TG contents were also markedly decreased (P < 0.01) in the FL group compared to cells in the PA group (Figure 3).
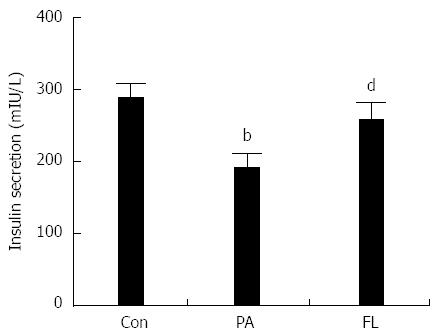
To ascertain whether PA damages insulin secretion in pancreatic islet cells, a radioimmunoassay kit was used to test insulin concentrations in the supernatants of the medium from different cell treatments. As shown in Figure 3, PA-treated NIT-1 cells exhibited decreased insulin levels compared to the control group (P < 0.01). However, FL significantly inhibited this effect (P < 0.01).
As shown in Table 2, NIT-1 cells treated with PA showed increased MDA levels and decreased T-SOD, GSH-PX and CAT levels compared with levels in the control group (P < 0.01). FL treatment markedly decreased the levels of MDA and increased the levels of SOD, CAT and GSH-PX (P < 0.01 or P < 0.05).
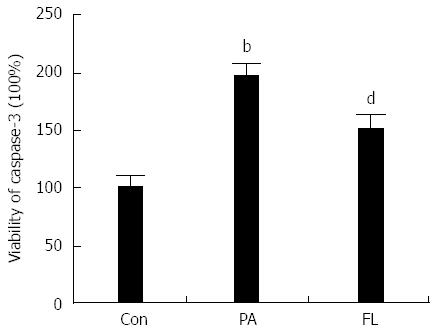
Caspase-3 is a crucial enzyme for apoptosis and cell survival. The activation of caspase-3 triggers the cell to undergo apoptosis. Therefore, we determined whether FL inhibits caspase-3 activation and PA-induced apoptosis in NIT-1 β-cells. Double staining using FITC-labeled annexin V and PI was performed. The result showed increased caspase-3 activity (Figure 4) and an increased apoptotic rate (Figure 5) in the PA group (P < 0.01). However, co-treatment with 1 μmol/L FL markedly inhibited caspase-3 activity and PA-induced apoptotic cell death (P < 0.01) (Figures 4 and 5).
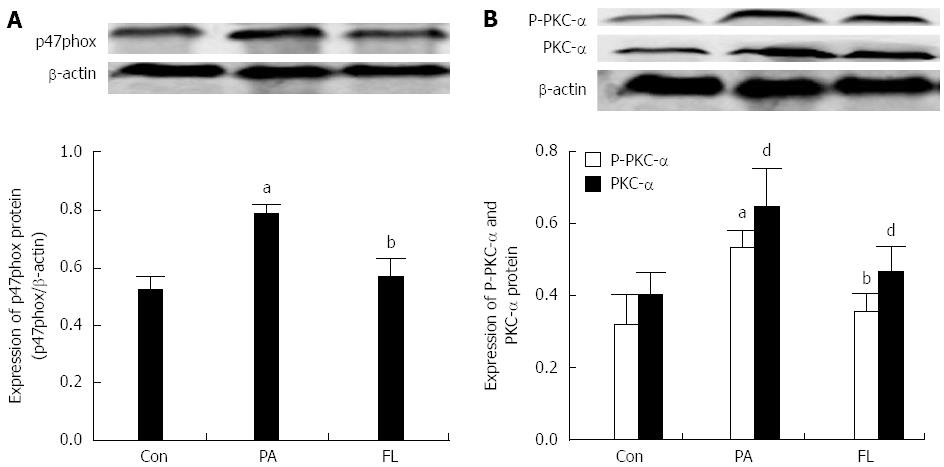
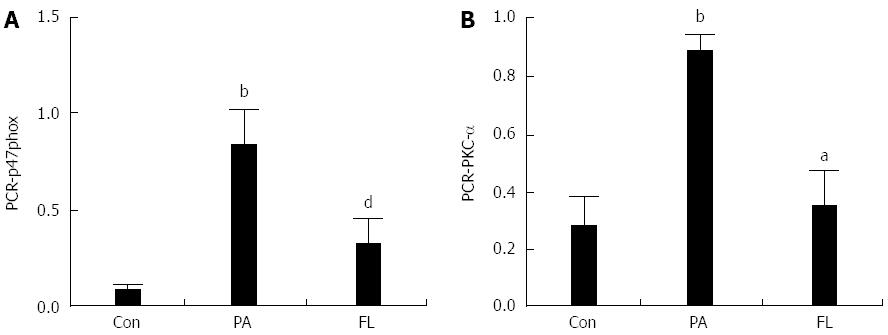
As shown in Figures 6 and 7, the protein and mRNA levels of PKC-α and p47phox were significantly increased in NIT-1 cells that were treated with PA compared with cells in the control group (P < 0.01). But there was a significant decrease in the expression of p47phox and PKC-α at both the protein and mRNA levels in the FL group. The protein levels of phosphorylated PKC-α also increased significantly (P < 0.01) following treatment with PA alone but were decreased by co-treatment with FL (P < 0.01).
T2DM, especially when combined with obesity, is always associated with the excessive release of FFA from proliferative adipose tissue, which leads to an increase in the plasma FFA concentration[18]. According to the Paris Prospective Study, a high plasma FFA concentration is a risk factor for the deterioration of glucose tolerance, with a relative risk of approximately 1.3 per 0.12 mmol/L change in the level of FFA[19].
Circulating FFA can sustain basal pancreatic β-cell function and assure efficient insulin secretion during fasting. FFA in islets has also been viewed as beneficial during the hypersecretion of insulin to protect against glucose intolerance in the early stages of obesity and diabetes[20]. However, under certain circumstances, it has the opposite or even a deleterious effect. When pancreatic beta cells were exposed to excessive quantities of FFA for long periods of time, islet cells malfunctioned and the biosynthesis and secretion of insulin were reduced in vitro in both rats and humans[21,22]. In addition to inhibiting insulin secretion, elevated plasma FFA might play a causal role in pancreatic β-cell apoptosis[22,23]. Apoptosis was observed in pancreatic cells that were exposed to 0.5 mmol/L PA for 48 h[6]. The PA concentration was 0.25 mmol/L in our present research, but our results were consistent with previous findings.
Oxidative stress has been considered to be involved in the pathogenesis of islet β-cell dysfunction and apoptosis induced by prolonged exposure to FFA[6]. FFA has been shown to initiate the production of ROS in islet β-cells in a number of studies. PA, at high doses, can pass through the cell membrane and into the mitochondria, where oxidative reactions occur[24]. During metabolic clearance of PA, β-oxidation and oxidative phosphorylation produce large amounts of ROS, and antioxidants are reduced in pancreatic islet β-cells[25]. It is widely accepted that excessive levels of ROS damage cells directly by oxidizing lipids, DNA and proteins. Our study also suggests that MDA, a lipid peroxidation product, showed raised levels and that antioxidant levels were diminished in pancreatic β-cells that were stimulated by PA.
How FFA induces oxidative stress is unknown. DAG is not only a metabolite of FFA but also a potent activator of PKC[26]. PKC-α-dependent NADPH oxidase might serve as a possible critical source of ROS in response to stimulation by PA[27]. The factor p47phox is a subunit that is critical for the activity of NADPH oxidase, which catalyzes the production of many ROS, breaks normal redox homeostasis, and results in cellular dysfunction and damage by oxidative stress[28]. Our results also confirm that PA provokes oxidative stress through the PKC-α/NADPH oxidase pathway. New therapeutic strategies aimed at reducing oxidative stress have attracted wide attention. Several lines of evidence have shown the potential value of antioxidant remedies and their advantageous impacts on islet cell protection[29]. For example, the antioxidant N-acetylcysteine prevented the decrease in insulin content that was induced by oleate in MIN6 islet cells[30].
Fenugreek is a traditional Chinese herb for the treatment of diabetes. Increasing experimental and clinical studies have supported the hypoglycemic effect of fenugreek, and a meta-analysis of human experiments also demonstrated the hypoglycemic effectiveness of fenugreek[31]. The active ingredients in fenugreek are not clear. FL [3-amino-4,5-dimethyl-2(5H)-furanone] is widely used as a flavor in foods due to its strong smell. However, its medical function is less well studied. Several furanones have been shown to display anticancer activity in mice and antioxidative effects to improve human health[12]. In our prior studies, fenugreek and Hu-Lu-Ba-Wan reduced the accumulation of oxidation products, improved insulin secretion and protected the function of the kidneys and testis in STZ-induced diabetic rats[8]. In these experiments, PA-treated NIT-1 cells were more prone to damage to their anti-oxidative defense systems, and administration of FL significantly augmented decreases in the levels of GSH-PX, CAT and T-SOD. FL also promoted insulin secretion and reduced apoptosis in islet β-cells by inhibiting the PKC-α/NAPDH oxidase pathway. This might be the mechanism for the protective effect of FL in NIT-1 pancreatic cells. Our findings may provide a new perspective on the use of FL as a potential new therapeutic medicine for preserving pancreatic β-cell mass and function.
However, some limitations remain. FFA can damage islet cells indirectly by activating several stress-sensitive signaling pathways, such as the JNK/Akt, NF-κB and p38MAPK pathways[6]. We did not examine other downstream pathways. Tests using DHE staining to show the activity of NADPH might be used to clarify the mechanisms of PA-induced oxidative stress. Some furanone compounds increase the DNA damage caused by oxidative stress, but FL relieves oxidative stress. The mechanism by which FL increases the activity of antioxidant enzymes remains to be further described. Other effective ingredients of fenugreek also need more study.
In brief, we have shown that 48-h treatment with PA impaired NIT-1 pancreatic β-cells via oxidative stress. The PA-induced lipid overload, decrease in insulin secretion and increase in cell apoptosis were prevented by FL. FL inhibited the PKC-α/NADPH oxidase pathway and protected islet cells from injury by oxidative stress. FL may be a promising antioxidant for use as a treatment for DM.
Fenugreek is a widely used traditional Chinese medicine which can improve hyperglycemia. However, the mechanism underlying its hypoglycemic effect remains not fully clarified. In the present study, we investigated the effect of fenugreek lactone (FL), a component of fenugreek, on palmitic acid (PA)-induced apoptosis and insulin secretion dysfunction in NIT-1 pancreatic β cells.
It has been established that diabetes mellitus is associated with oxidative stress. Previous studies have shown that FL reduced oxidative impairment, but its medical effect has been less studied.
Whether FFA improves or decreases insulin secretion is a controversial question. The present study suggests that 48 h of incubation with a high-dose of FFA restrains insulin secretion. In addition, this is the first study to evaluate the effect of FL on FFA-induced impairment of pancreatic cells. The results showed that FL attenuates PA-induced increased apoptosis and decreased insulin secretion in pancreatic β-cells. The mechanism for these actions may be related to improvements in oxidative stress.
FL might be promising as an antioxidant for the treatment of diabetes mellitus.
This is a good piece of work in which investigators have demonstrated the effect of fenugreek lactone on PA-induced apoptosis and insulin secretion dysfunction in pancreatic NIT-1 β-cells. This work has been well planned and results properly discussed.
P- Reviewer: Murtaza I S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1961] [Cited by in RCA: 2170] [Article Influence: 180.8] [Reference Citation Analysis (0)] |
| 2. | Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102-110. [PubMed] |
| 3. | Saisho Y. β-cell dysfunction: Its critical role in prevention and management of type 2 diabetes. World J Diabetes. 2015;6:109-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 145] [Cited by in RCA: 168] [Article Influence: 16.8] [Reference Citation Analysis (5)] |
| 4. | Poitout V. Glucolipotoxicity of the pancreatic beta-cell: myth or reality? Biochem Soc Trans. 2008;36:901-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463-466. [PubMed] |
| 6. | Yuan H, Zhang X, Huang X, Lu Y, Tang W, Man Y, Wang S, Xi J, Li J. NADPH oxidase 2-derived reactive oxygen species mediate FFAs-induced dysfunction and apoptosis of β-cells via JNK, p38 MAPK and p53 pathways. PLoS One. 2010;5:e15726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Graciano MF, Valle MM, Kowluru A, Curi R, Carpinelli AR. Regulation of insulin secretion and reactive oxygen species production by free fatty acids in pancreatic islets. Islets. 2011;3:213-223. [PubMed] |
| 8. | Zhou L, Dong H, Huang Y, Xu L, Zou X, Wang K, Chen G, Lu F. Hu-Lu-Ba-Wan Attenuates Diabetic Nephropathy in Type 2 Diabetic Rats through PKC- α /NADPH Oxidase Signaling Pathway. Evid Based Complement Alternat Med. 2013;2013:504642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Broca C, Manteghetti M, Gross R, Baissac Y, Jacob M, Petit P, Sauvaire Y, Ribes G. 4-Hydroxyisoleucine: effects of synthetic and natural analogues on insulin secretion. Eur J Pharmacol. 2000;390:339-345. [PubMed] |
| 10. | Gupta SK, Kumar B, Nag TC, Srinivasan BP, Srivastava S, Gaur S, Saxena R. Effects of Trigonella foenum-graecum (L.) on retinal oxidative stress, and proinflammatory and angiogenic molecular biomarkers in streptozotocin-induced diabetic rats. Mol Cell Biochem. 2014;388:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Kumar P, Bhandari U. Protective effect of Trigonella foenum-graecum Linn. on monosodium glutamate-induced dyslipidemia and oxidative stress in rats. Indian J Pharmacol. 2013;45:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Peraza-Luna F, Rodríguez-Mendiola M, Arias-Castro C, Bessiere JM, Calva-Calva G. Sotolone production by hairy root cultures of Trigonella foenum-graecum in airlift with mesh bioreactors. J Agric Food Chem. 2001;49:6012-6019. [PubMed] |
| 13. | Colin Slaughter J. The naturally occurring furanones: formation and function from pheromone to food. Biol Rev Camb Philos Soc. 1999;74:259-276. [PubMed] |
| 14. | Alexandre H. Flor yeasts of Saccharomyces cerevisiae--their ecology, genetics and metabolism. Int J Food Microbiol. 2013;167:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Wang D, Tian M, Qi Y, Chen G, Xu L, Zou X, Wang K, Dong H, Lu F. Jinlida granule inhibits palmitic acid induced-intracellular lipid accumulation and enhances autophagy in NIT-1 pancreatic β cells through AMPK activation. J Ethnopharmacol. 2015;161:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Lee JS, Kim YR, Song IG, Ha SJ, Kim YE, Baek NI, Hong EK. Cyanidin-3-glucoside isolated from mulberry fruit protects pancreatic β-cells against oxidative stress-induced apoptosis. Int J Mol Med. 2015;35:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Bikopoulos G, da Silva Pimenta A, Lee SC, Lakey JR, Der SD, Chan CB, Ceddia RB, Wheeler MB, Rozakis-Adcock M. Ex vivo transcriptional profiling of human pancreatic islets following chronic exposure to monounsaturated fatty acids. J Endocrinol. 2008;196:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Charles MA, Eschwège E, Thibult N, Claude JR, Warnet JM, Rosselin GE, Girard J, Balkau B. The role of non-esterified fatty acids in the deterioration of glucose tolerance in Caucasian subjects: results of the Paris Prospective Study. Diabetologia. 1997;40:1101-1106. [PubMed] |
| 20. | Amery CM, Nattrass M. Fatty acids and insulin secretion. Diabetes Obes Metab. 2000;2:213-221. [PubMed] |
| 21. | Paolisso G, Gambardella A, Amato L, Tortoriello R, D’Amore A, Varricchio M, D’Onofrio F. Opposite effects of short- and long-term fatty acid infusion on insulin secretion in healthy subjects. Diabetologia. 1995;38:1295-1299. [PubMed] |
| 22. | Mason TM, Goh T, Tchipashvili V, Sandhu H, Gupta N, Lewis GF, Giacca A. Prolonged elevation of plasma free fatty acids desensitizes the insulin secretory response to glucose in vivo in rats. Diabetes. 1999;48:524-530. [PubMed] |
| 23. | Unger RH, Zhou YT, Orci L. Regulation of fatty acid homeostasis in cells: novel role of leptin. Proc Natl Acad Sci USA. 1999;96:2327-2332. [PubMed] |
| 24. | Maestre I, Jordán J, Calvo S, Reig JA, Ceña V, Soria B, Prentki M, Roche E. Mitochondrial dysfunction is involved in apoptosis induced by serum withdrawal and fatty acids in the beta-cell line INS-1. Endocrinology. 2003;144:335-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Xu Q, Chen SY, Deng LD, Feng LP, Huang LZ, Yu RR. Antioxidant effect of mogrosides against oxidative stress induced by palmitic acid in mouse insulinoma NIT-1 cells. Braz J Med Biol Res. 2013;46:949-955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 627] [Cited by in RCA: 608] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 27. | Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484-496. [PubMed] |
| 28. | Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab. 2011;300:E145-E154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 29. | Rochette L, Zeller M, Cottin Y, Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta. 2014;1840:2709-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 349] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 30. | Oprescu AI, Bikopoulos G, Naassan A, Allister EM, Tang C, Park E, Uchino H, Lewis GF, Fantus IG, Rozakis-Adcock M. Free fatty acid-induced reduction in glucose-stimulated insulin secretion: evidence for a role of oxidative stress in vitro and in vivo. Diabetes. 2007;56:2927-2937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | Neelakantan N, Narayanan M, de Souza RJ, van Dam RM. Effect of fenugreek (Trigonella foenum-graecum L.) intake on glycemia: a meta-analysis of clinical trials. Nutr J. 2014;13:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |