Published online Feb 7, 2014. doi: 10.3748/wjg.v20.i5.1365
Revised: November 21, 2013
Accepted: December 5, 2013
Published online: February 7, 2014
Processing time: 160 Days and 6 Hours
Epstein-Barr virus (EBV)-associated lymphoepithelioma-like gastric carcinoma (LELC) is characterized by a lower lymph node (LN) metastasis rate and a higher survival rate than other forms of gastric cancer. Although current prognosis for LELC is favorable, the most common approach is radical gastrectomy involving an extensive D2 lymph node dissection. Here, we report four cases of EBV-associated early LELC that were treated by an alternative approach, endoscopic submucosal dissection (ESD). The long-term outcome of this procedure is discussed. All patients were treated by ESD en bloc, and all ESD specimens showed tumor-free lateral resection margins. None of the lesions showed lymphovascular invasion. A pathological examination of ESD specimens revealed submucosal invasion of more than 500 μm in all four cases. One patient underwent additional radical surgery post-ESD; no residual tumor or LN metastasis was noted in the surgical specimen. The other three patients did not undergo additional surgery, either because of severe comorbidity or their refusal to undergo operation, but were subjected to medical follow-up. None of the ESD-treated patients reported local recurrence or distant metastases during the 27-32 mo of follow-up after ESD.
Core tip: The present case studies indicate that treatment of Epstein-Barr virus (EBV)-associated early lymphoepithelioma-like gastric carcinoma (LELC) by endoscopic submucosal dissection (ESD) might have favorable long-term outcomes, despite deep submucosal invasion of tumor cells. Therefore, a conservative management strategy without additional surgery might be considered for EBV-positive early LELC with submucosal invasion treated by ESD, especially in patients with severe comorbidity and high operative risk.
- Citation: Lee JY, Kim KM, Min BH, Lee JH, Rhee PL, Kim JJ. Epstein-Barr virus-associated lymphoepithelioma-like early gastric carcinomas and endoscopic submucosal dissection: Case series. World J Gastroenterol 2014; 20(5): 1365-1370
- URL: https://www.wjgnet.com/1007-9327/full/v20/i5/1365.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i5.1365
Lymphoepithelioma-like gastric carcinoma (LELC) is a rare variant of gastric cancer (GC), constituting 1%-4% of all GCs[1,2]. More than 80% of the reported LELC cases are associated with an Epstein-Barr virus (EBV) infection. LELC is characterized by (1) a well-defined tumor margin; (2) dense lymphocytic infiltration, with the number of infiltrating lymphocytes exceeding the number of tumor cells; (3) indistinct cytoplasmic borders and a syncytial growth pattern with poorly formed glandular structures; and (4) lack of desmoplasia[3]. When compared to other forms of GC, LELC cases have shown a favorable prognosis, especially those involving an EBV infection[2-5]. This phenomenon could be due to the lower propensity of EBV-associated GC cells invading adjacent lymph nodes (LNs). However, despite the low rate of LN metastasis and a favorable prognosis, current treatment of LELC involves a radical gastrectomy procedure accompanied by extensive D2 LN dissection. Nakamura et al[4] reported that the 5-year survival rate of LELC and conventional adenocarcinoma patients undergoing surgical treatment was 84% and 58%, respectively. Few studies have focused on the outcomes of alternative therapeutic strategies for LELC, such as endoscopic submucosal dissection (ESD). Here, we report the long-term outcomes of four cases of EBV-associated early LELC treated with ESD.
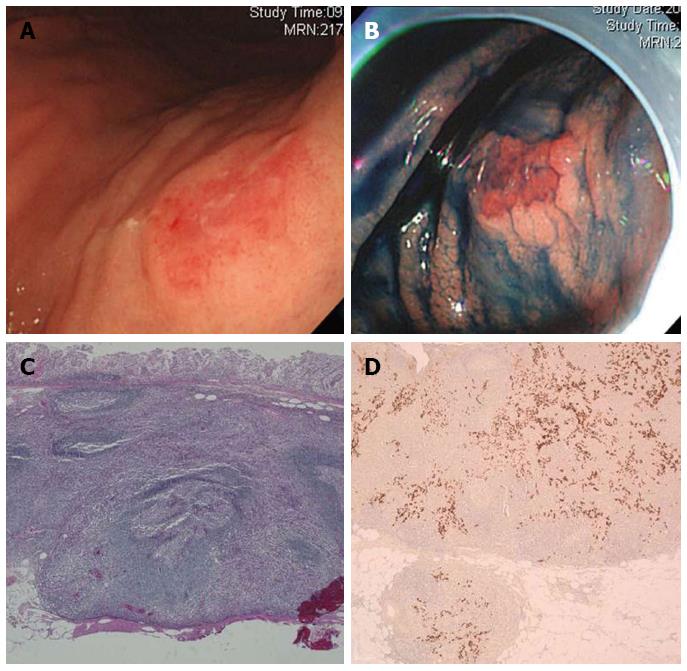
A 63-year-old man reporting poor oral intake underwent esophagogastroduodenoscopy (EGD) to diagnose the cause of his condition. The EGD resulted in the detection of two tumorous lesions in the high body (HB) and low body (LB) of the stomach. The tumor in the HB was a 2.0 cm large round, elevated lesion that displayed surface hyperemia (Figure 1A and B). The lesion in the LB was a 1.0 cm large round, mucosal elevation that displayed a central ulceration. The pathological examination of specimens collected by forceps biopsy of both lesions revealed them to be moderately differentiated adenocarcinomas. A similar biopsy from background mucosa identified the presence of chronic atrophic gastritis and Helicobacter pylori (H. pylori) infection. No significant enlargement of the LN was detected by a computed tomography (CT) scan of the abdomen. Therefore, ESD was performed on both lesions en bloc.
Pathological analysis of the ESD specimen showed that the HB lesion was a 1.6 cm large EBV-positive LELC invading the submucosal layer to a depth of 1800 μm (Figure 1C and D). The LB lesion was characterized as a 0.8 cm large, moderately differentiated adenocarcinoma confined within the mucosa layer. Neither lesion displayed lymphovascular invasion. The resection margins of both lesions were tumor-free. Since the LELC lesion showed deep submucosal invasion (1800 μm), the patient underwent total gastrectomy following ESD. No residual tumor or LN metastasis was observed in the surgical specimen after completion of the surgical treatment. In addition, no signs of recurrence were detected during the first 48 mo of follow-up post gastrectomy.
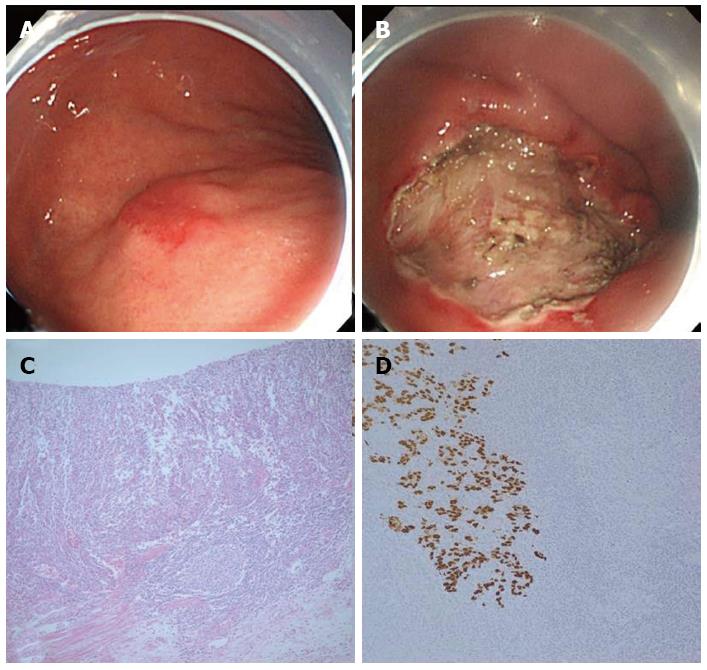
A 65-year-old man was referred to our hospital due to an incidentally found early GC on screening EGD. A second EGD performed in our hospital revealed slightly elevated 2.0 and 1.0 cm large lesions in the LB (Figure 2A) and mid body (MB) of the stomach, respectively. Analysis of specimens collected by forceps biopsy of the LB and MB lesions enabled classification of the two lesions as a moderately differentiated adenocarcinoma and a high-grade dysplasia, respectively. A similar pathological review of the background mucosa identified the presence of chronic atrophic gastritis and H. pylori infection. CT scan of the abdomen detected no definite enlargement of the LNs. ESD was thus performed on both lesions en bloc, and ESD specimens were further examined. Consequently, the LB lesion was described as a mixture of a 1.8 cm large well-differentiated adenocarcinoma within the mucosa layer, and a 0.6 cm large EBV-associated LELC with submucosal invasion of up to 1800 μm (Figure 2C and D). The MB lesion was characterized as a 0.8 cm large high-grade dysplasia. Neither lesion displayed lymphovascular invasion. The resection margins of both lesions were tumor-free. To treat the deep submucosal invasion of the LELC tumor, an additional radical surgery accompanied by LN dissection was recommended. However, the patient refused to undergo another operation, but requested medical follow-up. During the 32 mo of follow-up after ESD, no signs of local recurrence or distant metastases were found.
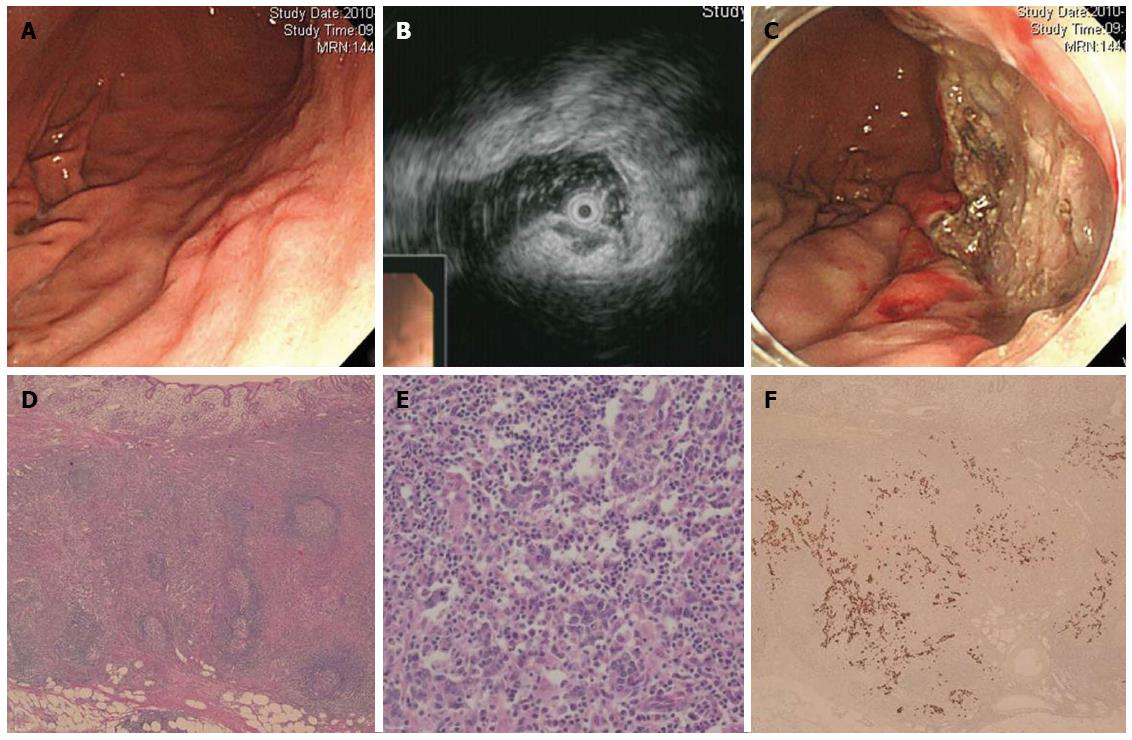
A 74-year-old man underwent EGD as part of a routine check-up. A 1.5 cm large, slightly elevated lesion with central dimpling was detected in the LB of the stomach. The lesion was covered with normal-looking mucosa and mimicked a subepithelial tumor (Figure 3A). Endoscopic ultrasound (EUS) revealed a homogeneous hypoechoic lesion growing from the muscularis mucosa layer, which also seemed to invade into the thickened submucosa (Figure 3B). Pathological examination of specimens collected by forceps biopsy identified a few markedly atypical cells in the submucosa, suggesting that the lesion could be an undifferentiated carcinoma. Chronic atrophic gastritis was found in the background mucosa. Unlike the previous cases, H. pylori infection was not detected. Results from the CT scan of the abdomen were inconclusive. To obtain a definite pathological diagnosis, ESD was performed en bloc (Figure 3C). Analysis of the ESD specimen showed a 1.8 cm large EBV-positive LELC invading the submucosal layer to a depth of 2500 μm (Figure 3D-F). No lymphovascular invasion was detected. Resection margins of the lesion were found to be tumor-free. Due to the lesion’s deep submucosal invasion, an additional radical surgery along with LN dissection was recommended. However, the patient refused to undergo another operation, but did agree to medical follow-up. During this 28-mo period following ESD, neither EGD nor abdominal CT scan detected signs of local recurrence or distant metastases.
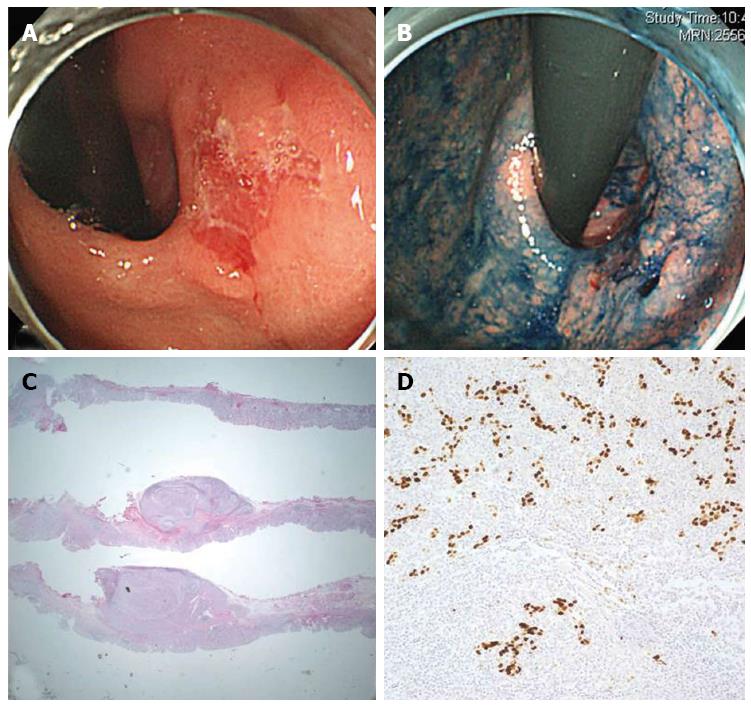
An 84-year-old man underwent EGD as part of a regular check-up. He had received concurrent chemoradiotherapy for treating a marginal zone B cell lymphoma of the hard palate two years ago, and was in complete remission at the time of the EGD. The EGD revealed a 1.5 cm large reddish and slightly depressed lesion in the cardia (Figure 4A and B), later characterized to be a moderately differentiated adenocarcinoma based upon examination of specimens collected by forceps biopsy. Chronic atrophic gastritis, but no H. pylori infection, was detected in the background mucosa. Abdominal CT scan identified no definite enlargement of the LNs. The patient was treated by ESD en bloc. Pathological analysis of the ESD specimen indicated the lesion was a 2.2 cm large EBV-associated LELC, with submucosal invasion of up to 2300 μm (Figure 4C and D). While lymphovascular invasion was not observed, the deep resection margin of the lesion was found to be invaded by tumor cells; the presence of a marked cautery artifact was also noted. Hence, an additional gastrectomy procedure was considered. However, the patient’s old age and poor pulmonary function (with forced expiratory volume of 1.09 L) complicated the radical surgery option, and after consulting with the patient the approach was decided against. Instead, the patient was closely monitored by EGDs and abdominal CT scans. During the 27 mo of follow-up after ESD, no signs of local recurrence or distant metastases were detected.
ESD is an effective therapeutic strategy for early GC, and is recognized as the preferred method of treatment in selected cases. Studies have demonstrated that the en bloc resection rate of ESD is greater than 90%, regardless of tumor size, and that the long-term survival rate following ESD is comparable to that of radical gastrectomy. The reported rates of postoperative bleeding and perforation range from 3.4%-7.6% and 1.0%-6.1%, respectively[6]. Few studies to date have examined the long-term outcomes of ESD treatment of early LELC[7,8]; among these, detailed pathological features of LELC have been described in only one case report. Lee et al[8] reported a case of EBV-associated LELC with submucosal invasion of up to 1538 μm, which was completely resected by ESD. No additional radical surgery was performed. During 24 mo of follow-up using EGD no local recurrence was detected. Our own cases of EBV-positive LELCs also showed favorable long-term outcomes following ESD, despite deep submucosal invasion of more than 500 μm. This favorable prognosis may be related to a lower propensity of EBV-associated GC to invade adjacent LNs, perhaps due to the immune response mounted against EBV proteins expressed by tumor cells[5]. LELC is characterized by a dense infiltration of lymphocytes in the tumor stroma. This high degree of infiltration could be another reason for the favorable prognosis. Song et al[3] reported that patients with EBV-associated LELC or GC accompanied by a Crohn’s disease-like lymphocyte reaction show significantly longer overall and disease-free survival following radical surgery than those with EBV-positive conventional adenocarcinoma. This in turn strongly suggests the importance of the host’s inflammatory response in determining the prognosis of EBV-associated GC. Nakamura et al[4] also reported that the 5-year overall survival rate following surgical treatment is significantly higher in patients diagnosed with LELC (84%) compared to those with conventional adenocarcinoma (58%).
The endoscopic features of LELC have not been well described. Several studies have reported cases of LELC histology mimicking features of subepithelial tumors[7,9,10]. Although rare, these studies have demonstrated the existence of hypoechoic lesion in the submucosa and the muscularis mucosa. This hypoechoic lesion might correspond to the lymphoid stroma composed of carcinoma cells and infiltrating lymphocytes. Results from EUS of Case 3 in our study corroborated the above findings (Figure 3B). Because of the dense lymphocytic aggregations and scattered foci of subepithelial neoplastic cells in LELC cases mimicking subepithelial tumors, pathological diagnosis of cancer using direct tissue sampling methods, such as fine needle aspiration is challenging. Therefore, ESD should be considered as a tool for a definite diagnosis and appropriate management of lesions if a lesion with endoscopic features of subepithelial tumor shows atypical cells in forceps biopsy specimen and above-mentioned findings in EUS.
In summary, our case series shows that ESD treatment of EBV-associated early LELC might have favorable long-term outcomes, despite deep submucosal invasion of tumor cells. Therefore, a conservative management strategy without additional surgery might be considered for EBV-positive early LELC with submucosal invasion treated by ESD, especially in patients with severe comorbidity or high operative risk. To enable conservative management, intensive medical follow-up should be performed using EGDs and abdominal CT scans. One shortcoming of this report is the limited number of cases; to confirm our findings, data from a larger number of case studies needs to be collected and analyzed.
Only one patient underwent esophagogastroduodenoscopy (EGD) to diagnose the cause of poor oral intake; the other three patients underwent EGD as part of a routine check-up, and did not display any symptoms requiring examination.
Since three of the patients were asymptomatic and only one patient complained of a non-specific symptom, clinical diagnosis of prior to endoscopic submucosal dissection (ESD) was difficult.
From EGD findings, early gastric cancer (GC) was diagnosed in three cases; the fourth patient was suspected to have a subepithelial tumor.
From the examination of specimens collected by forceps biopsy, three cases were diagnosed as moderately differentiated adenocarcinomas and one was described as a condition with few markedly atypical cells in the submucosa.
No definite enlargement of the lymph nodes was detected in any of the four cases, according to CT scanning of the abdomen.
Pathological examination of ESD specimens revealed dense lymphocytic infiltration in the tumor stroma. In situ hybridization detected Epstein-Barr virus (EBV)-encoded RNA in carcinoma cells.
ESD was successfully performed en bloc for all four cases.
LELC is a rare variant of GC characterized by a dense infiltration of lymphocytes in the tumor stroma.
The case series shows that ESD treatment of EBV-associated early LELC might have favorable long-term outcomes, despite deep submucosal invasion of tumor cells.
This report will help other clinicians in determining the prognosis of early LELC with deep submucosal invasion following an ESD treatment. To confirm the findings reported here, information from more case studies needs to be collected and analyzed.
P- Reviewers: Kita H, Limpaiboon T, Ooi LL, Sugimoto M, Yao H S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Corvalan A, Ding S, Koriyama C, Carrascal E, Carrasquilla G, Backhouse C, Urzua L, Argandoña J, Palma M, Eizuru Y. Association of a distinctive strain of Epstein-Barr virus with gastric cancer. Int J Cancer. 2006;118:1736-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Herath CH, Chetty R. Epstein-Barr virus-associated lymphoepithelioma-like gastric carcinoma. Arch Pathol Lab Med. 2008;132:706-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 3. | Song HJ, Srivastava A, Lee J, Kim YS, Kim KM, Ki Kang W, Kim M, Kim S, Park CK, Kim S. Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology. 2010;139:84-92.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Nakamura S, Ueki T, Yao T, Ueyama T, Tsuneyoshi M. Epstein-Barr virus in gastric carcinoma with lymphoid stroma. Special reference to its detection by the polymerase chain reaction and in situ hybridization in 99 tumors, including a morphologic analysis. Cancer. 1994;73:2239-2249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | van Beek J, zur Hausen A, Klein Kranenbarg E, van de Velde CJ, Middeldorp JM, van den Brule AJ, Meijer CJ, Bloemena E. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004;22:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Kakushima N, Fujishiro M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J Gastroenterol. 2008;14:2962-2967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Gromski MA, Miller CA, Lee SH, Lee TH, Chung IK, Park SH, Kim SJ, Cho HD. Gastric lymphoepithelioma-like carcinoma mimicking a subepithelial lesion treated by endoscopic submucosal dissection. Gastrointest Endosc. 2012;76:419-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Lee HL, Kim DC, Lee SP, Lee KN, Jun DW, Lee OY, Han DS, Yoon BC, Choi HS, Hahm JS. Treatment of Epstein-Barr virus-associated gastric carcinoma with endoscopic submucosal dissection. Gastrointest Endosc. 2012;76:913-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Song HJ, Kim KM. Pathology of epstein-barr virus-associated gastric carcinoma and its relationship to prognosis. Gut Liver. 2011;5:143-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Tang SJ, Ahmed N, Bhaijee F, Sheehan J, Subramony C, Jackson C, Lewin JR. Endoscopic mucosal resection of an Epstein-Barr virus-associated lymphoepithelioma-like gastric carcinoma. Dig Dis Sci. 2012;57:3032-3034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |