Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5513
Peer-review started: April 24, 2014
First decision: August 7, 2014
Revised: September 3, 2014
Accepted: November 8, 2014
Article in press: November 11, 2014
Published online: May 14, 2015
Processing time: 389 Days and 18.7 Hours
AIM: To study the cost-effectiveness of high-resolution microendoscopy (HRME) in an esophageal squamous cell carcinoma (ESCC) screening program in China.
METHODS: A decision analytic Markov model of ESCC was developed. Separate model analyses were conducted for cohorts consisting of an average-risk population or a high-risk population in China. Hypothetical 50-year-old individuals were followed until age 80 or death. We compared three different strategies for both cohorts: (1) no screening; (2) standard endoscopic screening with Lugol’s iodine staining; and (3) endoscopic screening with Lugol’s iodine staining and an HRME. Model parameters were estimated from the literature as well as from GLOBOCAN, the Cancer Incidence and Mortality Worldwide cancer database. Health states in the model included non-neoplasia, mild dysplasia, moderate dysplasia, high-grade dysplasia, intramucosal carcinoma, operable cancer, inoperable cancer, and death. Separate ESCC incidence transition rates were generated for the average-risk and high-risk populations. Costs in Chinese currency were converted to international dollars (I$) and were adjusted to 2012 dollars using the Consumer Price Index.
RESULTS: The main outcome measurements for this study were quality-adjusted life years (QALYs) and incremental cost-effectiveness ratio (ICER). For the average-risk population, the HRME screening strategy produced 0.043 more QALYs than the no screening strategy at an additional cost of I$646, resulting in an ICER of I$11808 per QALY gained. Standard endoscopic screening was weakly dominated. Among the high-risk population, when the HRME screening strategy was compared with the standard screening strategy, the ICER was I$8173 per QALY. For both the high-risk and average-risk screening populations, the HRME screening strategy appeared to be the most cost-effective strategy, producing ICERs below the willingness-to-pay threshold, I$23500 per QALY. One-way sensitivity analysis showed that, for the average-risk population, higher specificity of Lugol’s iodine (> 40%) and lower specificity of HRME (< 70%) could make Lugol’s iodine screening cost-effective. For the high-risk population, the results of the model were not substantially affected by varying the follow-up rate after Lugol’s iodine screening, Lugol’s iodine test characteristics (sensitivity and specificity), or HRME specificity.
CONCLUSION: The incorporation of HRME into an ESCC screening program could be cost-effective in China. Larger studies of HRME performance are needed to confirm these findings.
Core tip: Half of the cases accounting for the worldwide esophageal squamous cell carcinoma (ESCC) incidence occur in China, and there may be an opportunity to improve cancer survival with improved screening and surveillance. Our aim was to use a decision-analytic Markov model to study the cost-effectiveness of incorporating high-resolution microendoscopy (HRME) into an ESCC screening program in China. Our findings show that incorporating HRME into a screening program could be cost-effective, but larger studies confirming our preliminary estimates of HRME are necessary to confirm these results.
- Citation: Hur C, Choi SE, Kong CY, Wang GQ, Xu H, Polydorides AD, Xue LY, Perzan KE, Tramontano AC, Richards-Kortum RR, Anandasabapathy S. High-resolution microendoscopy for esophageal cancer screening in China: A cost-effectiveness analysis. World J Gastroenterol 2015; 21(18): 5513-5523
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5513.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5513
Esophageal cancer is the 6th most common cause of cancer-related mortality worldwide, with a notably high incidence rate in certain geographic regions, including Northern China, eastern Africa, Iran, and central Asia[1]. Half of the cases accounting for the worldwide esophageal squamous cell carcinoma (ESCC) incidence occur in China, and the uniformly poor five-year survival rates (< 15%) are a direct result of delayed diagnosis and the lack of standardized and effective screening and surveillance protocols worldwide[2,3]. The most widely accepted method of endoscopic evaluation for ESCC involves Lugol’s iodine mucosal staining with targeted biopsies of abnormal (unstained) areas. Although Lugol’s iodine staining has been shown to significantly increase the sensitivity of standard white-light endoscopy, specificity remains poor, as inflammation and other benign mucosal change can mimic neoplasia[4,5]. Recent studies suggest that use of confocal laser endomicroscopy, a technology which produces 1100 × magnified images of the epithelium at a subcellular level of resolution, can increase the accuracy of Lugol’s iodine staining to nearly 95% with a dramatic improvement in specificity[5]. Unfortunately, existing confocal platforms are costly (> $150000) and only available in a handful of Chinese academic medical centers[3].
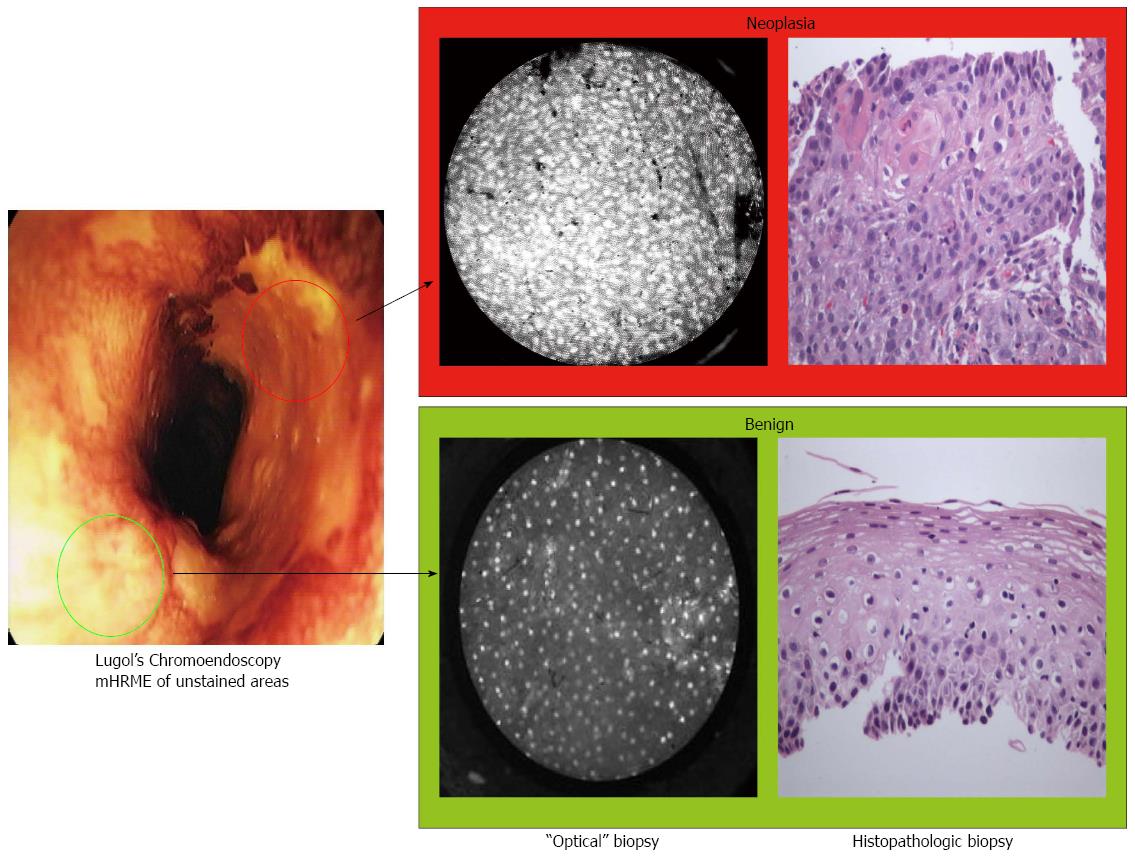
Given the limited availability and high cost of current high-resolution imaging platforms, our group successfully developed and preliminarily evaluated a prototype for high-resolution microendoscopy (HRME) that may serve as an alternative to confocal microendoscopy in low-resource or community-based settings. HRME offers a real-time, in vivo microscopic diagnosis so that more accurate and selective biopsy targeting can be performed[6,7]. The widefield and high-resolution images and corresponding histopathology are shown in Figure 1. In a single-arm pilot trial, the addition of HRME to Lugol’s iodine chromoendoscopy yielded a per biopsy sensitivity and specificity of 90% and 88%, respectively, and decreased the false-positive rate of Lugol’s iodine staining from 82% to 12%[8].
Preliminary studies show an improved specificity, and if an additional larger trial confirms an improvement in accuracy, this novel, low-cost imaging approach could improve the efficiency, clinical impact, and cost-effectiveness of the current standard of screening and surveillance in ESCC, allowing for national ESCC management programs in resource-restricted environments worldwide.
The aim of this analysis was to study the efficacy and cost-effectiveness of the novel HRME when applied to an ESCC screening and surveillance program in China.
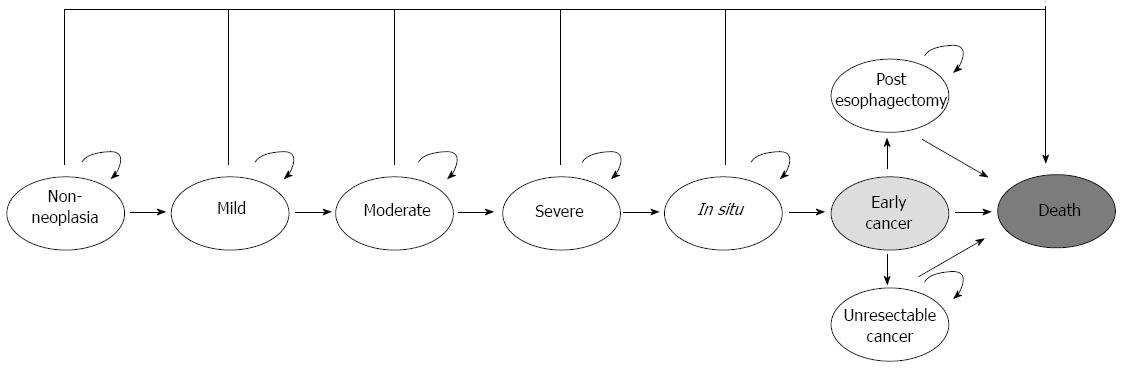
A decision analytic Markov model of ESCC was constructed in TreeAge Pro 2012 (TreeAge, Williamstown, MA, United States). Health states in the model included non-neoplasia, mild, moderate, and high-grade dysplasia (HGD; severe dysplasia and carcinoma in situ), intramucosal carcinoma (IMC), operable and inoperable cancer, and death. Initial prevalence rates of ESCC and precursor lesions were allocated based on published rates[9]. The simulation began with a hypothetical cohort of 50-year-old individuals who were followed until age 80 or death. Possible causes of death included age-related mortality, surgical mortality, squamous cell carcinoma, and endoscopic mucosal resection (EMR) complications. The Markov cycle length or time between state transitions was 1 mo. In each cycle, the simulated patient could stay in the same state, progress to the next state or die from age-related all-cause mortality. All patients were assumed to have the correct diagnosis of neoplastic states at the start of the model simulation. Separate model analyses were performed for cohorts consisting of the average-risk population or the high-risk region population. The average-risk cohort represents the general Chinese population at the risk of ESCC reported by the World Health Organization (WHO), and the high-risk cohort is at the risk informed by a prospective cohort study of patients from a high-risk population in Linxia, China[10-12]. Management options for the population were modeled to consist of no screening, endoscopic surveillance using Lugol’s iodine staining, and endoscopic surveillance using Lugol’s iodine and HRME.
The natural history of ESCC was modeled to examine the costs and outcomes related to the management of ESCC in the absence of surveillance, and compared with other intervention strategies. Figure 2 represents a sequence of monthly transitions among precancerous health states under natural history. Costs and discounted quality-adjusted life years (QALYs) without surveillance or other interventions for neoplastic states were determined. Cancer would be symptom-detected. Depending on the stage of cancer, the patients would receive either esophagectomy or palliative care. Age-related all-cause mortality was incorporated using Chinese life tables available from the Global Health Observatory Data Repository of the WHO (http://apps.who.int/gho/data/?vid=60340).
Screening was performed using Lugol’s iodine alone with targeted biopsy of Lugol’s iodine-voiding areas. Endoscopic surveillance continued at 3-mo intervals for HGD and IMC patients, at 1-year intervals for moderate dysplasia patients, at 3-year intervals for mild dysplasia patients, and at 5-year intervals for patients without dysplasia. The surveillance intervals for squamous neoplastic states were based on expert opinions in the absence of published guidelines. Patients diagnosed with HGD and IMC were followed up with EMR based on published compliance rates after the screening[13]. Those who underwent EMR would receive additional endoscopic treatments in order to achieve complete eradication of neoplasia if recurrence of malignancy was observed. Completely eradicated patients after EMR still had a possibility of developing neoplastic lesions. The model included complications of EMR, including perforation and stricture. Esophageal cancers that underwent surgery were modeled to be either surgically resectable or unresectable based on published rates[14,15].
Screening was performed using Lugol’s iodine staining and HRME with targeted biopsy of only areas abnormal on HRME. Endoscopic surveillance continued at the same intervals used for the Lugol’s iodine screening strategy. Patients with lesions identified as HGD and IMC based on visual interpretation of the HRME image were simultaneously treated with EMR. Those who underwent EMR would receive additional endoscopic treatments in order to achieve complete eradication of neoplasia if recurrence of malignancy was observed. Patients with completely eradicated neoplasia after EMR still had a possibility of developing neoplastic lesions. The model included complications of EMR, including perforation and stricture. Esophageal cancers that would undergo surgery were modeled to be either surgically resectable or unresectable based on published rates[14,15].
Model parameters or inputs were estimated from the literature. Base-case values and ranges used in sensitivity analyses are summarized in Table 1.
| Parameters | Base | Range | Ref. |
| Costs (I$: equivalent to 2012 USD) | |||
| Cost of cancer (annual) | I$3376 | [26-29] (conversion-ratio) | |
| Cost of screening (endoscopy + mucosal iodine staining + biopsy) | I$64 | I$58.5-63.6 | [13] |
| Cost of EGD | I$35.8 | [30] | |
| Cost of biopsy | I$28.2 | [30] | |
| Cost of HRME | I$35.8 | ||
| Cost of EMR | I$1292 | I$1292-1620 | [13] |
| Cost of EMR-related stricture | I$1111 | [31] (conversion-ratio) | |
| Cost of EMR-related perforation | I$1786 | [31] (conversion-ratio) | |
| Cost of esophagectomy | I$1768 | I$1485-2171 | [13] |
| Cost of post surgery state (annual) | I$136 | [19,27,28] (conversion-ratio) | |
| Discount rate, % | 0.03 | ||
| Transition probabilities | |||
| Non-neoplasia to mild dysplasia | Calibrated to overall annual ESCC incidence rate by age group-CI5[10]Overall cumulative incidence in follow-up study[11] | ||
| Mild to moderate dysplasia | |||
| Moderate to severe dysplasia | |||
| Severe dysplasia to IMC | |||
| IMC to operable cancer | |||
| Screening test characteristics (per patient) | |||
| Lugol’s iodine testing | |||
| Sensitivity | 0.99 | [8] | |
| Specificity | 0.15 | [8] | |
| HRME testing | |||
| Sensitivity | 0.99 | [8] | |
| Specificity | 0.82 | [8] | |
| Efficacy of EMR | |||
| Complete long-term remission | 0.62 | [32] | |
| Adherence rate (compliance of screening) | |||
| After positive biopsy | 0.70 | [13] | |
| Procedure characteristics | |||
| Operative candidate, cancer | 0.86 | [33] | |
| Surgical resectability rate | |||
| Surveillance | 0.76 | [33] | |
| No surveillance | 0.33 | [14,15] | |
| Complications of therapy | |||
| Post-EMR stricture rate | 0.05 | [34] | |
| Post-EMR perforation rate | 0.02 | [34] | |
| Post-RFA structure rate | 0.14 | [25] | |
| Complication rate from EGD | < 0.01 | [14,35,36] | |
| Mortality from EGD complication | < 0.01 | [14,35,36] |
The transition probabilities between the various health states are critical to the model’s validity. However, there is a wide range of estimates and uncertainty regarding transition rates between specific states (e.g., from non-neoplasia to mild or from mild to moderate). The best quality and amount of data exist for the annual incidence rate of ESCC in China. Because the incidence of esophageal cancer varies greatly across China and between high-risk vs average-risk populations, the transition probabilities between the health states were calibrated to generate two different overall ESCC incidence rate targets. One of the targets is based on the study by Wang et al[11], a prospective cohort study of patients from a high-risk population in Linxia, China. The study showed 16.7% incidence of ESCC over 13.5 years. The other target was obtained from the Cancer Incidence in Five Continents by the WHO[10,12]. This target provides age-dependent incidence rates pooled across five regions in China, which represent the average-risk population (Table 2).
| Age (yr) | Incidence (per 100000) |
| 50 | 17.32 |
| 55 | 26.61 |
| 60 | 36.35 |
| 65 | 56.58 |
| 70 | 77.50 |
| 75 | 117.48 |
| 80 | 143.29 |
| 85 | 143.17 |
Costs in Chinese currency were converted to international dollars (I$), a hypothetical unit of currency that has the same purchasing power parity that the US dollar had in the United States at a given point in time, using Purchasing Power Parity exchange rates from the WHO (http://www.who.int/choice/costs/ppp/en/). Published estimates of costs from prior years were converted to year 2012 dollars using the Consumer Price Index (Bureau of Labor Statistics, United States). When costs of procedures or treatments in China were not available, the cost estimates were based on expert opinions in China. Quality of life measures for various states in the model were adjusted to utility scores for the specific health states: cancer = 0.5 and post-esophagectomy = 0.97[16-19]. Costs and utility adjustments for chemoprevention and radiation were implemented in the model. All costs and expected life years were discounted at an annual rate of 3% to adjust for the relative value of present dollars or a present year of life[20].
The primary outcome of the analysis was the incremental cost-effectiveness ratio (ICER) per QALY between competing treatment strategies. ICERs are presented as the comparison of one intervention vs the next lowest cost alternative[21]. These comparisons are described with terms used for cost-effectiveness analyses, including “strongly dominated,” an option that is both less effective and more costly than another alternative, and “weakly dominated,” an option that is less effective and less costly than another alternative but has a higher ICER. A willingness-to-pay (WTP) threshold of 3 times the per-capita gross domestic product per QALY is recommended by the WHO; WTP of less than I$23500/QALY was used to determine cost-effectiveness[22,23]. Other outcomes assessed included costs, QALYs, and unadjusted life-years (life expectancy).
A base-case analysis using best estimates for all model parameters and transition probabilities was performed. Because of the variance in incidence of ESCC, we chose to have two base-case analyses corresponding to two target ESCC incidence rates which encompass a wide range of values from the average-risk population to the high-risk population[11]. One-way sensitivity analyses were performed to investigate the effects of changes in model parameters on estimated outcomes across a wide range of values, including performance characteristics of screening techniques, compliance rate to the endoscopic treatment under Lugol’s iodine screening, and efficacy of EMR. Additionally, probabilistic sensitivity analysis was performed. Distributions for specific parameters or model input variables were assigned and 1000 iterations were performed to gain further insight into the optimal strategy under uncertain conditions within the range of WTP thresholds.
The base-case analyses of the high-risk and average-risk population cohorts are presented in Table 3. For the average-risk population analysis, the Lugol’s iodine screening strategy was weakly dominated by the HRME screening strategy. When HRME screening was compared to the no screening strategy, the ICER was I$11808/QALY. For the high-risk region analysis, compared with no screening, Lugol’s iodine screening produced 1.12 more QALYs at a cost of I$2449, resulting in an ICER of I$1027/QALY. When HRME screening was compared to Lugol’s iodine screening, the ICER was I$8173/QALY, and was therefore a cost-effective alternative to Lugol’s iodine screening, assuming a WTP threshold of I$23500 per QALY. For both the high-risk and average-risk populations, the HRME screening strategy seemed to be the cost-effective strategy, producing ICERs below our WTP threshold.
| Strategy | Cost (I$) | QALYs | ICER (I$) | Unadjusted LYs |
| Average-risk population | ||||
| No screening | 50 | 15.6725 | - | 22.1245 |
| Lugol’s iodine screening | 665 | 15.7158 | Weakly dominated | 22.1989 |
| HRME screening | 696 | 15.7184 | 11808 | 22.2032 |
| High-risk population | ||||
| No screening | 1297 | 13.6188 | - | 18.8274 |
| Lugol’s iodine screening | 2449 | 14.7408 | 1027 | 20.6889 |
| HRME screening | 2911 | 14.7973 | 8173 | 20.7764 |
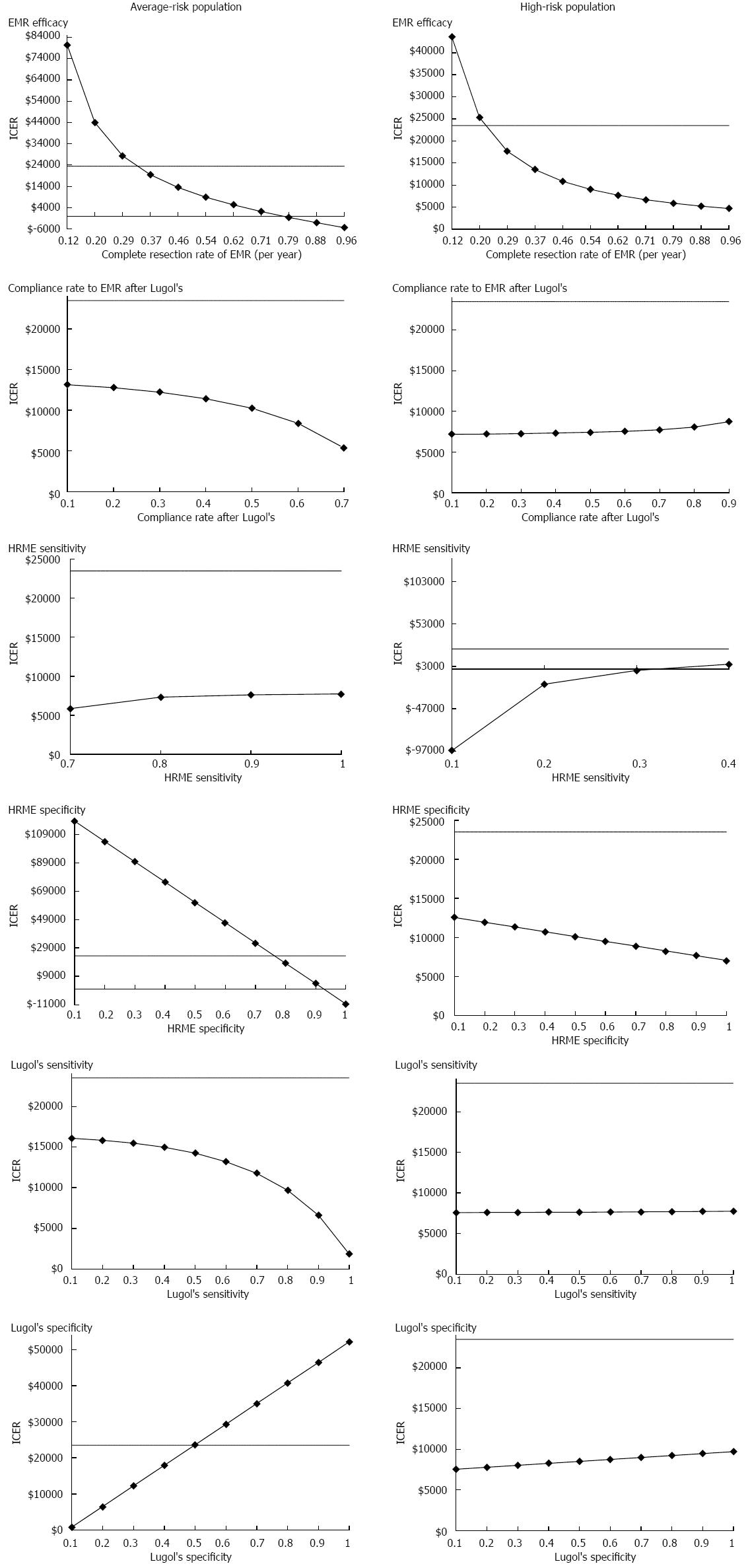
The results of the key sensitivity analyses for both high-risk and average-risk screening populations are summarized in Figure 3. The ICERs calculated in the tables compare the HRME screening strategy to Lugol’s iodine screening strategy.
Among the average-risk screening population, Lugol’s iodine screening strategy became cost-effective when EMR efficacy rate was lower than 35%. Higher specificity of Lugol’s iodine (> 40%) and lower specificity of HRME (< 70%) could also make Lugol’s iodine screening cost-effective. However, higher EMR efficacy rate (> 79%) and follow-up rate after the Lugol’s iodine (> 80%) resulted in HRME dominating the Lugol’s iodine screening strategy.
For the high-risk population, the results of the model were not substantially affected by varying the follow-up rate after Lugol’s iodine screening, Lugol’s iodine test characteristics (sensitivity and specificity), or HRME specificity. If the sensitivity of HRME is less than 70%, the Lugol’s iodine screening strategy may become cost-effective. Lower EMR efficacy (< 24% complete resection of neoplasia) could also make Lugol’s iodine screening strategy more cost-effective.
In addition, we performed one-way sensitivity analyses on the overall ESCC incidence rate per year in the range of 0.04% to approximately 2.00%. The incidence rate in the high-risk region was 1.20% per year and 16.20% over 13.5 years. In the average-risk screening population, the weighted average incidence rate across the age groups was 0.04% per year. HRME was the preferred strategy at all incidence rates within the range, assuming a WTP of I$23500/QALY. At rates below 0.04%, no screening seemed to be appropriate.
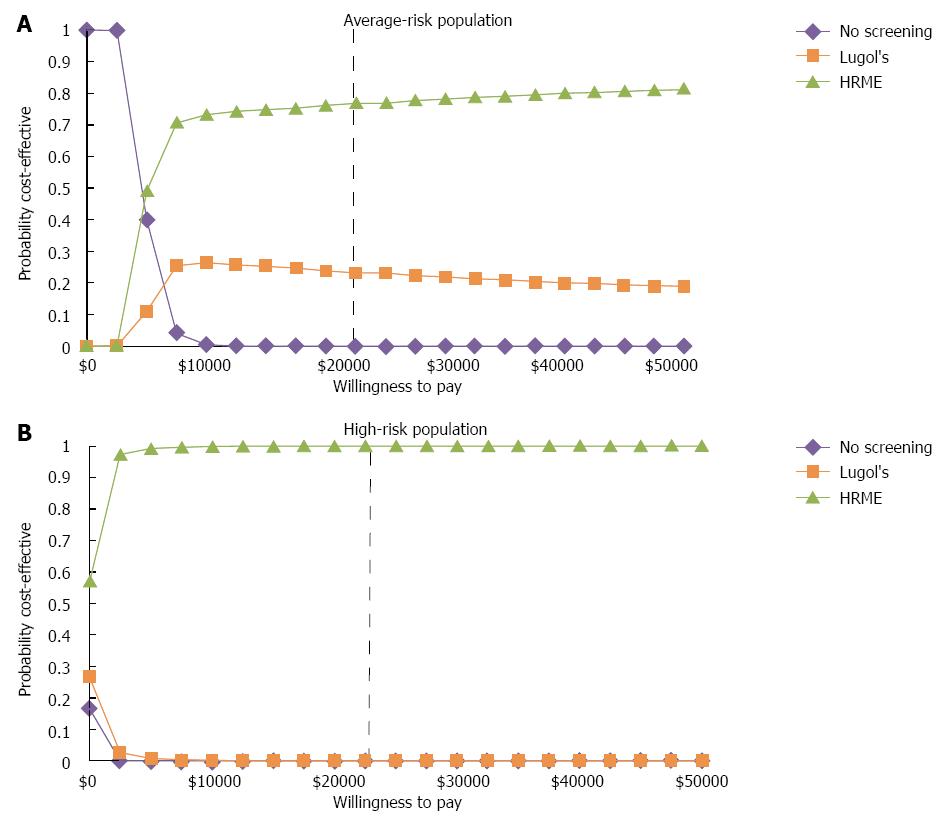
Probabilistic sensitivity analyses (see results in Figure 4) found that at a WTP between I$5000 and I$50000 per QALY, HRME was the preferred strategy for both the high and average-risk populations. When WTP was set at < I$5000 per QALY, no screening was preferred in the average-risk population. For the high-risk screening population, Lugol’s iodine screening was only preferred in at a WTP < I$2350, and only for 3.2% of trials.
This study shows that an HRME platform could be useful and cost-effective in endoscopic screening and surveillance programs for both average-risk and high-risk populations. Performance characteristics of the HRME platform were obtained and derived from a study performed in China and incorporated into the simulation model. With its higher specificity compared to Lugol’s iodine-directed endoscopy and biopsy, the incorporation of HRME can reduce the number of biopsies performed during the endoscopic screening. Also, by treating neoplastic lesions with EMR at the time of screening, the HRME technique could prevent the loss of diagnosed patients to EMR treatment follow-up as a result of patient non-compliance, an issue that is documented in China[13].
In both high- and average-risk population settings, the HRME screening strategy could be more effective than the Lugol’s iodine screening strategy by resulting in 0.0043 more unadjusted life years for the average-risk population, and 0.0875 more unadjusted life years for the high-risk population. These relatively small differences in life years gained are typical of what is seen in cancer screening programs, as the effects are the net benefits from a minority of cancer patients averaged over the entire population[19]. When HRME was compared to Lugol’s iodine screening, the ICERs were considerably below the WTP threshold of I$23500/QALY, making HRME the most plausible strategy in terms of cost-effectiveness.
Yang et al[24] published a cost-benefit analysis that studied standard endoscopic screening strategies of esophageal cancer in high-risk areas of China. They found that, compared with no screening, all screening strategies with varying screening age, frequencies, and follow-up intervals could save more life years. Strategies with higher screening frequencies were more cost-beneficial than those with lower screening frequencies. Although the present study focused on the incorporation of HRME into a screening program, the results are consistent with those of Yang et al[24]. Additionally, in an attempt to make our findings more generalizable to the average-risk population, separate analyses were conducted for both average- and high-risk populations in China. The analysis was based on the WHO’s Cancer Incidence in Five Continents esophageal cancer data in China, which is not derived from one local region or province, and thus can be applied to the country as a whole.
The analysis presented here have limitations. As with any analysis that uses a disease model, limited data of the natural history and other model inputs lead to uncertainty in the model and raise concerns regarding the validity of the model results and projections. Although more complex versions of cancer models are possible, we chose to construct a model that was as simple as possible in order to maintain a high level of model transparency and minimize the “black box” phenomenon. Moreover, sensitivity analyses were performed, as well as base-case analyses targeting two different populations (average- and high-risk) in acknowledgement of the uncertainty and generalizability of the findings. Although these measures do not eliminate model uncertainty, this approach was aimed to fully delineate these areas within the analysis, to disclose, but perhaps more importantly, to explore, their impact.
In addition, HRME test characteristics were based on screening performed by experts[8], based on the assumption that HRME would be performed in a referral endoscopy setting in conjunction with interventional endoscopic capabilities. Additional analyses using novice HRME found that HRME screening continued to be cost-effective in the high-risk population, although slightly above the WTP threshold of I$23500 in the average-risk population (ICER I$42193).
Radiofrequency ablation was not incorporated into the model as a treatment strategy because EMR was the preferred management strategy among the Chinese endoscopists in our pilot study, and also, there is limited data beyond the study by Bergmann et al[25] on the efficacy of radiofrequency ablation in treating squamous cell carcinoma.
The modeling analysis presented here also serves to highlight the new high-resolution screening technology that could allow for national ESCC screening programs in resource-restricted environments worldwide. This technology could improve the efficiency, clinical impact, and cost-effectiveness of the current standard of endoscopic screening of ESCC by offering a real-time in vivo diagnosis that reduces biopsy number and repeat procedures, while preserving accuracy. As better data for various model inputs become available, particularly if pivotal parameters change significantly from the current estimates, another analysis would be warranted.
In conclusion, the results of this analysis show that the incorporation of HRME into an ESCC management program could be cost-effective in China. Larger studies of HRME performance are needed to confirm these findings. Additionally, an HRME screening program could also be cost-effective in other regions or settings with high ESCC incidence.
Esophageal squamous cell cancer (ESCC) is the fifth most common cancer in China and is associated with significant morbidity. The current technique in ESCC management program, Lugol’s iodine chronoendoscopy, has poor specificity. Whereas existing confocal microendoscopy provides higher accuracy, the platform is costly and not widely available.
This study developed and analyzed a simulation model to assess the efficacy and cost-effectiveness of an ESCC screening program in China incorporating a prototype for high-resolution microendoscopy (HRME) that may serve as an alternative to confocal microendoscopy in community-based settings. By providing a real-time, in vivo microscopic diagnosis, the HRME technique coupled with Lugol’s iodine chronoendoscopy could offer selective biopsies and treatments with higher specificity.
This analysis found that an ESCC screening and surveillance program in China that incorporates HRME could be cost-effective.
The findings show that the incorporation of HRME into an ESCC management program could be cost-effective for both average- and high-risk individuals in China. This finding may help inform clinical management and guide policy decisions in China, but also demonstrates the applicability of HRME in other countries with high ESCC incidence. Preliminary estimates of HRME performance need to be validated in larger studies.
A Markov model is a model that includes different health states in which hypothetical patients can change over time. This model can be used to perform decision and cost-effectiveness analyses. The incremental cost-effectiveness ratio is the ratio of the change in costs to incremental benefits of the intervention. The quality-adjusted life-year is a measure of the burden of diseases, taking into consideration both quantity and quality of life.
This article is a well-designed, elegant, and much needed cost-benefit analysis of an ESCC cancer screening tool.
P- Reviewer: An HX, Aldrich MB S- Editor: Yu J L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Ferlay JSH, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer 2010; . |
| 2. | Ni K. Cancer of the Esophagus and Stomach. Mayo Clinic Proceedings. 2008;83:712-722. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Freitag CP, Barros SG, Kruel CD, Putten AC, Dietz J, Gruber AC, Diehl AS, Meurer L, Breyer HP, Wolff F. Esophageal dysplasias are detected by endoscopy with Lugol in patients at risk for squamous cell carcinoma in southern Brazil. Dis Esophagus. 1999;12:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Dawsey SM, Fleischer DE, Wang GQ, Zhou B, Kidwell JA, Lu N, Lewin KJ, Roth MJ, Tio TL, Taylor PR. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83:220-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Pech O, Rabenstein T, Manner H, Petrone MC, Pohl J, Vieth M, Stolte M, Ell C. Confocal laser endomicroscopy for in vivo diagnosis of early squamous cell carcinoma in the esophagus. Clin Gastroenterol Hepatol. 2008;6:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Collier T, Lacy A, Richards-Kortum R, Malpica A, Follen M. Near real-time confocal microscopy of amelanotic tissue: detection of dysplasia in ex vivo cervical tissue. Acad Radiol. 2002;9:504-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Collier T, Shen P, de Pradier B, Sung KB, Richards-Kortum R, Follen M, Malpica A. Near real time confocal microscopy of amelanotic tissue: dynamics of aceto-whitening enable nuclear segmentation. Opt Express. 2000;6:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Protano MA, Xu H, Wang GQ, Polydorides A, Dawsey SM, Cui J, Xue L, Zhang FZ, Quang T, Pierce M. Accuracy of a high resolution, low-cost microendoscope for the early detection of esophageal squamous cell neoplasia: A Prospective, International, Multicenter Trial. Gastroenterology. 2015;In press. |
| 9. | He Z, Zhao Y, Guo C, Liu Y, Sun M, Liu F, Wang X, Guo F, Chen K, Gao L. Prevalence and risk factors for esophageal squamous cell cancer and precursor lesions in Anyang, China: a population-based endoscopic survey. Br J Cancer. 2010;103:1085-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Bai SX. [Primary esophageal adenocarcinoma--report of 19 cases]. Zhonghua Zhong Liu Zazhi. 1989;11:383-385. [PubMed] |
| 11. | Wang GQ, Abnet CC, Shen Q, Lewin KJ, Sun XD, Roth MJ, Qiao YL, Mark SD, Dong ZW, Taylor PR. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 272] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Ferlay J, Bray F, Steliarova-Foucher E, Forman D. Cancer Incidence in Five Continents, CI5plus. IARC CancerBase No. 9 [Internet]. Lyon: International Agency for Research on Cancer 2014; . |
| 13. | Yang J, Wei WQ, Niu J, He YT, Liu ZC, Song GH, Zhao de L, Qiao YL, Yang CX. Estimating the costs of esophageal cancer screening, early diagnosis and treatment in three high risk areas in China. Asian Pac J Cancer Prev. 2011;12:1245-1250. [PubMed] |
| 14. | Hur C, Nishioka NS, Gazelle GS. Cost-effectiveness of aspirin chemoprevention for Barrett’s esophagus. J Natl Cancer Inst. 2004;96:316-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1144] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 16. | de Boer AG, Stalmeier PF, Sprangers MA, de Haes JC, van Sandick JW, Hulscher JB, van Lanschot JJ. Transhiatal vs extended transthoracic resection in oesophageal carcinoma: patients’ utilities and treatment preferences. Br J Cancer. 2002;86:851-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Fisher D, Jeffreys A, Bosworth H, Wang J, Lipscomb J, Provenzale D. Quality of life in patients with Barrett’s esophagus undergoing surveillance. Am J Gastroenterol. 2002;97:2193-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Gerson LB, Ullah N, Hastie T, Triadafilopoulos G, Goldstein M. Patient-derived health state utilities for gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:524-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Inadomi JM, Somsouk M, Madanick RD, Thomas JP, Shaheen NJ. A cost-utility analysis of ablative therapy for Barrett’s esophagus. Gastroenterology. 2009;136:2101-2114.e1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 217] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Gold M. Panel on cost-effectiveness in health and medicine. Med Care. 1996;34:DS197-DS199. [PubMed] |
| 22. | Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7:518-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 549] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 23. | International Monetary Fund. World Economic and Financial Surveys World Economic Outlook Database (April 2010 Edition). [Internet] Washington D.C. : International Monetary Fund 2010; . |
| 24. | Yang J, Wei WQ, Niu J, Liu ZC, Yang CX, Qiao YL. Cost-benefit analysis of esophageal cancer endoscopic screening in high-risk areas of China. World J Gastroenterol. 2012;18:2493-2501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Bergman JJ, Zhang YM, He S, Weusten B, Xue L, Fleischer DE, Lu N, Dawsey SM, Wang GQ. Outcomes from a prospective trial of endoscopic radiofrequency ablation of early squamous cell neoplasia of the esophagus. Gastrointest Endosc. 2011;74:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Gorelick AB, Inadomi JM, Barnett JL. Unsedated small-caliber esophagogastroduodenoscopy (EGD): less expensive and less time-consuming than conventional EGD. J Clin Gastroenterol. 2001;33:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Provenzale D, Kemp JA, Arora S, Wong JB. A guide for surveillance of patients with Barrett’s esophagus. Am J Gastroenterol. 1994;89:670-680. [PubMed] |
| 28. | Provenzale D, Schmitt C, Wong JB. Barrett’s esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 229] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Soni A, Sampliner RE, Sonnenberg A. Screening for high-grade dysplasia in gastroesophageal reflux disease: is it cost-effective? Am J Gastroenterol. 2000;95:2086-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Miller SM, Goldstein JL, Gerson LB. Cost-effectiveness model of endoscopic biopsy for eosinophilic esophagitis in patients with refractory GERD. Am J Gastroenterol. 2011;106:1439-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Gerson LB, Groeneveld PW, Triadafilopoulos G. Cost-effectiveness model of endoscopic screening and surveillance in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2004;2:868-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Pech O, May A, Gossner L, Rabenstein T, Manner H, Huijsmans J, Vieth M, Stolte M, Berres M, Ell C. Curative endoscopic therapy in patients with early esophageal squamous-cell carcinoma or high-grade intraepithelial neoplasia. Endoscopy. 2007;39:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Zhang DW, Cheng GY, Huang GJ, Zhang RG, Liu XY, Mao YS, Wang YG, Chen SJ, Zhang LZ, Wang LJ. Operable squamous esophageal cancer: current results from the East. World J Surg. 1994;18:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Takahashi H, Arimura Y, Masao H, Okahara S, Tanuma T, Kodaira J, Kagaya H, Shimizu Y, Hokari K, Tsukagoshi H. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc. 2010;72:255-64, 264.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 35. | Falk GW, Chittajallu R, Goldblum JR, Biscotti CV, Geisinger KR, Petras RE, Birgisson S, Rice TW, Richter JE. Surveillance of patients with Barrett’s esophagus for dysplasia and cancer with balloon cytology. Gastroenterology. 1997;112:1787-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Silvis SE, Nebel O, Rogers G, Sugawa C, Mandelstam P. Endoscopic complications. Results of the 1974 American Society for Gastrointestinal Endoscopy Survey. JAMA. 1976;235:928-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 333] [Article Influence: 6.8] [Reference Citation Analysis (0)] |