Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8636
Peer-review started: February 2, 2015
First decision: March 10, 2015
Revised: March 30, 2015
Accepted: May 7, 2015
Article in press: May 7, 2015
Published online: July 28, 2015
Processing time: 178 Days and 15.5 Hours
AIM: To investigate the clinical characteristics and outcomes of idiopathic Helicobacter pylori (H. pylori)-negative and drug-negative] peptic ulcer bleeding (PUB).
METHODS: A consecutive series of patients who experienced PUB between 2006 and 2012 was retrospectively analyzed. A total of 232 patients were enrolled in this study. The patients were divided into four groups according to the etiologies of PUB: idiopathic, H. pylori-associated, drug-induced and combined (H. pylori-associated and drug-induced) types. We compared the clinical characteristics and outcomes between the groups. When the silver stain or rapid urease tests were H. pylori-negative, we obtained an additional biopsy specimen by endoscopic re-examination and performed an H. pylori antibody test 6-8 wk after the initial endoscopic examination. For a diagnosis of idiopathic PUB, a negative result of an H. pylori antibody test was confirmed. In all cases, re-bleeding was confirmed by endoscopic examination. For the risk assessment, the Blatchford and the Rockall scores were calculated for all patients.
RESULTS: For PUB, the frequency of H. pylori infection was 59.5% (138/232), whereas the frequency of idiopathic cases was 8.6% (20/232). When idiopathic PUB was compared to H. pylori-associated PUB, the idiopathic PUB group showed a higher rate of re-bleeding after initial hemostasis during the hospital stay (30% vs 7.4%, P = 0.02). When idiopathic PUB was compared to drug-induced PUB, the patients in the idiopathic PUB group showed a higher rate of re-bleeding after initial hemostasis upon admission (30% vs 2.7%, P < 0.01). When drug-induced PUB was compared to H. pylori-associated PUB, the patients in the drug-induced PUB were older (68.49 ± 14.76 years vs 47.83 ± 15.15 years, P < 0.01) and showed a higher proportion of gastric ulcer (77% vs 49%, P < 0.01). However, the Blatchford and the Rockall scores were not significantly different between the two groups. Among the patients who experienced drug-induced PUB, no significant differences were found with respect to clinical characteristics, irrespective of H. pylori infection.
CONCLUSION: Idiopathic PUB has unique clinical characteristics such as re-bleeding after initial hemostasis upon admission. Therefore, these patients need to undergo close surveillance upon admission.
Core tip: Recently, the number of Helicobacter pylori (H. pylori)-negative and drug-negative “idiopathic” peptic ulcers has increased. This study analyzed the clinical characteristics of idiopathic peptic ulcer bleeding (PUB) and compared different etiologies, including H. pylori infection and drug use. In conclusion, definite etiologic factors of PUB including drug and H. pylori infection seemed to play an insignificant role in the severity of PUB. Idiopathic PUB has unique clinical characteristics such as re-bleeding after initial hemostasis upon admission. Therefore, these patients need to undergo close surveillance upon admission.
-
Citation: Chung WC, Jeon EJ, Kim DB, Sung HJ, Kim YJ, Lim ES, Kim MA, Oh JH. Clinical characteristics of
Helicobacter pylori -negative drug-negative peptic ulcer bleeding. World J Gastroenterol 2015; 21(28): 8636-8643 - URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8636.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8636
Acute upper gastrointestinal bleeding is a common complication of peptic ulcer disease, often caused by Helicobacter pylori (H. pylori) and non-steroidal anti-inflammatory drug (NSAID)/aspirin use. Recently, with the declining prevalence of H. pylori infection, H. pylori-negative ulcers have been frequently reported. Epidemiological studies have been performed to investigate whether the number of H. pylori-negative and drug-negative ulcers, the so called ‘‘idiopathic ulcers”, are increasing[1-4]. For a diagnosis of an idiopathic ulcer, a negative result of an H. pylori tests performed during at least two different time points has to be confirmed, and the validity of the drug use history should be checked. Then, conditions associated with malignancy and stressful conditions such as trauma, burns and multi-organ failure should be excluded.
Previously, several studies have reported that idiopathic ulcer disease has a higher recurrence rate than H. pylori-associated peptic ulcers, and its long-term recurrence rate is significantly higher than that of drug-induced peptic ulcers as well as H. pylori-associated peptic ulcers[3-6]. However, to date, the natural history of idiopathic PUB is unknown; the optimal management of these patients remains uncertain. In this study, we analyzed the clinical characteristics and outcomes of idiopathic peptic ulcer bleeding (PUB) and compared different etiologies, including H. pylori infection and drug use.
The Blatchford scores use pre-endoscopic clinical and laboratory variables to predict the need for clinical intervention (blood transfusion, endoscopy, surgery)[7]. The Rockall scores have been developed from mathematical models to predict the risk of death or re-bleeding[8]. Although the Blatchford and the Rockall scores lack subjective variables such as the severity of systemic diseases, these scoring systems have served as risk stratification tools and seem to perform well in predicting mortality[9-11]. We also aimed to assess the severity of PUB among groups categorized by different etiologies using these scoring systems.
The study was conducted at St. Vincent and St. Paul Hospital, the Catholic University of Korea. The medical records, charts and the digitalized picture archived images of consecutive patients who were admitted with PUB between January 2006 and January 2012 were collected. The patients presented with objective evidence of upper gastrointestinal bleeding (hematemesis, melena or blood in nasogastric aspirates).
All of the patients underwent an emergency esophagogastroduodenoscopy within 24 h of initial presentation. No systemic sedative agent was given to any of the patients. The stigmata of bleeding were classified according to the Forrest classification[12]. When the base of the ulcer was categorized as Forrest classification I or IIa, endoscopic treatment was performed. All of the patients underwent second look endoscopy within 48 h of the initial endoscopic examination. During the second look endoscopy, two biopsy specimens were taken from the antrum (the greater curvature of the mid-antrum) and the corpus (the greater curvature of the mid-body) for histological assessment. The diagnosis of H. pylori infection was based on histological results, including a rapid urease test (CLO test®, Kimberly-Clark, Utah, United States) or a Warthin-Starry silver stain in any of the two specimens from the antrum and body. Alcohol use was defined as consumption of at least 20 g of alcohol/d for up to three times/week. Smoking was defined as current smoking.
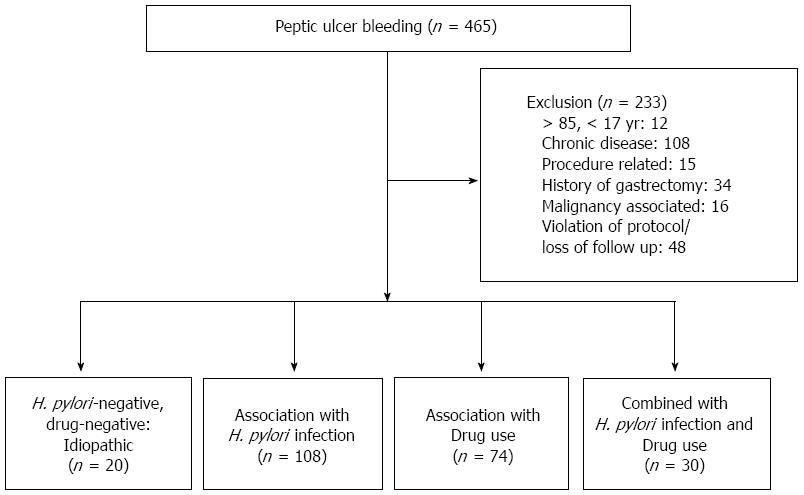
A total of 465 patients had bleeding from a peptic ulcer as confirmed by endoscopy. Patients less than 17 years and more than 85 years of age were excluded from the study. We excluded patients with procedure related bleeding (e.g., after gastric polypectomy, endoscopic mucosal resection or endoscopic submucosal dissection) and patients with medical co-morbidities consisting of serious systemic diseases (heart failure, liver cirrhosis with a Child-Pugh score > 7, chronic obstructive pulmonary disorder, sepsis, hematologic disorder, etc.). Individuals with conditions that might have substantial effects on our study results (e.g., serum creatinine > 2.5 mg/dL and total bilirubin > 3.0 mg/dL) and bleeding associated with malignancy were excluded. We also excluded patients with a history of gastrectomy. However, patients with diabetes mellitus without any complications or well-controlled hypertension were included.
All of the patients with PUB were prescribed proton pump inhibitor (PPI) therapy for 4 wk or longer. If the silver stain and rapid urease test were H. pylori-negative, we obtained an additional biopsy specimen by performing endoscopy 6-8 wk after the initial examination. Patients did not take PPIs for at least 2 wk before the re-endoscopy. For the diagnosis of idiopathic ulcer bleeding, a negative result of an H. pylori antibody test was confirmed.
Variables including patient age, sex, smoking and alcohol history, initial hemoglobin level, duration of admission, ulcer size, need for transfusion, evidence of re-bleeding, use of anti-thrombotic agents, use of NSAIDs and the status of H. pylori infection were reviewed. Anti-thrombotic agents included low dose aspirin and anti-platelet agents such as clopidogrel and ticlopidine. Re-bleeding was defined as objective evidence of bleeding with continuous melena, hematochezia, or the presence of fresh blood in vomit while in the hospital. When hemodynamic instability (systolic blood pressure < 90 mmHg or heart rate > 120 beats/min) developed or there was an abrupt drop of more than 2 g/dL in the hemoglobin level, these episodes were also defined as re-bleeding. In all cases, re-bleeding was confirmed by endoscopy. The Blatchford and the Rockall scores were calculated for all patients for the risk assessment.
For the quantitative variables, the mean ± SD was calculated. One-way ANOVA along with a post-hoc test such as Dunnett test was used to compare continuous variables among subgroups of PUB. For the qualitative variables, the number of subjects in the subgroups was calculated. In addition, the χ2 and/or Fisher’s exact test were used to investigate the association with other variables. The binary logistic regression model was used for the univariate and the multivariate analysis. SPSS statistical package version 21.0 (SPSS Inc., Chicago, IL, United States) and R language ver. 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria) were used for all statistical analyses. Statistical significance was defined as P < 0.05. The statistical methods of this study were reviewed by Jinho Yoo from Bio-Age Inc. in the Republic of Korea.
Of the 465 patients with PUB, 233 were excluded due to extreme age (n = 12), significant medical co-morbidity (n = 108), procedure related bleeding (n = 15), a history of gastrectomy (n = 34), bleeding from gastric cancer (n = 16), and violation of protocol/loss of follow up (n = 48). Surgical intervention was performed in 7 patients (4 cases of gastric ulcer bleeding and 3 cases of duodenal ulcer bleeding), and these participants were excluded due to incomplete evaluation of H. pylori infection. Finally, a total of 232 patients (162 males and 70 females) were enrolled in this study. The mean age of the subjects was 57.13 ± 17.42 years.
For PUB, the frequency of H. pylori infection was 59.5% (138/232), whereas 20 patients who had negative results for H. pylori infection and no definite drug history were allocated into the idiopathic (H. pylori-negative and drug-negative) PUB group (Figure 1). The frequency of idiopathic PUB was 8.6% (20/232). The patients were divided into the following four groups according to the etiologies of PUB: idiopathic, H. pylori-associated, drug-induced and combined (H. pylori-associated and drug-induced) type. The H. pylori-associated PUB group included 108 patients, whereas the drug induced PUB group included 74 patients who used aspirin (n = 35), anti-platelet agents (n = 17) and NSAIDs (n = 43). Twenty-one patients (28.4%) took two or more drugs. The combined type PUB group included 30 patients who used aspirin (n = 13), anti-platelet agents (n = 5) and NSAIDs (n = 18). Six patients (20.0%) took two or more drugs. Significant differences were found for age, sex, location of the ulcer, duration of admission, rate of re-bleeding after initial hemostasis, and the Blatchford and the Rockall scores among the four groups (Table 1).
| Etiology of PUB | Idiopathic (n = 20) | H. pylori-associated (n = 108) | Drug-induced (n = 74) | Combined (H. pylori, Drug) (n = 30) | P value | P value1 | P value2 | P value3 |
| Age | 55.15 ± 12.33 | 47.83 ± 15.15 | 68.49 ± 14.76 | 63.87 ± 14.79 | < 0.01 | 0.09 | < 0.01 | 0.09 |
| Sex (M:F) | 19:1 | 89:19 | 37:37 | 17:13 | < 0.01 | 0.24 | < 0.01 | < 0.01 |
| Smoking (Yes:No) | 10:10 | 53:55 | 21:53 | 7:23 | < 0.01 | 1 | 0.12 | 0.10 |
| Alcohol (Yes:No) | 6:14 | 27:81 | 14:60 | 5:25 | 0.554 | 0.85 | 0.364 | 0.314 |
| Gastric ulcer (GU) | 12 | 53 | 57 | 21 | < 0.01 | 0.644 | 0.044 | 0.084 |
| Duodenal ulcer (DU) | 6 | 44 | 6 | 2 | ||||
| GU + DU | 2 | 11 | 11 | 7 | ||||
| Initial hemoglobin (g/dL) | 9.98 ± 3.15 | 9.36 ± 2.56 | 8.75 ± 2.73 | 8.84 ± 2.39 | 0.19 | 0.57 | 0.14 | 0.26 |
| Creatinine (mg/dL) | 1.040 ± 0.179 | 1.018 ± 0.435 | 1.255 ± 0.845 | 1.03 ± 0.356 | 0.05 | 0.99 | 0.27 | 1.00 |
| Duration of admission (d) | 6.15 ± 2.277 | 5.602 ± 2.411 | 8.473 ± 8.919 | 5.5 ± 2.556 | < 0.01 | 0.93 | 0.18 | 0.93 |
| Ulcer size (cm) | 1.21 ± 0.65 | 0.86 ± 0.51 | 1.25 ± 0.92 | 1.07 ± 0.80 | < 0.01 | 0.12 | 0.99 | 0.80 |
| Needs for transfusion(PRC no.) | 2.35 ± 2.46 | 2.21 ± 1.98 | 3.53 ± 4.94 | 2.53 ± 1.74 | 0.06 | 0.99 | 0.29 | 0.99 |
| Re-bleeding caseupon admission: | 6 | 8 | 2 | 2 | < 0.014 | < 0.014 | < 0.014 | < 0.054 |
| Re-admission | 2 | 3 | 4 | 2 | 0.304 | 0.174 | 0.604 | 14 |
| Past history of ulcer | 6 | 25 | 2 | 8 | < 0.014 | 0.574 | < 0.014 | 1 |
| Blatchford score | 8.85 ± 4.06 | 8.56 ± 3.71 | 9.78 ± 3.77 | 10.40 ± 3.94 | 0.05 | 0.97 | 0.56 | 0.29 |
| Rockall score | 3.50 ± 1.05 | 3.05 ± 1.54 | 4.73 ± 2.21 | 4.73 ± 1.87 | < 0.01 | 0.52 | 0.02 | 0.04 |
The patient group consisted of 19 men (95%) and 1 woman (5%), and the mean age was 55.15 ± 12.33 years. The frequencies of gastric ulcer and duodenal ulcer were 60% (12/20) and 30% (6/20), respectively. The frequency of combined gastric and duodenal ulcers was 10% (2/20). When idiopathic PUB was compared to H. pylori-associated PUB, the idiopathic PUB group showed a higher rate of re-bleeding after initial hemostasis during the hospital stay (30% vs 7.4%, P < 0.01) (Table 2). When idiopathic PUB was compared to drug-induced PUB, the patients in the drug-induced PUB group were older (55.15 ± 12.33 years vs 68.49 ± 14.76 years, P < 0.01). In addition, the idiopathic PUB group showed a higher rate of re-bleeding after initial hemostasis upon admission (30% vs 2.7%, P < 0.01) (Table 3).
| Idiopathic PUB (n = 20) | Helicobacter pylori-associated PUB (n = 108) | P value1 | OR (95%CI)1 | P value2 | OR (95%CI)2 | |
| Age | 0.71 | 1.224 (0.43-3.49) | 0.94 | 1.04 (0.34-3.18) | ||
| ≥ 60 yr | 6 | 28 | ||||
| < 60 yr | 14 | 80 | ||||
| Ulcer size | 0.06 | 2.55 (0.96-6.77) | 0.15 | 2.13 (0.76-5.95) | ||
| ≥ 1.0 cm | 12 | 40 | ||||
| < 1.0 cm | 8 | 68 | ||||
| Re-bleeding case upon admission | ||||||
| Yes | 6 | 8 | < 0.01 | 5.36 (1.62-17.74) | 0.02 | 4.56 (1.34-15.54) |
| No | 14 | 100 |
| Idiopathic PUB (n = 20) | Drug-induced PUB (n = 74) | P value1 | OR (95%CI)1 | P value2 | OR (95%CI)2 | |
| Age | < 0.01 | 0.16 (0.05-0.47) | < 0.01 | 0.12 (0.03-0.55) | ||
| ≥ 60 yr | 6 | 54 | ||||
| < 60 yr | 14 | 20 | ||||
| Sex | < 0.01 | 19.00 (2.42-149.36) | 0.06 | 8.59 (0.94-83.82) | ||
| Male | 19 | 37 | ||||
| Female | 1 | 37 | ||||
| Ulcer type | 0.02 | 4.86 (1.37-17.29) | 0.08 | 3.86 (0.85-17.55) | ||
| Duodenal ulcer | 6 | 6 | ||||
| Others | 14 | 68 | ||||
| Ulcer size | 0.60 | 1.31 (0.48-3.58) | 0.16 | 2.65 (0.68-10.29) | ||
| ≥ 1.0 cm | 12 | 39 | ||||
| < 1.0 cm | 8 | 35 | ||||
| Re-bleeding case upon admission | ||||||
| Yes | 6 | 2 | < 0.01 | 15.43 (2.82-84.43) | < 0.01 | 19.76 (2.39-162.91) |
| No | 14 | 72 |
When drug-induced PUB was compared to H. pylori-associated PUB, significant differences in age, sex, proportion of gastric ulcers, ulcer size, duration of admission, and Blatchford and Rockall scores were found. A multivariate analysis of the covariates that showed statistical significance in the univariate analysis was performed; the results showed that drug-induced PUB was more common in older patients (68.49 ± 14.76 years vs 47.83 ± 15.15 years, P < 0.01). The drug-induced PUB group had equal numbers of men and women, whereas the H. pylori-associated PUB group had a predominantly male pattern (50% vs 82%, P = 0.03). In the drug-induced PUB group, ulcers were more likely to occur in the stomach (77% vs 49%, P < 0.01), and the duration of admission was longer (8.47 ± 8.92 d vs 5.60 ± 2.41 d, P = 0.04). However, the Blatchford and the Rockall scores were not significantly different between the two groups (Table 4).
| Drug-induced PUB (n = 74) | Helicobacter pylori-associated PUB (n = 108) | P value1 | OR (95%CI)1 | P value2 | OR (95%CI)2 | |
| Age | < 0.01 | 7.71 (3.95-15.07) | < 0.01 | 4.51 (1.83-11.11) | ||
| ≥ 60 yr | 54 | 28 | ||||
| < 60 yr | 20 | 80 | ||||
| Sex | < 0.01 | 0.21 (0.11-0.42) | 0.03 | 0.39 (0.17-0.91) | ||
| Male | 37 | 89 | ||||
| Female | 37 | 19 | ||||
| Ulcer type | < 0.01 | 3.48 (1.80-6.73) | < 0.01 | 4.09 (1.73-9.64) | ||
| Gastric ulcer | 57 | 53 | ||||
| Others | 17 | 55 | ||||
| Ulcer size | 0.03 | 1.95 (1.07-3.57) | 0.62 | 0.82 (0.37-1.81) | ||
| ≥ 1.0 cm | 39 | 40 | ||||
| < 1.0 cm | 35 | 68 | ||||
| Creatinine | 0.61 | 0.85 (0.47-1.55) | 0.11 | 0.52 (0.23-1.16) | ||
| ≥ 1.0 mg/dL | 41 | 64 | ||||
| < 1.0 mg/dL | 33 | 44 | ||||
| Duration of admission | < 0.01 | 2.71 (1.45-5.07) | 0.04 | 2.28 (1.04-5.00) | ||
| ≥ 7 d | 36 | 28 | ||||
| < 7 d | 38 | 80 | ||||
| Needs for transfusion | 0.43 | 1.28 (0.70-2.34) | 0.62 | 1.23 (0.55-2.76) | ||
| ≥ 3 PRCs | 31 | 39 | ||||
| < 3 PRCs | 43 | 69 | ||||
| Blatchford score | 0.02 | 2.15 (1.16-3.96) | 0.2 | 1.70 (0.76-3.82) | ||
| ≥ 10 points | 48 | 51 | ||||
| < 10 points | 26 | 57 | ||||
| Rockall score | < 0.01 | 3.94 (2.09-7.43) | 0.49 | 1.35 (0.58-3.13) | ||
| ≥ 4 points | 51 | 40 | ||||
| < 4 points | 23 | 68 |
When the combined type PUB was compared to H. pylori-associated PUB, the patients in the combined type PUB group were older (63.87 ± 14.79 years vs 47.83 ± 15.15 years, P < 0.01) and had a dominant pattern of gastric ulcers (70% vs 49%, P < 0.01). Additionally, the Blatchford and the Rockall scores were higher (P < 0.01). When the combined type PUB was compared to drug-induced PUB, no significant differences in the characteristics and clinical outcomes were found. The pattern of combined type of PUB was similar to that of drug-induced PUB.
The progressive decline of H. pylori infection and the increasing use of NSAIDs and aspirin have changed the etiologic distribution of PUB in the last two decades. In this study, the prevalence of H. pylori infection was 59.5%. This finding is consistent with other studies that have found a prevalence of H. pylori infection ranging from 61%-68%[13,14]. In the diagnosis of an idiopathic ulcer, the exclusion of an H. pylori infection is crucial, and the validity of the drug use history should be checked. Recently, Yoon et al[6] reported that idiopathic peptic ulcer disease was an independent risk factor for ulcer recurrence. However, in this study, 14.3% of idiopathic ulcer patients were determined only by rapid urease test without histologic evaluation, it may be difficult to completely exclude the possibility that H-pylori positive patients might be included in the idiopathic peptic ulcer group[6]. In the present study, to avoid an inaccurate diagnosis of H. pylori infection, we determined the H. pylori status twice by delayed diagnostic methods including histology. Furthermore, we performed the different types of diagnostic methods, such as H. pylori antibody tests, and confirmed the negative results. Then, we excluded malignancy, and other stressful conditions such as trauma, burns and multi-organ failure. Previously, several studies have reported that idiopathic ulcer disease has a higher recurrence rate than H. pylori-associated peptic ulcers, and its long-term recurrence rate is significantly higher than that of drug-induced peptic ulcers as well as H. pylori-associated peptic ulcers[3-6]. However, our results revealed no significant difference in long term recurrence rates. Further studies that assess the long term results are needed to determine the recurrence of idiopathic PUB compared to H. pylori-associated PUB. We also showed that patients with idiopathic PUB had a higher proportion of patients experienced re-bleeding during the hospital stay. Similar results were previously observed, suggesting that patients with idiopathic PUB have a substantial risk of recurrent bleeding[3,4]. Although we did not evaluate the mortality of PUB directly, recurrent bleeding is known as an independent risk factor that potentially leads to mortality. In particular, in-hospital bleeders were hemodynamically more unstable and had significantly more deaths due to bleeding-related cause[15,16]. Therefore, patients with idiopathic PUB have a potentially high risk of bleeding related mortality and need to undergo close surveillance upon admission.
Herein, we hypothesized the possible presence of an unidentified pathway that participates in the formation of idiopathic ulcers. Few data regarding the pathogenesis of idiopathic peptic ulcers exist in the literature. Recent studies have suggested that gastroduodenal ulcerations are related to sex hormone secretion. In this study, a predominantly male pattern was typically observed in the idiopathic PUB group, although this is not statistically significant. Previous studies in different animal species and humans have suggested that sex hormones influence gastric acid secretion and contribute to the integrity of the oral and gastroduodenal mucosa[17,18]. Another possibility is that the genetic and epigenetic changes in the mucin molecule may be responsible for idiopathic peptic ulcer disease[19,20]. Iijima et al[21] proposed several factors including age, systemic complications such as hepatocirrhosis and psychological stress were related to the idiopathic ulcers. However, Kanno et al[22] reported that older age itself is not a risk factor for idiopathic peptic ulcers. Instead, this study revealed that the presence of multiple underlying comorbidities (such as hypertension, hyperlipidemia and diabetes mellitus) is an important risk factor for idiopathic peptic ulcers compared with simple H. pylori-positive ulcers[22]. In the present study, we excluded hepatocirrhosis patients with moderate to severe portal hypertension, because this condition could affect the severity of PUB. In addition, we did not analyze that underlying comorbid disease between the four PUB subgroups that could be a cause of idiopathic peptic ulcers at least partially through local ischemia of the GI mucosa[22]. Further research using a prospective design is needed in this area.
One potential limitation of our study was its retrospective design; therefore, possible inherent biases existed. Second, H. pylori duodenal colonization or infection with Helicobacter heilmannii was not ruled out[23,24]. Third, a higher drop-out rate was observed in this study, which could be considered a limiting factor. However, we applied strict inclusion criteria for the enrolled patients and eliminated the other confounding factors. Generally, factors that affected the severity of PUB included the presence of conditions related to cardiac or multi-organ failure, particularly in older and comorbid patients, rather than the bleeding itself. In this study, it was noteworthy that we solely compared the severity of the bleeding from the peptic ulcer after excluding underlying diseases such as ischemic heart disease and chronic liver, lung and renal disease.
The high number of emergency admissions for upper gastrointestinal bleeding is due to drug use, especially in older patients[25,26]. Furthermore, patients with PUB receiving aspirin or NSAIDs therapy are exposed to a greater risk of severe bleeding, which requires a transfusion[27-29]. In this study, we examined whether the etiologic factors of peptic ulcer disease may be related to more severe clinical outcomes of PUB and whether H. pylori infection may influence the severity of PUB. To date, the characteristics and clinical outcomes of PUB according its etiology have not been clearly defined. Our results revealed that no significant difference in the severity of PUB in terms of the Blatchford and the Rockall scores between the drug-induced and H. pylori-associated group. Additionally, among the patients who experienced drug-induced PUB, no significant differences in clinical characteristics and clinical outcomes were observed, irrespective of H. pylori infection. Altogether, H. pylori infection does not play an important role in the severity of PUB.
In conclusion, definite etiologic factors of PUB including drug and H. pylori infection seemed to play an insignificant role in the severity of PUB. Idiopathic PUB had unique and distinct clinical characteristics and outcomes compared to other etiologies of PUB. Clinically, it had a tendency for re-bleeding after initial hemostasis during the hospital stay. Therefore, the patients with idiopathic PUB should be under close surveillance.
Currently, the number of Helicobacter pylori (H. pylori)-negative and drug-negative ulcers, the so called ‘‘idiopathic” peptic ulcers, is increasing. However, the natural history and the optimal management of idiopathic peptic ulcer bleeding (PUB) remain uncertain.
Previously, several studies have reported that idiopathic ulcer disease has a higher recurrence rate than H. pylori-associated peptic ulcers, and its long-term recurrence rate is significantly higher than that of drug-induced peptic ulcers as well as H. pylori-associated peptic ulcers.
In this study, the authors analyzed the clinical characteristics and outcomes of idiopathic PUB and compared different etiologies, including H. pylori infection and drug use. We also aimed to assess the severity of PUB among the groups categorized by the different etiologies using the Blatchford and the Rockall scoring systems.
Idiopathic PUB has unique and distinct clinical characteristics and outcomes compared to other etiologies of PUB. Clinically, it has a tendency for re-bleeding after initial hemostasis during the hospital stay. Therefore, the patients with idiopathic PUB should be under close surveillance.
The Blatchford score uses pre-endoscopic clinical and laboratory variables to predict the need for clinical intervention (blood transfusion, endoscopy, surgery). The Rockall score has been developed from mathematical models to predict the risk of death or re-bleeding. Although the Blatchford and the Rockall scores lack subjective variables such as the severity of systemic diseases, these scoring systems have served as risk stratification tools and seem to perform well for predicting mortality.
The study is well written and organized. The issue is very interesting.
P- Reviewer: Guadagni S S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Adamopoulos AB, Efstathiou SP, Tsioulos DI, Tzamouranis DG, Tsiakou AG, Tiniakos D, Mountokalakis TD. Bleeding duodenal ulcer: comparison between Helicobacter pylori positive and Helicobacter pylori negative bleeders. Dig Liver Dis. 2004;36:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Gisbert JP, Calvet X. Review article: Helicobacter pylori-negative duodenal ulcer disease. Aliment Pharmacol Ther. 2009;30:791-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Hung LC, Ching JY, Sung JJ, To KF, Hui AJ, Wong VW, Leong RW, Chan HL, Wu JC, Leung WK. Long-term outcome of Helicobacter pylori-negative idiopathic bleeding ulcers: a prospective cohort study. Gastroenterology. 2005;128:1845-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Wong GL, Wong VW, Chan Y, Ching JY, Au K, Hui AJ, Lai LH, Chow DK, Siu DK, Lui YN. High incidence of mortality and recurrent bleeding in patients with Helicobacter pylori-negative idiopathic bleeding ulcers. Gastroenterology. 2009;137:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Chason RD, Reisch JS, Rockey DC. More favorable outcomes with peptic ulcer bleeding due to Helicobacter pylori. Am J Med. 2013;126:811-818.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Yoon H, Kim SG, Jung HC, Song IS. High Recurrence Rate of Idiopathic Peptic Ulcers in Long-Term Follow-up. Gut Liver. 2013;7:175-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356:1318-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 684] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 8. | Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 896] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 9. | Vreeburg EM, Terwee CB, Snel P, Rauws EA, Bartelsman JF, Meulen JH, Tytgat GN. Validation of the Rockall risk scoring system in upper gastrointestinal bleeding. Gut. 1999;44:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 160] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Church NI, Dallal HJ, Masson J, Mowat NA, Johnston DA, Radin E, Turner M, Fullarton G, Prescott RJ, Palmer KR. Validity of the Rockall scoring system after endoscopic therapy for bleeding peptic ulcer: a prospective cohort study. Gastrointest Endosc. 2006;63:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Sanders DS, Carter MJ, Goodchap RJ, Cross SS, Gleeson DC, Lobo AJ. Prospective validation of the Rockall risk scoring system for upper GI hemorrhage in subgroups of patients with varices and peptic ulcers. Am J Gastroenterol. 2002;97:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | de Groot NL, van Oijen MG, Kessels K, Hemmink M, Weusten BL, Timmer R, Hazen WL, van Lelyveld N, Vermeijden RR, Curvers WL. Reassessment of the predictive value of the Forrest classification for peptic ulcer rebleeding and mortality: can classification be simplified? Endoscopy. 2014;46:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Gisbert JP, Gonzalez L, de Pedro A, Valbuena M, Prieto B, Llorca I, Briz R, Khorrami S, Garcia-Gravalos R, Pajares JM. Helicobacter pylori and bleeding duodenal ulcer: prevalence of the infection and role of non-steroidal anti-inflammatory drugs. Scand J Gastroenterol. 2001;36:717-724. [PubMed] |
| 14. | Schilling D, Demel A, Nüsse T, Weidmann E, Riemann JF. Helicobacter pylori infection does not affect the early rebleeding rate in patients with peptic ulcer bleeding after successful endoscopic hemostasis: a prospective single-center trial. Endoscopy. 2003;35:393-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Marmo R, Koch M, Cipolletta L, Bianco MA, Grossi E, Rotondano G. Predicting mortality in patients with in-hospital nonvariceal upper GI bleeding: a prospective, multicenter database study. Gastrointest Endosc. 2014;79:741-749.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Müller T, Barkun AN, Martel M. Non-variceal upper GI bleeding in patients already hospitalized for another condition. Am J Gastroenterol. 2009;104:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Baron JH. Sex, gonads, sex hormones and histamine-stimulated gastric acid and serum pepsinogen. Inflamm Res. 1997;46:260-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 18. | Machowska A, Szlachcic A, Pawlik M, Brzozowski T, Konturek SJ, Pawlik WW. The role of female and male sex hormones in the healing process of preexisting lingual and gastric ulcerations. J Physiol Pharmacol. 2004;55 Suppl 2:91-104. [PubMed] |
| 19. | Niv Y. H. pylori/NSAID--negative peptic ulcer--the mucin theory. Med Hypotheses. 2010;75:433-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Boltin D, Halpern M, Levi Z, Vilkin A, Morgenstern S, Ho SB, Niv Y. Gastric mucin expression in Helicobacter pylori-related, nonsteroidal anti-inflammatory drug-related and idiopathic ulcers. World J Gastroenterol. 2012;18:4597-4603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Iijima K, Kanno T, Koike T, Shimosegawa T. Helicobacter pylori-negative, non-steroidal anti-inflammatory drug: negative idiopathic ulcers in Asia. World J Gastroenterol. 2014;20:706-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Kanno T, Iijima K, Abe Y, Yagi M, Asonuma S, Ohyauchi M, Ito H, Koike T, Shimosegawa T. A multicenter prospective study on the prevalence of Helicobacter pylori-negative and nonsteroidal anti-inflammatory drugs-negative idiopathic peptic ulcers in Japan. J Gastroenterol Hepatol. 2015;30:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Seo WJ, Park CS, Cho YJ, Cha KW, Lee SW, Lim ST, Sung YH, Baek AR. [A case of gastric ulcer induced by Helicobacter heilmannii-like organism]. Korean J Gastroenterol. 2003;42:63-66. [PubMed] |
| 24. | Kato S, Ozawa K, Sekine H, Ohyauchi M, Shimosegawa T, Minoura T, Iinuma K. Helicobacter heilmannii infection in a child after successful eradication of Helicobacter pylori: case report and review of literature. J Gastroenterol. 2005;40:94-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Nakamura K, Akahoshi K, Ochiai T, Komori K, Haraguchi K, Tanaka M, Nakamura N, Tanaka Y, Kakigao K, Ogino H. Characteristics of hemorrhagic peptic ulcers in patients receiving antithrombotic/nonsteroidal antiinflammatory drug therapy. Gut Liver. 2012;6:423-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Yamaguchi D, Sakata Y, Tsuruoka N, Shimoda R, Higuchi T, Sakata H, Fujimoto K, Iwakiri R. Characteristics of patients with non-variceal upper gastrointestinal bleeding taking antithrombotic agents. Dig Endosc. 2015;27:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Chan FK, Sung JJ. How does Helicobacter pylori infection interact with non-steroidal anti-inflammatory drugs? Baillieres Best Pract Res Clin Gastroenterol. 2000;14:161-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Hawkey CJ. Nonsteroidal anti-inflammatory drug gastropathy. Gastroenterology. 2000;119:521-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 220] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Laine L. Review article: the effect of Helicobacter pylori infection on nonsteroidal anti-inflammatory drug-induced upper gastrointestinal tract injury. Aliment Pharmacol Ther. 2002;16 Suppl 1:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |