Published online Feb 7, 2014. doi: 10.3748/wjg.v20.i5.1348
Revised: November 6, 2013
Accepted: December 5, 2013
Published online: February 7, 2014
Processing time: 164 Days and 4.4 Hours
AIM: To investigate H2O2-induced promotion proliferation and malignant transformation in WB-F344 cells and anti-tumor effects of ursolic acid (UA) and oleanolic acid (OA).
METHODS: WB-F344 cells were continuously exposed to 7 x 10-7 mol/L H2O2 for 21 d. Observations of cell morphology, colony formation rates, flow cytometric analysis of cell cycle changes and aneuploidy formation indicated that H2O2 was able to induce malignant transformation of WB-F344 cells. We treated malignantly transformed WB-F344 cells with 4 μmol/L OA or 8 μmol/L UA for 72 h and analyzed the cell cycle distribution by flow cytometry.
RESULTS: MTT assay showed that 7 x 10-7 mol/L H2O2 decreased G1 phase subpopulation from 73.8% to 49.6% compared with the control group, and increased S phase subpopulation from 14.5% to 31.8% (P < 0.05 vs control group). Cell morphology showed that nucleus to cytoplasm ratio increased, many mitotic cells, prokaryotes and even tumor giant cells were shown in H2O2-induced WB-F344 cells. Fluorescence activated cell sorting analysis showed that WB-F344 cell aneuploidy increased to 12% following H2O2 treatment. Flow cytometric analysis of the transformed WB-F344 cells following treatment with OA (4 μmol/L) and UA (8 μmol/L) showed that OA increased G1 subpopulation to 68.6%, compared to 49.7% in unexposed cells. UA increased G1 subpopulation to 67.4% compared to 49.7% in unexposed cells (P < 0.05 vs H2O2 model group).
CONCLUSION: H2O2 causes the malignant transformation of WB-F344 cells. OA and UA exert anti-tumor effects by inhibiting the proliferation in malignantly transformed WB-F344 cells.
Core tip: It is known that H2O2 can promote tumorigenesis. Here, we used H2O2 as a premalignant and malignant agent to induce proliferation and malignant transformation in quiescent rat liver oval cell line WB-F344. Multistage carcinogenic processes provide the basis for interrupting and reversing precancerous changes; therefore, reversing precancerous changes is critical for tumor prevention and treatment. The salient and novel findings of the study are that ursolic acid (UA) and oleanolic acid (OA) induced malignantly transformed WB-F344 cell arrest in the G1 phase and apparently inhibited the proliferation of these cells. These results better our understanding of the antitumor effects of OA and UA.
- Citation: Han YY, Xue XW, Shi ZM, Wang PY, Wu XR, Wang XJ. Oleanolic acid and ursolic acid inhibit proliferation in transformed rat hepatic oval cells. World J Gastroenterol 2014; 20(5): 1348-1356
- URL: https://www.wjgnet.com/1007-9327/full/v20/i5/1348.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i5.1348
Hepatocarcinogenesis is a multistage developmental process that is divided into three stages: initiation, promotion and progression[1]. The initiation stage is characterized by irreversible genetic mutations. The promotion stage is characterized by a process of clonal cell hyperplasia, a process that is generally considered to be reversible, thus being an ideal target for cancer prevention[2]. The promotion stage is also referred to as the stage of hepatic precancerous lesions, and its principle pathological feature is oval cell hyperplasia. Oval cells are involved in liver regeneration and can mobilize and differentiate into hepatocytes and bile duct cells when the liver is injured. However, changes in the liver microenvironment can lead to the malignant transformation of oval cells during the process of hyperplasia, which is involved in the initiation and promotion stages of liver cancer development[3-5].
Reactive oxygen species (ROS) induce oxidative stress, serve as a major intrinsic factor in multistage carcinogenic processes and are involved in every step of this progression. Therefore, ROS and their downstream effects may be targets for cancer prevention[6,7]. Our previous studies demonstrated that ROS levels were significantly increased in aflatoxin- and diethylnitrosamine-induced rat hepatic precancerous lesions, whereas the activity of antioxidant enzymes decreased significantly[8]. To further explore the mechanism of ROS-induced hepatocellular carcinoma (HCC) development, we used 7 × 10-7 mol/L hydrogen peroxide (H2O2) as a premalignant and malignant agent to induce proliferation and malignant transformation in quiescent rat hepatic oval cells (WB-F344 cells). In this way, a model of oval cell malignant transformation was established[9]. Multistage carcinogenic processes provide the basis for interrupting and reversing precancerous changes. Ursolic acid (UA) and its isomer oleanolic acid (OA) are triterpenoic acids that naturally coexist in many Chinese herbs, such as cornel[10], glossy privet fruit[11] and Chinese hawthorn[12]. On the basis of our previous studies, we here explored the efficacy of OA and UA in the prevention and treatment of precancerous changes. Flow cytometry analysis of cell cycle distribution revealed differences in malignantly transformed WB-F344 cells following UA and OA treatment. We observed that OA and UA can inhibit the proliferation of malignantly transformed WB-F344 cells, which may explain the anti-tumor effects of UA and OA.
The rat oval cell line WB-F344 (a gift from Dr. Geng-Tao Liu, Peking Union Medical College) was grown in high-glucose Dulbecco’s Modified Eagle Medium (DMEM) with 100 U/mL penicillin and 100 μg/mL streptomycin and with or without 10% fetal bovine serum (FBS). The cells were maintained in the logarithmic growth phase at 37 °C in 5% CO2. The buffalo rat liver cells (BRL) rat hepatocyte cell line (purchased from Life Research Institute, Chinese Academy of Sciences, Shanghai, China) was cultured in high-glucose DMEM media that was supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. These cells were maintained in the logarithmic growth phase at 37 °C in 5% CO2. The WB-F344 cells were stimulated with 7 × 10-7 mol/L H2O2 for 12 h per day. After 21 d, these cells served as the malignant transformation model group cells (H2O2). The WB-F344 cells that were cultured in complete medium were used as one control group. The cells in both of these groups were cultured for Western blotting and flow cytometric analyses.
OA, UA and fluorouracil (5-FU) were purchased from Sigma (St Louis, MO, United States). OA and UA were dissolved in dimethyl sulfoxide (DMSO) and added to the cultures at a final concentration of 4 μmol/L or 8 μmol/L for 24, 48, 72 or 96 h. For the control experiments, an equal volume of DMSO was added to the control group. Fluorouracil (5-FU), a positive control, was added to the cultures at a final concentration of 0.05 mmol/L for 24, 48, 72 or 96 h.
The model group and the control group cells were resuspended in DMEM that was mixed with methylcellulose, which consisted of 1% methylcellulose, 10% bovine serum albumin, 100 U/mL penicillin and 100 μg/mL streptomycin. Next, 500 cells were suspended in 1.0 mL and were then plated in 24-well plates and cultured for 7-10 d. The colonies were counted at 400 × magnification under microscope.
This assay was performed as described previously[13], with slight modifications. Briefly, 24 h prior to the experiment, the WB-F344 cells and the transformed WB-F344 cells were added into 96-well dishes. The WB-F344 cells were exposed to 7 × 10-4-7 × 10-9 mol/L H2O2 for 6, 9, 12, 15, 18 h. The transformed WB-F344 cells were treated with OA at 0.5, 1, 2, 4 or 8 μmol/L OR WITH UA at 1, 2, 4, 8 or 16 μmol/L and allowed to incubate for 24, 48, 72 or 96 h. Prior to harvesting, 20 μL of 5 mg/mL MTT [3-(4,5-dimethythiazolzyl)-2,5-diphenyl tetrazolium bromide; Sigma, St Louis, MO, United States] was added to each well. After incubating for 4 h, 0.2 mL of DMSO was added to stop the reactions. The absorbance values for each well were determined spectrophotometrically at 490 nm on a Microplate Reader (BIO-TEK, Rockville, MA, United States).
The cell cycle analysis was performed as previously described[14]. Briefly, aliquots of cells (1.5 × 106) were pelleted (1300 rpm × 5 min at 4 °C) and were washed twice in ice-cold phosphate-buffered saline (PBS). The cells were fixed in 70% ethanol overnight at 4 °C, washed in PBS and digested with DNase-free RNase A (10 μg/mL) at 37 °C for 30 min. Prior to FACS analysis, the cells were resuspended in 200 μL propidium iodide (PI, 10 μg/mL; Sigma, St Louis, MO, United States) for DNA staining. A BD FACSCalibur (Becton Dickinson, Franklin Lakes, NJ, United States) flow cytometer was used to analyze cellular DNA contents.
The experimental data were expressed as the mean ± SD, and the SPSS 11.5 statistical package was used for data analysis. The data were compared using single factor analysis of variance (one way). P values < 0.05 were considered statistically significant.
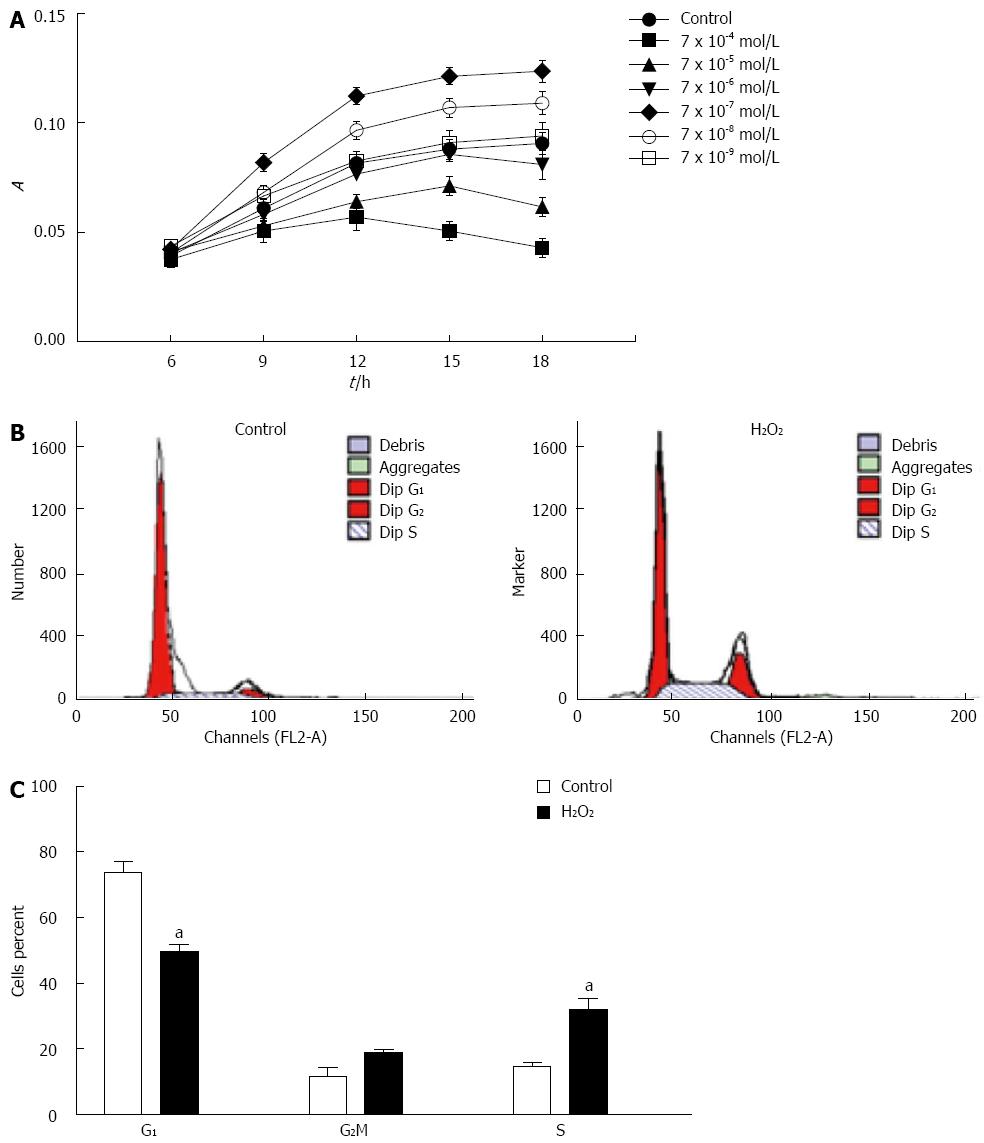
To estimate the effects of H2O2 on cell proliferation, WB-F344 cells were exposed to 7 × 10-4-7 × 10-9 mol/L H2O2 for 6, 9, 12, 15 and 18 h, respectively. Cell proliferation was evaluated using the MTT assay. Our results showed that 7 × 10-7 mol/L H2O2 promoted WB-F344 cell proliferation obviously (Figure 1A), so we used 7 × 10-7 mol/L H2O2 as a premalignant and malignant agent to induce proliferation and malignant transformation in quiescent rat hepatic oval cells. To determine whether the H2O2-induced effect on cell growth was closely related to cell cycle control, we determined the cell cycle distribution of WB-F344 cells using FACS analysis. In H2O2-exposed WB-F344 cells, the G1 phase subpopulation decreased from 73.8% to 49.6% compared with the control group, and the S phase subpopulation increased from 14.5% to 31.8% (Figure 1B and C). These results indicated that H2O2 promoted WB-F344 cell proliferation, an effect that is potentially involved in the carcinogenic effects of this ROS.
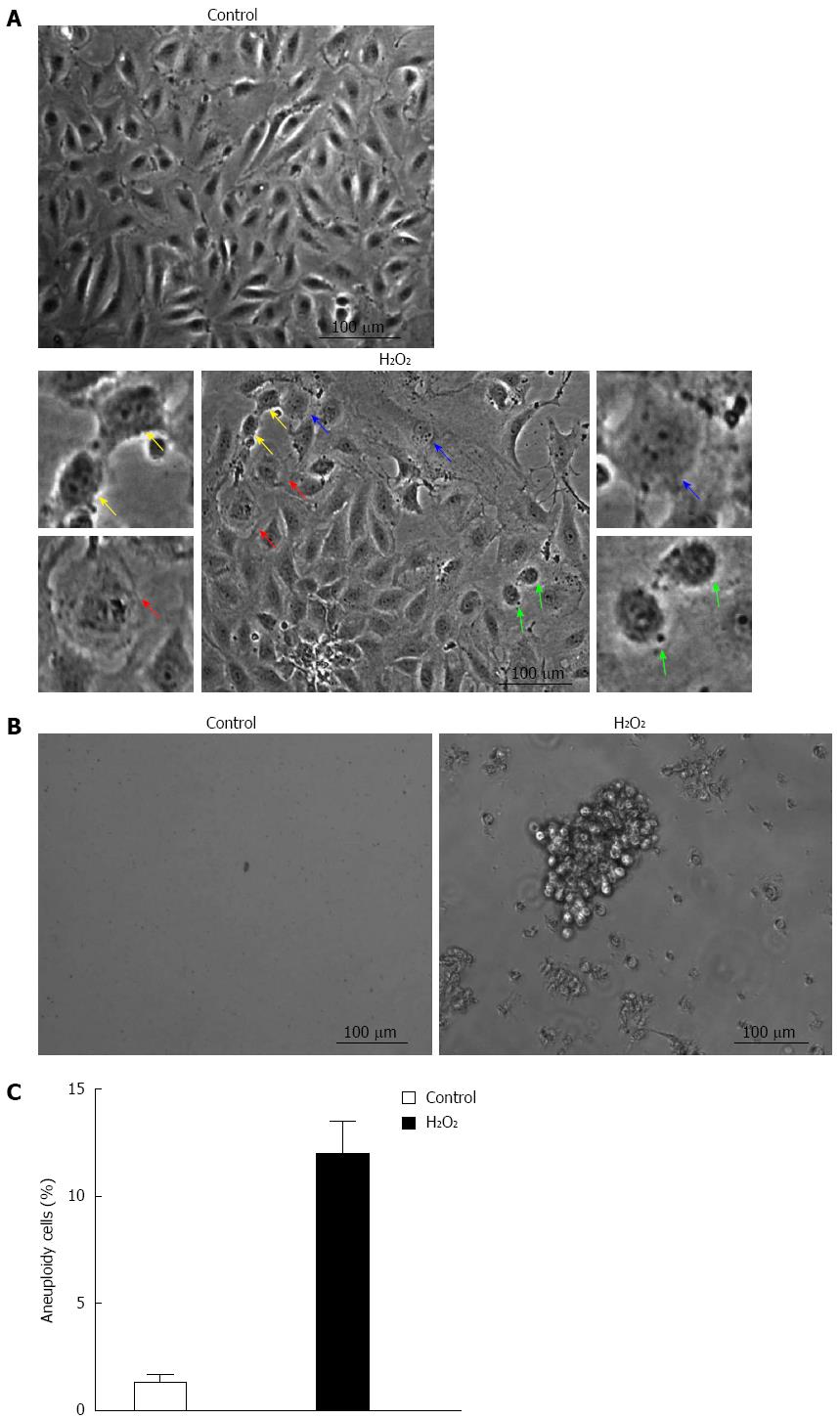
Cell morphology was observed under microscope to further investigate the H2O2-induced tumorigenicity of WB-F344 cells. The cells in the control group exhibited a regular shape and abundant cytoplasm and grew with contact inhibition. After H2O2 stimulation for 21 d, the cells became anomalous and changed in size. An increasing nucleus to cytoplasm ratio was observed (Figure 2A), as were many mitotic cells (Figure 2A), prokaryotes (Figure 2A) and even tumor giant cells (Figure 2A). Compared with normal WB-F344 cells, there was no contact inhibition between the cells, and overlapping growth was often present (Figure 2A). The cell morphologic changes indicated that H2O2 had induced the malignant transformation of WB-F344 cells. Moreover, H2O2-treated WB-F344 cells formed clones in methylcellulose medium culture (Figure 2B). These results indicate that oxidative stress plays an important role in the progression of hepatocarcinogenesis.
FACS analysis was used to examine the tumorigenicity of H2O2 in WB-F344 cells. DNA was stained with propidium iodide to analyze cellular DNA content. The population of > 4N cells represent aneuploidy cells. WB-F344 cell aneuploidy increased to 12% following H2O2 treatment (Figure 2C). Thus, H2O2 significantly induced aneuploidy in WB-F344 cells. These results suggest that H2O2 could cause the malignant transformation of WB-F344 cells.
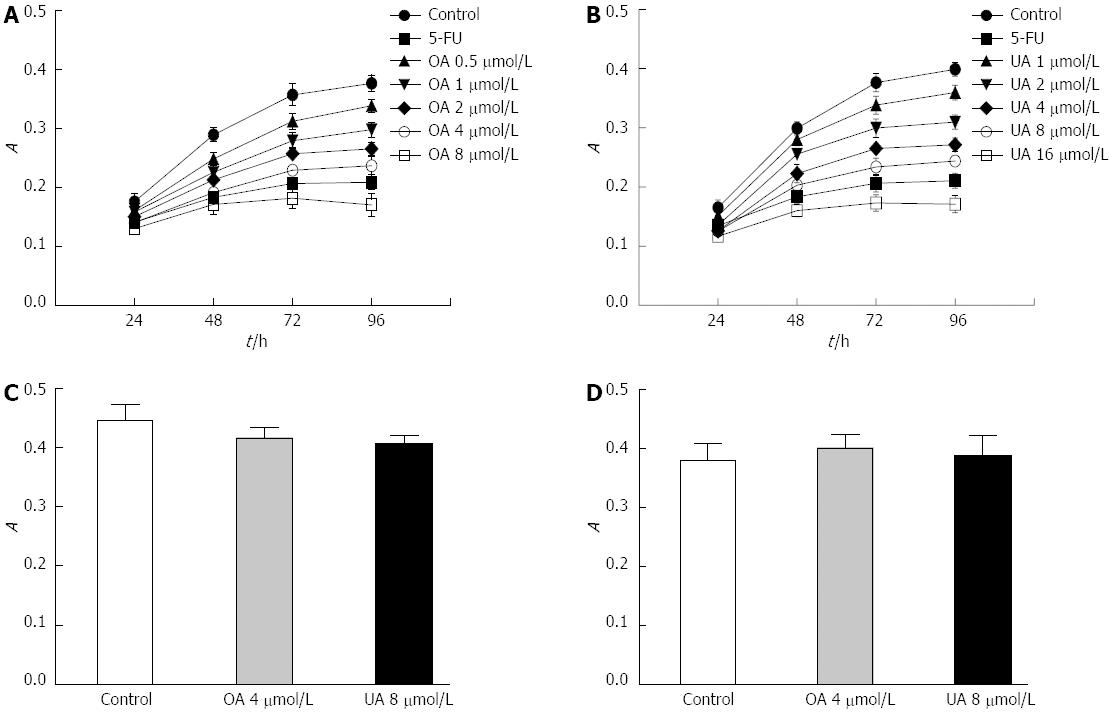
We analyzed the inhibitory effects of OA and UA on the proliferation of malignantly transformed WB-F344 cells using the MTT method. The time- and dose-effect curves revealed that 4 μmol/L OA or 8 μmol/L UA caused significant growth inhibition in malignantly transformed WB-F344 cells within 24-96 h (Figure 3A and B). Based on these results, we chose 4 μmol/L OA or 8 μmol/L UA as the final concentrations and 72 h as the drug intervention time. To estimate the genotoxicity of OA and UA, the normal rat liver cell BRL line was also exposed to 4 μmol/L OA or 8 μmol/L UA for 72 h. However, no obvious inhibitory effect on the growth of BRL cells was observed (Figure 3C). To estimate the genotoxicity of OA and UA on quiescent WB-344 cells, the quiescent WB-344 cells were also exposed to 4 μmol/L OA or 8 μmol/L UA for 72 h. Also no obvious inhibitory effect on the growth of quiescent WB-344 cells was observed (Figure 3D).
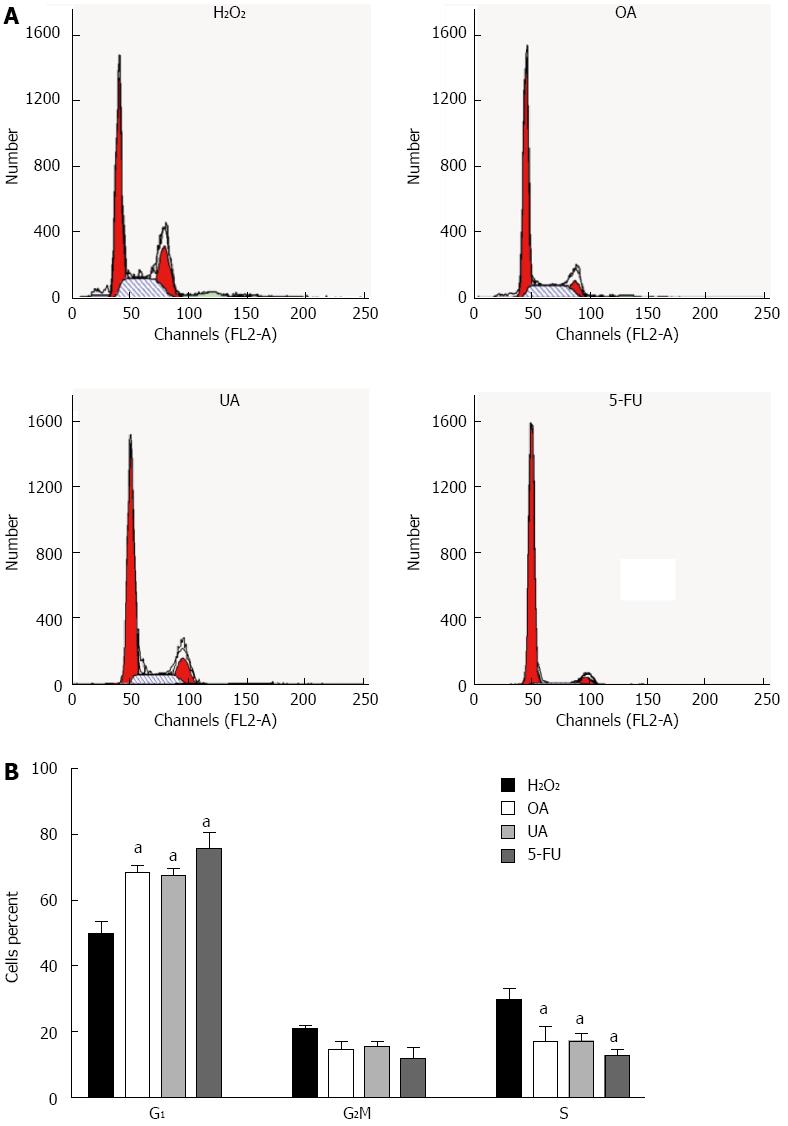
Using FACS analysis, we observed that treatment with 4 μmol/L OA increased the G1 subpopulation of H2O2-treated WB-F344 cells to 68.6% within 72 h, compared to 49.7% in unexposed cells (Figure 4A and B). In H2O2-treated WB-F344 cells, 8 μmol/L UA increased the G1 subpopulation to 67.4% at 72 h, compared to 49.7% in unexposed cells. As a positive control, 5-FU also increased the G1 subpopulation to 75.5% compared to unexposed cells (49.7%). In addition, OA, UA and 5-FU decreased the size of the G2/M phase and S phase subpopulations in transformed WB-F344 cells. These results indicate that OA and UA could inhibit DNA replication in malignantly transformed WB-F344 cells, leading to cell cycle arrest in the G1 phase.
The hypothesis that cancers arise from stem cells is currently an active research field. The main premise of this hypothesis is that cancer results from a subset of cancer stem cells that do not mature into terminally differentiated cells but instead continue to proliferate[15]. Certain cancer cells can express the same surface markers as do stem cells, providing support for this theory[16,17]. Stem cell differentiation is affected by microenvironment changes in tissues and organs. When the stem cell microenvironment changes, it is possible for stem cells to malignantly differentiate, providing a certain degree of evidence that these cells are involved in tumorigenesis[18,19]. Many studies have demonstrated that oxidative stress produces excess ROS, which can be involved in various stages of tumor development[20]. ROS, as an important microenvironment factor, can play important roles in stem cell differentiation. Oval cells are hepatic stem cells with a distinct phenotype and have been demonstrated to be a bipotential progenitor of epithelial cells that are found in the liver, namely, hepatocytes and bile duct cells[21]. In the present study, we used a low dose of H2O2 to continuously stimulate WB-F344 cells (an in vitro model of liver oval cells) to explore the effects of oxidative stress on the malignant transformation of oval cells.
The MTT colorimetric experiments led us to select the concentration of 7 × 10-7 mol/L H2O2 to stimulate WB-F344 cells. This concentration, when applied to WB-F344 cells for 12 h per day for 21 d, clearly stimulated proliferation. Specifically, the treated cells exhibited characteristics of transformed cells, e.g., mitotic activity, a loss of contact inhibition, and multinucleation, with tumor giant cells also being observed. The H2O2-treated cells were able to form colonies cultured in methylcellulose semi-solid medium, indicating that the anchorage dependence of cells was lost following H2O2 stimulation, which is an important feature of transformed cells[22]. It has been reported that cancer is a disease that is caused by a cell cycle disorder[23,24]. Mutations can be clonally amplified in the promotion, or precancerous lesion stage; therefore, cancer cells exhibit active proliferation and an increase in the S phase fraction[25]. Our results demonstrated that continuous stimulation of WB-F344 cells with H2O2 significantly increased the S phase fraction and the number of aneuploid cells. Increased aneuploidy is an important feature of tumor cells[26]. Flow cytometric analysis indicated that the proportion of hypotetraploid cells may have increased, but specific karyotype changes require further confirmation. The important features that were observed in the malignantly transformed cells provide strong evidence that the continuous stimulation of liver oval cells with H2O2 causes these cells to transform. Thus, a malignant transformation model was successfully created; however, the behavior of the transformed cells should be further confirmed by experiments in nude mice.
The prevention and treatment of liver cancer has been receiving a great deal of attention. Traditional Chinese medicines (TCMs), with their low toxicity and many targets, have huge advantages as potential treatments[27,28]. However, the pharmacological effects of TCM are complex. To further explore the mechanism of tumor suppression by TCM, the purification of the active ingredients and their use in research has become a popular area of research. UA and its isomer OA are triterpenoic acids that coexist in many Chinese herbs. There are multiple uses of TCM herbs that contain UA and OA. To further examine the role of OA and UA in hepatic precancerous lesions, we designed experiments to investigate the abnormal proliferation of oval cells in hepatic precancerous lesions.
Cell cycle checkpoint disorders are closely related to the development of tumors. Once the function of the cell cycle monitoring point is weakened, mutant genes can accumulate (i.e., the accumulation of genetic instability). Additionally, the accumulation of mutant genes can destroy the normal progress of the cell cycle, resulting in a vicious cycle. Thus, genetically modified tumor cells are not like normal cells, which are controlled by all cell cycle checkpoints, but instead exhibit uncontrolled growth[29]. One mechanism of anticancer drugs is to control the growth of tumor cells. The present study demonstrated that the cell cycle distribution changed in malignantly transformed WB-F344 cells following UA and OA treatment. The S phase fraction was significantly reduced compared with the control group, and the proportion of cells in G1 phase was significantly increased, indicating that an inhibition of cell proliferation may be involved in the anti-tumor effects of UA and OA.
We thank Dr. Geng-Tao Liu from Peking Union Medical College for providing the rat oval cell line WB-F344.
Hepatocarcinogenesis is a multistage developmental process that is divided into three stages: initiation, promotion and progression. The promotion stage is also referred to as the stage of hepatic precancerous lesions, and its principal pathological feature is oval cell hyperplasia. However, changes in the liver microenvironment can lead to the malignant transformation of oval cells during the process of hyperplasia, which is involved in the initiation and promotion of liver cancer development.
Oleanolic acid (OA) and ursolic acid (UA) naturally coexist in many plants and herbs and have been reported to possess hepatoprotective effect and anticancer activities. Based on a large number of chemical and pharmacological studies, numerous bioactive compounds have been found in Chinese medicinal plants for prevention and treatment of tumor. OA and UA as monomer drugs showed potential preventive and anti-tumor properties in this study.
It is known that H2O2 can promote tumorigenesis. Here, the authors used H2O2 as a premalignant and malignant agent to induce proliferation and malignant transformation in quiescent WB-F344 cells. Multistage carcinogenic processes provide the basis for interrupting and reversing precancerous changes; therefore, reversing precancerous changes is critical for tumor prevention and treatment. The salient and novel findings of the study are that OA and UA induced malignantly transformed WB-F344 cell arrest in the G1 phase and apparently inhibited the proliferation of these cells. These results improve their understanding of the anti-tumor effects of OA and UA.
Hepatocellular carcinoma is one of the most prevalent human malignancies. The prevention and treatment of liver cancer has been receiving a great deal of attention. Traditional Chinese medicines (TCMs), with low toxicity and many targets, have huge advantages. The separation and extraction of effective monomer from TCM is an important direction of anti-tumor drug discovery currently. OA and UA as monomer drugs show potential preventive and anti-tumor properties in hepatocarcinogenesis.
Premalignant lesion is a morphologically altered tissue or cells in which cancer is more likely to occur than its apparently normal counterpart. Malignant lesion is a state in which cancer has occurred.
The authors induced malignant transformation into rat hepatic oval cells WB-F344 by low-dose H2O2 treatment and evaluated the effects of OA and UA. Overall, the data is convincing. The manuscript describes the effect of the phytochemicals OA and UA on the malignant phenotype of tumor cells. The authors analyzed cell morphology and colony formation rates. The data presented in this manuscript is with great interest.
P- Reviewers: Khatib AM, Sarkar D S- Editor: Ma YJ L- Editor: Ma JY E- Editor: Zhang DN
| 1. | Pitot HC. Stage-specific gene expression during hepatocarcinogenesis in the rat. J Cancer Res Clin Oncol. 1996;122:257-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, Reddy BS. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597-601. [PubMed] |
| 3. | Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3’-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142-148. [PubMed] |
| 4. | Mishra L, Banker T, Murray J, Byers S, Thenappan A, He AR, Shetty K, Johnson L, Reddy EP. Liver stem cells and hepatocellular carcinoma. Hepatology. 2009;49:318-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 5. | Sell S. On the stem cell origin of cancer. Am J Pathol. 2010;176:2584-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 6. | Nakae D, Umemura T, Kurokawa Y. Reactive Oxygen and Nitrogen Oxide Species-induced Stress, a Major Intrinsic Factor Involved in Carcinogenic Processes and a Possible Target for Cancer Prevention. Asian Pac J Cancer Prev. 2002;3:313-318. [PubMed] |
| 7. | Oberley LW, Oberley TD, Buettner GR. Cell division in normal and transformed cells: the possible role of superoxide and hydrogen peroxide. Med Hypotheses. 1981;7:21-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Shi ZM, Feng P, Jiang DQ, Wang XJ. Mistletoe alkali inhibits peroxidation in rat liver and kidney. World J Gastroenterol. 2006;12:4052-4055. [PubMed] |
| 9. | Shi GP, Li Y, Wang QY, Wu YD. [Role of hydrogen peroxide in promoting proliferation and transformation of rat liver oval cell line WB-F344]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2001;23:346-350. [PubMed] |
| 10. | Zhang G, Qi Y, Lou Z, Liu C, Wu X, Chai Y. Determination of oleanolic acid and ursolic acid in cornel by cyclodextrin-modified micellar electrokinetic chromatography. Biomed Chromatogr. 2005;19:529-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Yin MC, Chan KC. Nonenzymatic antioxidative and antiglycative effects of oleanolic acid and ursolic acid. J Agric Food Chem. 2007;55:7177-7181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Cui T, Li JZ, Kayahara H, Ma L, Wu LX, Nakamura K. Quantification of the polyphenols and triterpene acids in chinese hawthorn fruit by high-performance liquid chromatography. J Agric Food Chem. 2006;54:4574-4581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Jia XZ, Yang SY, Zhou J, Li SY, Ni JH, An GS, Jia HT. Inhibition of CHK1 kinase by Gö6976 converts 8-chloro-adenosine-induced G2/M arrest into S arrest in human myelocytic leukemia K562 cells. Biochem Pharmacol. 2009;77:770-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Zhang HY, Gu YY, Li ZG, Jia YH, Yuan L, Li SY, An GS, Ni JH, Jia HT. Exposure of human lung cancer cells to 8-chloro-adenosine induces G2/M arrest and mitotic catastrophe. Neoplasia. 2004;6:802-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Sell S, Leffert HL. Liver cancer stem cells. J Clin Oncol. 2008;26:2800-2805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Ishikawa K, Sasaki A, Haraguchi N, Yoshikawa Y, Mori M. A case of an alpha-fetoprotein-producing intrahepatic cholangiocarcinoma suggests probable cancer stem cell origin. Oncologist. 2007;12:320-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Crowe DL, Parsa B, Sinha UK. Relationships between stem cells and cancer stem cells. Histol Histopathol. 2004;19:505-509. [PubMed] |
| 18. | Zhu Y, Liu T, Ye H, Song K, Ma X, Cui Z. Enhancement of adipose-derived stem cell differentiation in scaffolds with IGF-I gene impregnation under dynamic microenvironment. Stem Cells Dev. 2010;19:1547-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Yan X, Liu Y, Han Q, Jia M, Liao L, Qi M, Zhao RC. Injured microenvironment directly guides the differentiation of engrafted Flk-1(+) mesenchymal stem cell in lung. Exp Hematol. 2007;35:1466-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 919] [Cited by in RCA: 965] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 21. | Gennero L, Roos MA, Sperber K, Denysenko T, Bernabei P, Calisti GF, Papotti M, Cappia S, Pagni R, Aimo G. Pluripotent plasticity of stem cells and liver repopulation. Cell Biochem Funct. 2010;28:178-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Condon MS, Kaplan LA, Crivello JF, Horton L, Bosland MC. Multiple pathways of prostate carcinogenesis analyzed by using cultured cells isolated from rats treated with N-methyl-N-nitrosourea and testosterone. Mol Carcinog. 1999;25:179-186. [PubMed] |
| 23. | Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1494] [Cited by in RCA: 1405] [Article Influence: 93.7] [Reference Citation Analysis (0)] |
| 24. | Mateen S, Tyagi A, Agarwal C, Singh RP, Agarwal R. Silibinin inhibits human nonsmall cell lung cancer cell growth through cell-cycle arrest by modulating expression and function of key cell-cycle regulators. Mol Carcinog. 2010;49:247-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Jakesz R, Smith CA, Aitken S, Huff K, Schuette W, Shackney S, Lippman M. Influence of cell proliferation and cell cycle phase on expression of estrogen receptor in MCF-7 breast cancer cells. Cancer Res. 1984;44:619-625. [PubMed] |
| 26. | Fang X, Zhang P. Aneuploidy and tumorigenesis. Semin Cell Dev Biol. 2011;22:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Li X, Shi ZM, Feng P, Wen ZY, Wang XJ. Effect of Qi-protecting powder (Huqi San) on expression of c-jun, c-fos and c-myc in diethylnitrosamine-mediated hepatocarcinogenesis. World J Gastroenterol. 2007;13:4192-4198. [PubMed] |
| 28. | Wen Z, Shi Z, Feng P, Xue X, Dong K, Wang X. Modulation of energy metabolic enzyme expression in N-nitrosodiethylamine-mediated hepatocarcinogenesis by Chinese herbs, Huqi San. Biofactors. 2008;34:303-312. [PubMed] [DOI] [Full Text] |