Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5454
Peer-review started: November 22, 2014
First decision: December 26, 2014
Revised: January 21, 2015
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: May 14, 2015
Processing time: 177 Days and 20 Hours
AIM: To investigate the roles and interactions of rho-associated protein kinase (ROCK)1 and miR-124 in human colorectal cancer (CRC).
METHODS: Expression of ROCK1 protein was examined by Western blotting, and quantitative reverse transcriptase PCR was performed to measure expression of ROCK1 mRNA and miR-124. Two cancer cell lines were transfected with pre-miR-124 (mimic) and anti-miR-124 (inhibitor) and the effects on ROCK1 protein and mRNA expression were observed. In addition, cell proliferation was assessed via a 5-ethynyl-2′ deoxyuridine assay. Soft agar formation assay, and cell migration and invasion assays were used to determine the effect of survivin on the transformation and invasion activity of CRC cells.
RESULTS: miR-124 was significantly downregulated in CRC compared to normal specimens (0.603 ± 0.092 vs 1.147 ± 0.286, P = 0.016) and in metastatic compared to nonmetastatic CRC specimens (0.416 ± 0.047 vs 0.696 ± 0.089, P = 0.020). Expression of miR-124 was significantly associated with CRC metastasis, tumor T and N stages, and tumor grade (all P < 0.05). ROCK1 protein was significantly increased in CRC compared to normal tissues (1.896 ± 0.258 vs 0.866 ± 0.136, P = 0.026), whereas ROCK1 mRNA expression was unaltered (2.613 ± 0.251 vs 2.325 ± 0.246). miR-124 and ROCK1 were inversely expressed in CRC tissues and cell lines. ROCK1 mRNA was unaltered in cells transfected with miR-124 mimic and miR-124 inhibitor, compared to normal controls. There was a significant reduction in ROCK1 protein in cells transfected with miR-124 mimic and a significant increase in cells transfected with miR-124 inhibitor (Ps < 0.05). Transformation and invasion of cells transfected with miR-124 inhibitor were significantly increased compared to those in normal controls (P < 0.05). Cells transfected with miR-124 inhibitor showed increased cell proliferation.
CONCLUSION: miR-124 promotes hyperplasia and contributes to invasion of CRC cells, but downregulates ROCK1. ROCK1 and miR-124 may play important roles in CRC.
Core tip: miR-124 inhibits neoplastic transformation, cell proliferation, and metastasis, and downregulates rho-associated protein kinase (ROCK)1 in some cancers. In this study, we investigated the roles and interactions of ROCK1 and miR-124 in human colorectal cancer (CRC). miR-124 promoted cell hyperplasia and contributed to invasion, but downregulated ROCK1 in CRC. ROCK1 and miR-124 may play important roles in CRC.
- Citation: Xi ZW, Xin SY, Zhou LQ, Yuan HX, Wang Q, Chen KX. Downregulation of rho-associated protein kinase 1 by miR-124 in colorectal cancer. World J Gastroenterol 2015; 21(18): 5454-5464
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5454.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5454
Colorectal cancer (CRC) is one of the most common malignances and the third leading cause of cancer-related death worldwide, with an estimated incidence of 1 million new cases and mortality of > 600000 deaths annually[1,2]. Recent progress in diagnosis and therapy has helped to save the lives of many patients at early stages of this malignancy, but the prognosis for patients with advanced disease or metastasis is still poor[1,2]. Therefore, further investigation into the molecular pathogenesis of CRC and the consequential development of novel targeted therapeutics are needed.
miRNAs are non-protein-coding small RNAs of 19-25 nucleotides that are cleaved from 70-100-nucleotide hairpin pre-miRNA precursors by the enzyme Drosha[3,4]. miRNAs bind to complementary sequences in the 3’-untranslated regions of their target mRNAs and induce mRNA degradation or translational repression[5]. Recent evidence shows that abnormal expression levels of miRNAs are associated with several human cancers, and that they play crucial roles in cell proliferation, differentiation, and apoptosis[6]. Increasing evidence reveals that miRNA dysfunction is associated with several human cancers[7,8]. Volinia et al[9] studied the miRNA expression pattern in solid cancers (2532 samples, 31 cancer types, 120 miRNAs), and found that miR-124 was expressed at a low level in many solid cancers[9-11], and acted as a tumor suppressor[12,13]. However, its role in CRC remains elusive.
Rho-associated protein kinase (ROCK)1 is a member of the rho-associated serine/threonine kinase family, which facilitates reorganization of the actin cytoskeleton during motion[14]. ROCK1 functions as an oncogene, and possesses a wide range of functions, including cellular migration, invasion, and metastasis[15]. ROCK1 is increased in many cancers, including glioma, osteosarcoma, prostate cancer, and gastric cancer[13,16]. ROCK1 is positively correlated with tumor-node-metastasis (TNM) stage and lymph node metastasis in gastric cancer[17]. ROCK1 is targeted by several miRNAs, including miR-135a, miR-145, and miR-148a[12,15,17]. Hu et al[18] found that ROCK1 was a direct target of miR-124 in gastric cancer. However, relatively little is known regarding the underlying mechanisms through which miR-124 regulates ROCK1 in CRC. In this study, we aimed to elucidate the roles and interactions between ROCK1 and miR-124 in human CRC.
Tissue specimens (68 tissue pairs) from CRC patients were obtained and histologically confirmed by a pathologist at the Second Affiliated Hospital of Henan University of Traditional Chinese Medicine (Zhengzhou, China). Written informed consent was obtained from all patients and the study was approved by the Ethics Committee of Henan University of Traditional Chinese Medicine on Human Research (No. H20130816).
Three colon cancer cell lines (HCT116, HT29, and SW620) and one human colonic mucosa epithelial cell line (NCM460) were obtained from the Xie He Cell Bank of the Chinese Academy of Medical Sciences (Beijing, China). HCT116 and HT29 cells were cultured in McCoy’s 5A medium (Invitrogen of Thermo Fisher Scientific, Waltham, MA, United States), and SW620 cells were cultured in RPMI 1640 containing 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, United States). All the cells were cultured in a humidified 37 °C incubator supplemented with 5% CO2.
Paired resected surgical specimens from primary tumor and adjacent non-tumor sites were obtained from CRC patients [male (n = 47), female (n = 21); median age: 57 years; colon cancer (n = 37) and rectal cancer (n = 31)] who underwent surgery at the Affiliated Hospital of Henan University of Traditional Chinese Medicine, according to a standard protocol, before any therapeutic intervention. Adjacent non-tumor mucosa, ≥ 6 cm from the tumor, was removed. The specimens were snap-frozen in liquid nitrogen and stored at -80 °C for molecular analyses. The remaining tissue specimens were fixed in 10% formalin and embedded in paraffin for routine histologic examination.
Total proteins were extracted from tissues using a total protein extraction kit (Keygen, Nanjing, China). The concentrations of total proteins were measured using a BCA Protein Assay Kit (Keygen). A total of 80 μg protein was separated using SDS-PAGE and transferred onto polyvinylidene difluoride membranes; the membranes were then blocked in 5% fat-free milk at room temperature for 2 h. After incubation with rabbit or goat primary antibodies against ROCK1 (ab80590; Abcam, Cambridge, United Kingdom) at a dilution of 1:10000 or GAPDH (Santa Cruz Biotechnology, Dallas, TX, United States) at a dilution of 1:200 at 4 °C overnight, the membranes were probed with goat anti-rabbit or mouse anti-goat secondary antibodies at a dilution of 1:5000 at room temperature for 2 h. The signals were detected using a Super ECL Plus Kit (Keygen) and determined by quantitative analysis using UVP software (UVP, LLC, Upland, CA, United States). The integral optical density ratio of ROCK1/GAPDH indicated the relative expression of ROCK1 protein.
TRIzol reagent (CWbio, Beijing, China) was used to isolate total RNA from the snap-frozen tissues. The isolated RNA was treated with DNase I (Invitrogen). The RNA concentration and purity were determined using a NanoDrop ND-1000 (NanoDrop Products, Wilmington, DE, United States). The ratio of 28S/18S was analyzed by Glyko Bandscan 5.0. RNA quality and quantity were determined spectrophotometrically at 260 and 280 nm, respectively. Reverse transcription of RNA was performed using the NCode miRNA First-Strand cDNA Synthesis Kit (Invitrogen).
Quantitative reverse transcriptase (qRT)-PCR was performed using the Light Cycler 2.0 Real-Time PCR System (Roche, Penzberg, Germany) in a total volume of 20 μL in glass capillaries containing 2 μL cDNA, 0.8 μL each primer, and 10 μL Light Cycler TaqMan Master Mix (Invitrogen). PCR for miR-124 was initiated using a 10-min denaturation step at 95 °C, followed by termination with a 30-s cooling step at 40 °C. The cycling protocol consisted of denaturation at 95 °C for 15 s and annealing at 60 °C for 1 min for 40 cycles. Fluorescence detection was performed at the end of each step. PCR for ROCK1 was initiated with a 10-min denaturation at 95 °C. Amplification was carried out for 40 cycles of 15 s at 95 °C and 1 min at 60 °C, followed by an extension step of 5 min at 72 °C. All reactions were performed in duplicate. The PCR products were confirmed by melting curve analysis. We used the mathematical delta-delta method (ratio = 2-ΔΔCT) developed by PE Applied Biosystems (Foster City, CA, United States) to compare relative expression between treatments.
HCT-116 and HT-29 cells were incubated in a six-well tissue culture dish without antibiotics for 24 h prior to transfection, when they had reached 60%-80% confluence. Negative control siRNA, specific miR-124 inhibitor and mimic siRNA (Invitrogen) transfection reagent complexes were mixed with Lipofectamine 2000 (Invitrogen) and then added to the cells. After 6 h at 37 °C, the medium was changed and the cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated FBS. Silencing of miRNA-124 and ROCK1 was determined by qRT-PCR and Western blotting.
Transfected HCT-116 and HT-29 cells were plated in 24-well plates at 4 × 104 cells/well, allowed to adhere, washed with PBS, and incubated for 2 h in serum-free RPMI containing 10 μmol/L EdU (Guangzhou RiboBio Co. Ltd., Guangzhou, China). The cells were washed with PBS, fixed, and permeabilized in PBS containing 2% formaldehyde, 0.5% Triton ×100, and 300 mmol/L sucrose for 15 min. After washing with PBS, cells were blocked using 10% FBS in PBS for 30 min, and incorporated EdU was detected by incubation with a fluorescent azide coupling solution (Apollo; Guangzhou RiboBio Co. Ltd.) for 30 min. The cells were washed three times with PBS containing 0.05% Tween 20, incubated with the DNA staining dye Hoechst 33342 for 30 min, and washed in PBS. Images were captured using a fluorescent microscope, and the average nuclear fluorescent intensity was calculated from at least 50 non-S phase cells randomly selected in five different fields of view.
A bottom layer (0.6% low-melt agarose) was prepared with RPMI 1640 medium containing 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin. A top layer (0.3% low-melt agarose) was prepared with the same RPMI 1640 medium as described above plus 5000 cells. Plates were incubated at 37.8 °C in 5% CO2 in a humidified incubator for approximately 2 wk. The plates were scanned and photographed, and the number of colonies was quantified using Quantity one version 4.0.3 software (Bio-Rad Laboratories, Hercules, CA, United States).
Cell migration and invasion assays were performed using a Transwell chamber. For migration, 2 × 104 transfected cells in serum-free medium were plated into the top chamber (BD Biosciences, Franklin Lakes, NJ, United States). For invasion, the same density of cells was seeded into the top chamber, which was precoated with Matrigel (BD Biosciences). After incubation for 24 h, the membranes were fixed and stained with 0.1% crystal violet. Cells passing through the membranes were counted under a microscope (Olympus Corp., Shinjuku, Tokyo, Japan).
SPSS version 13.0 software (SPSS Inc., Chicago, IL, United States) was used for the data analysis. Each assay was performed a minimum of three times. The data are expressed as the mean ± SD and Student’s t test and one-way analysis of variance were used to determine the significance of the difference in multiple comparisons. The Mann-Whitney U test was used to determine the associations of miR-124 expression and CRC clinicopathologic features. P < 0.05 indicated statistical significance.
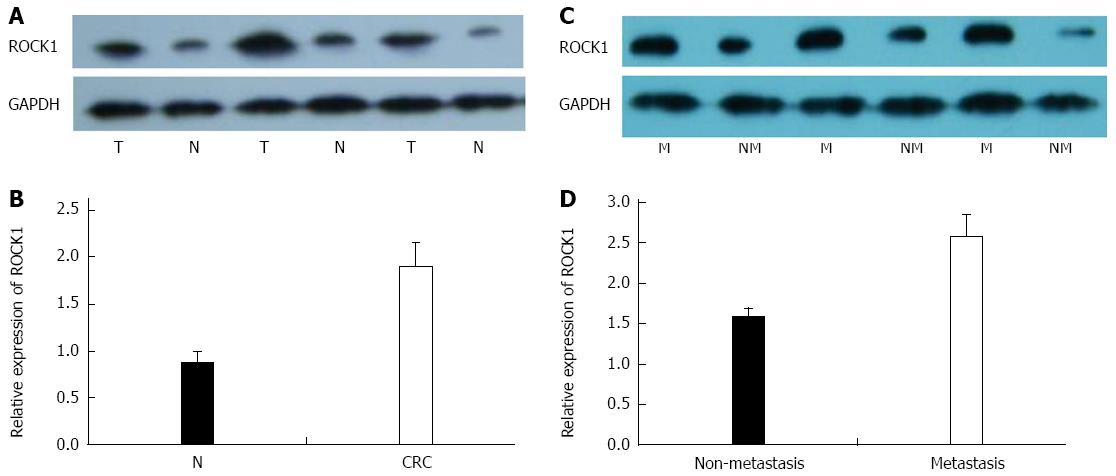
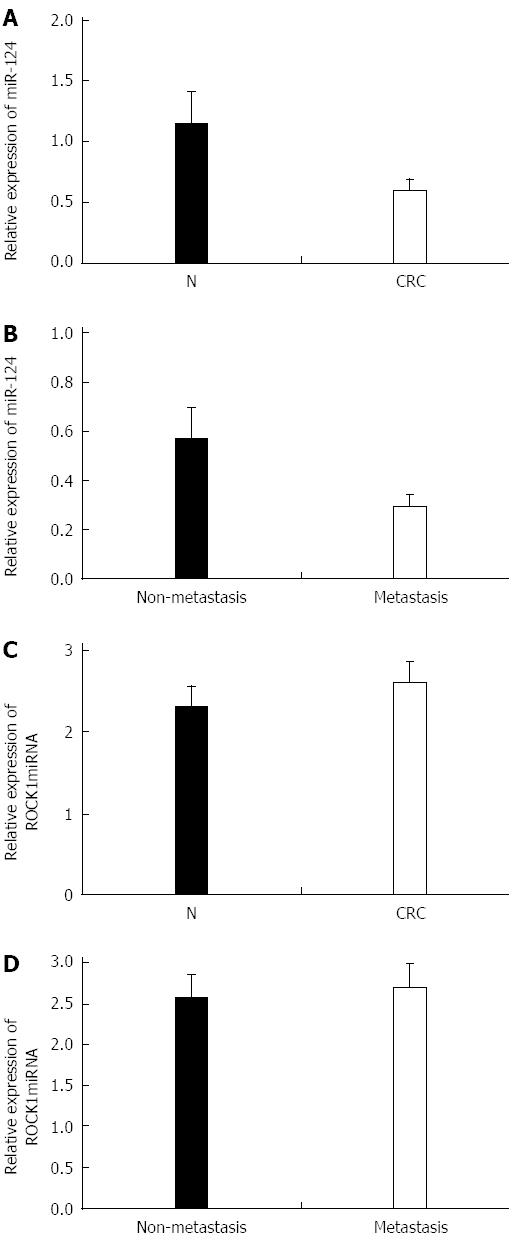
We investigated miR-124 and ROCK1 expression in 68 paired tissue specimens, including 38 cases of nonmetastatic CRC and 30 cases of metastatic CRC. Expression of ROCK1 was significantly increased in CRC compared with noncancerous tissues (1.896 ± 0.258 vs 0.866 ± 0.136, P = 0.026) (Figure 1A and B) and between metastatic tissues and non-metastatic CRC tissues (2.576 ± 0.269 vs 1.566 ± 0.126, P = 0.017) (Figure 1C and D). In contrast, higher expression of miR-124 was found in normal compared with CRC tissues (1.147 ± 0.286 vs 0.603 ± 0.092, P = 0.016), and in nonmetastatic compared to metastatic CRC tissues (0.696 ± 0.089 vs 0.416 ± 0.047, P = 0.020) (Figure 2A and B). For expression of ROCK1 mRNA, there was no difference between the CRC and the normal tissues (2.325 ± 0.246 vs 2.613 ± 0.251) or between metastatic and nonmetastatic CRC tissues (2.723 ± 0.283 vs 2.577 ± 0.276) (Figure 2C and D).
The relationships between miR-124 expression and clinicopathologic variables of CRC are summarized in Table 1. There was no significant relationship between miR-124 expression and patient age or sex. However, miR-124 was significantly associated with CRC metastasis, tumor T and N stages, and tumor grade (all P < 0.05).
| Parameters | n | miR-124 expression1 | P value | ||
| Low | Middle | High | |||
| Age | 0.823 | ||||
| ≤ mean | 23 | 11 | 8 | 4 | |
| > mean | 45 | 24 | 16 | 5 | |
| Sex | 0.921 | ||||
| Male | 47 | 24 | 19 | 4 | |
| Female | 21 | 11 | 5 | 5 | |
| T stage2 | 0.022 | ||||
| T1 | 19 | 4 | 12 | 3 | |
| T2 | 17 | 9 | 6 | 2 | |
| T3 | 15 | 10 | 3 | 2 | |
| T4 | 15 | 12 | 2 | 1 | |
| N stage2 | 0.019 | ||||
| N0 | 23 | 6 | 10 | 7 | |
| N1 | 19 | 8 | 7 | 4 | |
| N2 | 17 | 8 | 6 | 3 | |
| N3 | 9 | 6 | 3 | 0 | |
| Metastasis | 0.006 | ||||
| Metastasis | 30 | 22 | 6 | 2 | |
| Non-metastasis | 38 | 14 | 17 | 7 | |
| Grade3 | 0.015 | ||||
| Well differentiated | 26 | 10 | 13 | 3 | |
| Moderately differentiated | 12 | 10 | 9 | 3 | |
| Poorly differentiated | 18 | 15 | 1 | 2 | |
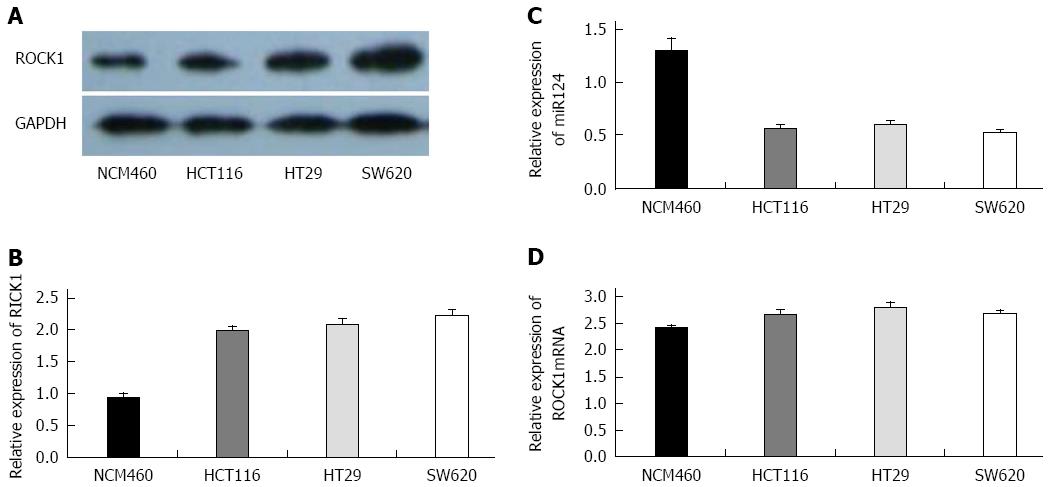
We determined the expression of miR-124 and ROCK1 (mRNA and protein) in three CRC cell lines and one normal colorectal cell line (NCM460) (Figure 3). Compared to NCM460 (1.306 ± 0.116), miR-124 expression was significantly reduced in HCT116, HT29, and SW620 cell lines (0.566 ± 0.036, 0.608 ± 0.032, and 0.526 ± 0.028, respectively; all P < 0.01) (Figure 3C). Western blotting showed a higher expression of ROCK1 protein in the three CRC cell lines compared to the NCM460 (HCT116: 1.983 ± 0.0768, HT29: 2.086 ± 0.078, and SW620: 2.226 ± 0.086 vs 0.936 ± 0.066; all P < 0.01) (Figure 3A and B). For ROCK1 mRNA, however, there was no significant difference between NCM460 and cancer cell lines (HCT116: 2.679 ± 0.076, HT29: 2.814 ± 0.086, SW620: 2.703 ± 0.046, NCM460: 2.426 ± 0.056) (Figure 3D).
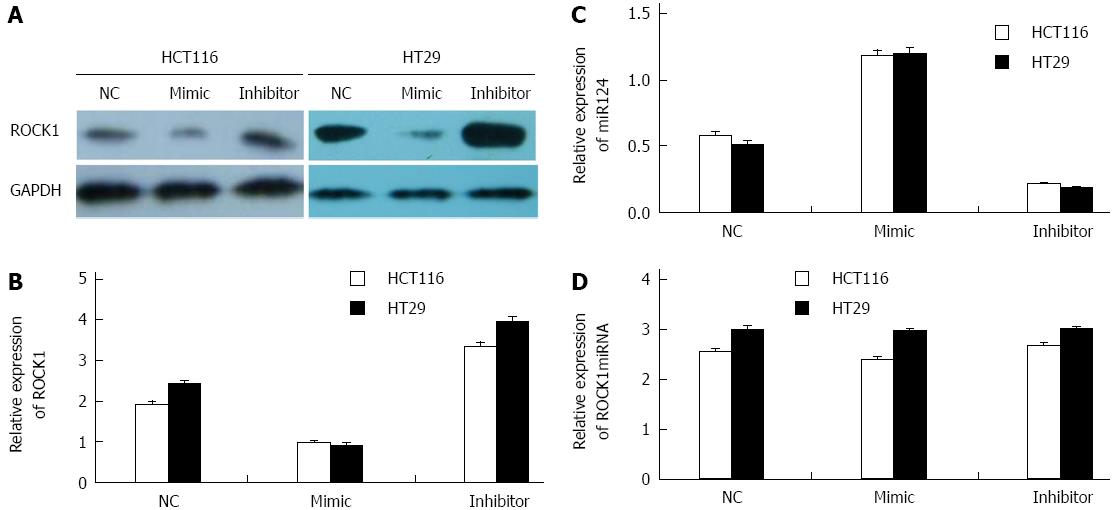
We determined whether transfection of HCT116 and HT29 cells with miR-124 mimic or miR-124 inhibitor affected ROCK1 expression. In HCT116 and HT29 cells characterized by low miR-124 expression, downregulation of endogenous miR-124 with miR-124 inhibitor led to a significant increase in ROCK1 protein levels compared to the negative controls (HCT116: 3.356 ± 0.0916 vs 1.956 ± 0.516, HT29: 3.996 ± 0.0981 vs 2.456 ± 0.0706; both P < 0.01) (Figure 4A and B). In contrast, there was a significant decrease in ROCK1 protein levels in cells transfected with miR-124 mimic (HCT116: 0.986 ± 0.0506 vs 1.956 ± 0.516, HT29: 0.916 ± 0.0483 vs 2.456 ± 0.0706; both P < 0.01). For ROCK1 mRNA, there was no difference in cells transfected with miR-124 inhibitor or mimic compare to the negative controls (HCT116: 2.686 ± 0.0515 vs 2.567 ± 0.062, HT29: 3.016 ± 0.0538 vs 3.012 ± 0.072) (Figure 4D).
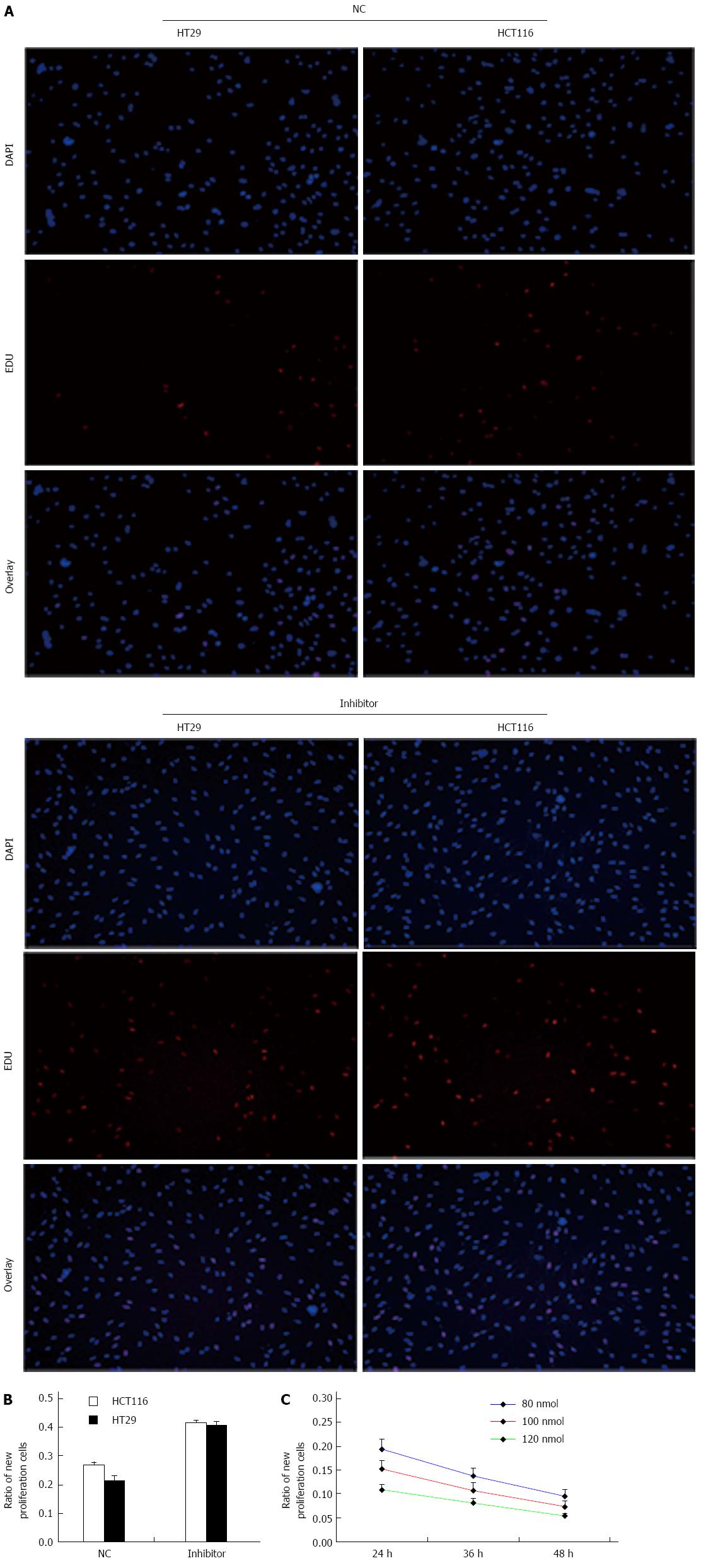
We determined the effect of knockdown of miR-124 on cell viability and proliferation using an EdU assay (Figure 4). To confirm the increased number of HCT116 and HT29 cells following treatment with miR-124 inhibitor, cells were labeled with EdU to measure active DNA synthesis and Hoechst 33342 to show all cell nuclei (Figure 4A). According to fluorescent microscopy, the mean percentage of new cells that incorporated EdU was 26.6% and 21.6% in the negative control siRNA group, 41.6% and 40.8% in cells transfected with miR-124 inhibitor (HCT116: 0.416 ± 0.0105 vs 0.266 ± 0.0121, HT29: 0.408 ± 0.0131 vs 0.216 ± 0.0147; both P < 0.01) (Figure 5A and B). We observed that the proliferative ability of HCT116 cells transfected with miR-124 mimic decreased with increasing concentrations of miR-124 mimic from 80 nmol/μL to 120 nmol/μL, with a time of transfection from 1 d to 2 d (P < 0.05) (Figure 5C).
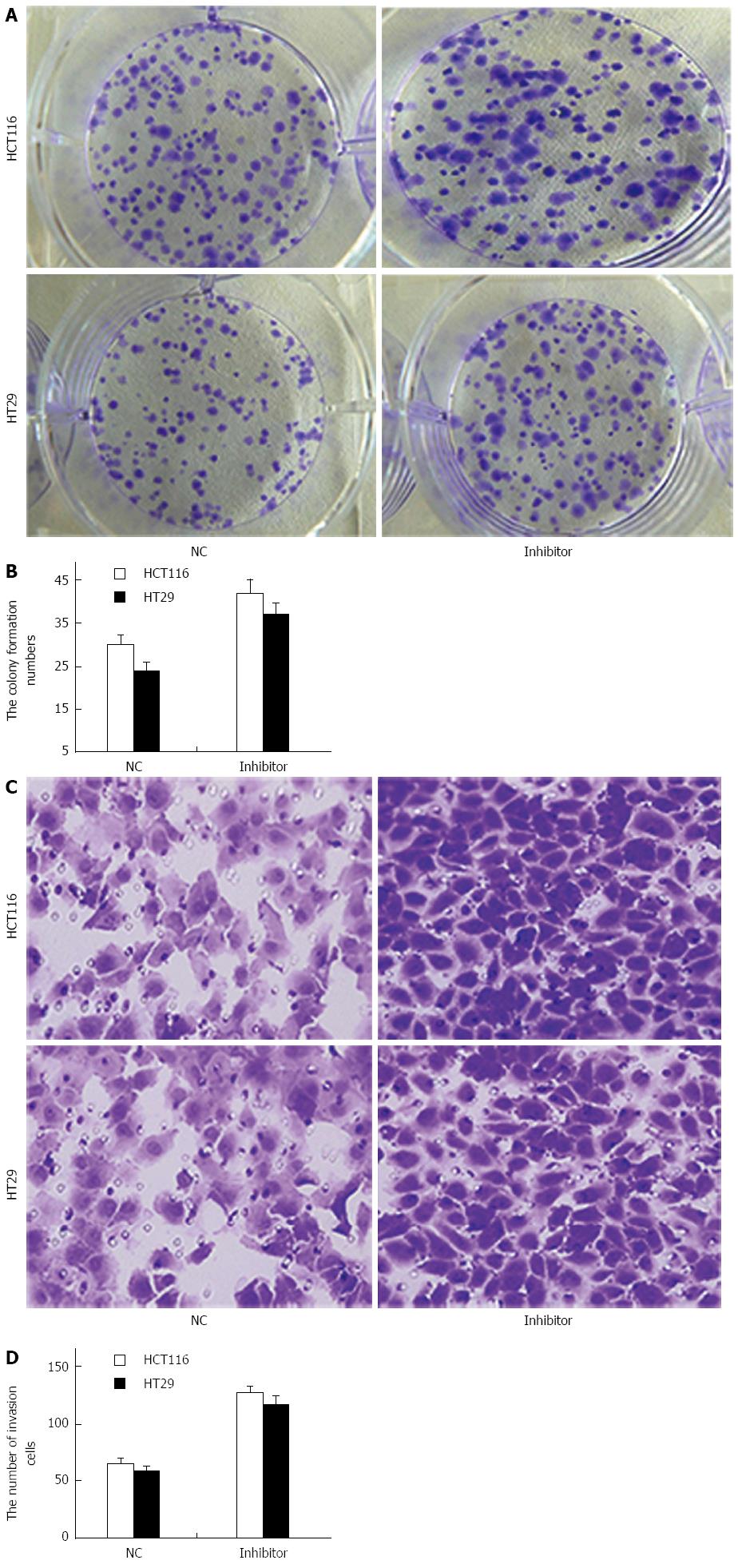
To study the role of miR-124 in the regulation of growth and invasion of CRC cells, a colony-formation assay was performed to evaluate whether miR-124 knockdown synergistically promoted HCT116 and HT29 cell transformation (Figure 6A). The assay demonstrated that the number of colonies generated by cells transfected with the miR-124 inhibitor was further increased compared to that generated by the controls (HCT116: 42 ± 3.2 vs 30 ± 2.2, HT29: 37 ± 2.6 vs 24 ± 2, both P < 0.01) (Figure 6A and B).
Migration and invasion assays showed that downregulation of miR-124 markedly increased proliferation of HCT116 and HT29 cells compare to negative controls (HCT116: 126 ± 7 vs 65 ± 4, HT29: 115 ± 8 vs 58 ± 5, both P < 0.01) (Figure 6C and D).
There are few CRC-specific biomarkers that have been used clinically for diagnosis and prediction of prognosis. Although many miRNAs are aberrantly altered in CRC, their underlying molecular mechanisms in the development and progression of CRC remain poorly understood[19]. Lewis et al[20] identified the targets of vertebrate miRNAs using an algorithm called TargetScan. They found that the conserved 5′ region of mammalian miRNAs is most important for target identification. Karginov et al[21] devised a direct biochemical method for miRNA target discovery that combined RNA-induced silencing complex purification with microarray analysis of bound mRNAs. They found that examining the complete spectrum of miR-124 targets in 293 cells yielded a set that were downregulated at the mRNA level, as previously observed, and a set whose mRNA levels were unaffected by miR-124a. Therefore, to explore the function of miRNAs specifically involved in CRC development and progression would greatly help expand our knowledge of CRC, and may provide new targets for its diagnosis and therapy. miR-124 and ROCK1 may not be specific markers for CRC, but they may be used to benefit patients due to their significance in prediction of tumor prognosis. An inverse association between miR-124 and ROCK1 protein was observed in CRC. miR-124 expression was significantly downregulated in CRC tissues and cell lines. In contrast, ROCK1 protein expression was significantly increased, whereas ROCK1 mRNA expression showed consistent results. We observed a significant reduction in ROCK1 protein and mRNA levels in cells transfected with miR-124 mimic, but a significant increase in cells transfected with miR-124 inhibitor. The results suggest that miR-124 post-transcriptionally and negatively regulates ROCK1 by repressing translation in CRC.
As a tumor suppressor, miR-124 is significantly decreased in a variety of human malignancies, including CRC, glioblastoma, hepatocellular carcinoma, medulloblastoma, and gastric cancer[22,23]. Zhao et al[24] found that downregulation of miR-124 promoted malignant progression of glioblastoma cells via increasing expression of a novel protein similar to human protein phosphatase 1, regulatory (inhibitor) subunit 13 like. Xie et al[23] reported that miR-124 inhibited proliferation and induced apoptosis by targeting enhancer of zeste homolog 2 in gastric cancer. In our study, expression of miR-124 was associated with CRC metastasis, supporting our observation that miR-124 inhibits invasion-related processes. Cells transfected with miR-124 inhibitor showed increased cell proliferation and transformation capacity, according to EdU and soft agar formation assays. Therefore, we assume that downregulation of ROCK1 by miR-124 may result in proliferation or metastasis in CRC. Our biologic evidence suggests that targeting miR-124 provides a good strategy to block tumor proliferation and metastasis. From the clinical viewpoint, low expression of miR-124 or high expression of ROCK1 might be considered as a risk factor for tumor progression. Thus, strict systemic therapy after surgery, such as immunotherapy, angiogenesis inhibitors, chemotherapy, and radiotherapy with regular investigation might improve prognosis.
In conclusion, our present study indicates that ROCK1 is negatively regulated at the post-transcriptional level by miR-124, and that miR-124 reduces proliferation and invasion/metastasis in CRC. The results suggest that, as a tumor suppressor, miR-124 may play a role in tumor growth and metastasis. Our findings further suggest that rescue strategies against miR-124, or strategies interfering with the miR-124/ROCK1 interaction, or that inhibit ROCK1 expression, may have potential therapeutic application in CRC in the future.
miR-124 is decreased in many cancers and acts as a tumor suppressor. Rho-associated protein kinase (ROCK)1 functions as an oncogene and possesses a wide range of functions, including cellular migration, invasion, and metastasis. miR-124 inhibits neoplastic transformation, cell proliferation, and metastasis, and downregulates ROCK1 in gastric cancer.
There are few colorectal cancer (CRC)-specific biomarkers that have been used clinically for diagnosis and prediction of prognosis. Although many miRNAs are aberrantly altered in CRC, their underlying molecular mechanisms in CRC development and progression remain poorly understood.
In the present study, ROCK1 was a direct target of miR-124 in gastric cancer. However, relatively little is known regarding the underlying mechanisms through which miR-124 regulates ROCK1 in CRC. In this study, the authors elucidated the roles and interactions between ROCK1 and miR-124 in human CRC.
As a tumor suppressor, miR-124 may play a role in tumor growth and metastasis. The results for miR-124 and ROCK1 suggest that rescue strategies against miR-124, or strategies interfering with the miR-124/ROCK1 interaction, or inhibition of ROCK1 expression, may provide therapeutic potential in CRC in the future.
miRNAs are non-protein-coding small RNAs of 19-25 nucleotides that are cleaved from 70-100-nucleotide hairpin pre-miRNA precursors by the enzyme Drosha. ROCK1 is a member of the Rho-associated serine/threonine kinase family, which facilitates reorganization of the actin cytoskeleton during motion.
This is an interesting work about a potential linkage between mir-124 and ROCK1. The authors studied expression of and relationship between miRNA-124 and ROCK1 in colorectal cancer specimens and in normal colorectal cells in vitro.
P- Reviewer: De Nardi P, Haier J, Linnebacher M S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Ma S
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 3. | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-297. [PubMed] |
| 4. | Zeng Y. Principles of micro-RNA production and maturation. Oncogene. 2006;25:6156-6162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Engels BM, Hutvagner G. Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene. 2006;25:6163-6169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 344] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 6. | Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5384] [Cited by in RCA: 5607] [Article Influence: 295.1] [Reference Citation Analysis (0)] |
| 7. | Chu D, Zhao Z, Li Y, Li J, Zheng J, Wang W, Zhao Q, Ji G. Increased microRNA-630 expression in gastric cancer is associated with poor overall survival. PLoS One. 2014;9:e90526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Ding H, Wu YL, Wang YX, Zhu FF. Characterization of the microRNA expression profile of cervical squamous cell carcinoma metastases. Asian Pac J Cancer Prev. 2014;15:1675-1679. [PubMed] |
| 9. | Volinia S, Galasso M, Costinean S, Tagliavini L, Gamberoni G, Drusco A, Marchesini J, Mascellani N, Sana ME, Abu Jarour R. Reprogramming of miRNA networks in cancer and leukemia. Genome Res. 2010;20:589-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 278] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 10. | Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373-4379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 563] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 11. | Medina PP, Slack FJ. microRNAs and cancer: an overview. Cell Cycle. 2008;7:2485-2492. [PubMed] |
| 12. | Li J, Song Y, Wang Y, Luo J, Yu W. MicroRNA-148a suppresses epithelial-to-mesenchymal transition by targeting ROCK1 in non-small cell lung cancer cells. Mol Cell Biochem. 2013;380:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Zhang H, Wang Q, Zhao Q, Di W. MiR-124 inhibits the migration and invasion of ovarian cancer cells by targeting SphK1. J Ovarian Res. 2013;6:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Wen X, Ding L, Wang JJ, Qi M, Hammonds J, Chu H, Chen X, Hunter E, Spearman P. ROCK1 and LIM kinase modulate retrovirus particle release and cell-cell transmission events. J Virol. 2014;88:6906-6921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Shin JY, Kim YI, Cho SJ, Lee MK, Kook MC, Lee JH, Lee SS, Ashktorab H, Smoot DT, Ryu KW. MicroRNA 135a suppresses lymph node metastasis through down-regulation of ROCK1 in early gastric cancer. PLoS One. 2014;9:e85205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Zhou P, Huang G, Zhao Y, Zhong D, Xu Z, Zeng Y, Zhang Y, Li S, He F. MicroRNA-363-mediated downregulation of S1PR1 suppresses the proliferation of hepatocellular carcinoma cells. Cell Signal. 2014;26:1347-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Wan X, Cheng Q, Peng R, Ma Z, Chen Z, Cao Y, Jiang B. ROCK1, a novel target of miR-145, promotes glioma cell invasion. Mol Med Rep. 2014;9:1877-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Hu CB, Li QL, Hu JF, Zhang Q, Xie JP, Deng L. miR-124 inhibits growth and invasion of gastric cancer by targeting ROCK1. Asian Pac J Cancer Prev. 2014;15:6543-6546. [PubMed] |
| 19. | Hong L, Han Y, Yang J, Zhang H, Zhao Q, Wu K, Fan D. MicroRNAs in gastrointestinal cancer: prognostic significance and potential role in chemoresistance. Expert Opin Biol Ther. 2014;14:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787-798. [PubMed] |
| 21. | Karginov FV, Conaco C, Xuan Z, Schmidt BH, Parker JS, Mandel G, Hannon GJ. A biochemical approach to identifying microRNA targets. Proc Natl Acad Sci USA. 2007;104:19291-19296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 22. | Li KK, Pang JC, Ching AK, Wong CK, Kong X, Wang Y, Zhou L, Chen Z, Ng HK. miR-124 is frequently down-regulated in medulloblastoma and is a negative regulator of SLC16A1. Hum Pathol. 2009;40:1234-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Xie L, Zhang Z, Tan Z, He R, Zeng X, Xie Y, Li S, Tang G, Tang H, He X. MicroRNA-124 inhibits proliferation and induces apoptosis by directly repressing EZH2 in gastric cancer. Mol Cell Biochem. 2014;392:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Zhao WH, Wu SQ, Zhang YD. Downregulation of miR-124 promotes the growth and invasiveness of glioblastoma cells involving upregulation of PPP1R13L. Int J Mol Med. 2013;32:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |