Published online Sep 28, 2014. doi: 10.3748/wjg.v20.i36.13167
Revised: April 14, 2014
Accepted: June 14, 2014
Published online: September 28, 2014
Processing time: 245 Days and 13.8 Hours
AIM: To assess the value of D-dimer level in determining resectability of pancreatic cancer.
METHODS: Preoperative prediction of pancreatic head cancer resectability remains inaccurate. The use of hemostatic factors may be of potential help, since D-dimers correlate with tumor stage. Single center clinical trial study comprised patients with potentially resectable pancreatic head tumor and without detectable venous thrombosis (n = 64). Resectability was defined as no evidence of nodal involvement, distant spread and no invasion of mesenteric vessels. Final decision of resectability was confirmed intraoperatively. Experienced pancreatic surgeon performed all surgeries. Following the dissection of hepatoduodenal ligament, samples of portal blood and bile were taken. Peripheral blood via central line and urine via Foley catheter were sampled. D-dimer levels were further measured.
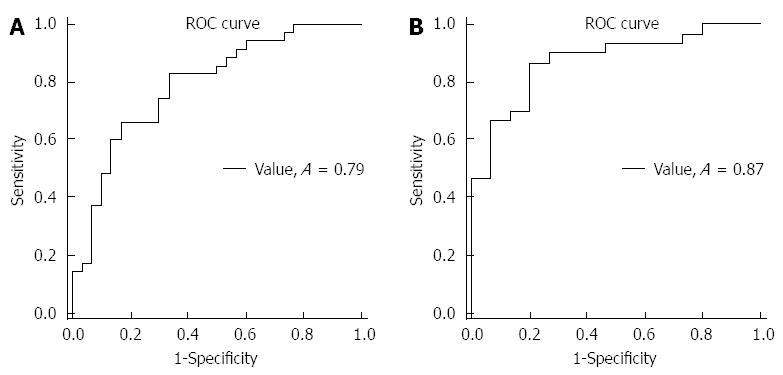
RESULTS: At laparotomy only 29 (45.3%) tumors were found to be resectable. Our analysis showed higher by 57.5% (P < 0.001) mean D-dimer values in peripheral and 43.7% (P = 0.035) in portal blood of patients with unresectable pancreatic cancer. Significant differences were not observed when analyzing D-dimer levels in bile and urine. Peripheral D-dimer level correlated with pancreatic cancer resectability. When cut-off D-dimer value of 570.6 μg/L was used, the sensitivity for assessment of tumor unresectability was 82.8%. Furthermore, D-dimer level in peripheral blood of metastatic disease (n = 15) was significantly higher when compared to locally advanced (n = 20) pancreatic cancer (2470 vs 1168, P = 0.029). The area under ROC curve for this subgroup of patients was 0.87; for determination of unresectable disease when threshold of 769.8 μg/L was used, sensitivity and specificity was 86.6% and 80%, respectively.
CONCLUSION: Patients with resectable pancreatic head cancer based on preoperative imaging studies and high D-dimer level may be considered unresectable due to occult hepatic metastases. These patients may benefit from diagnostic laparoscopy to avoid exploratory laparotomy.
Core tip: High D-dimer level in patients with resectable pancreatic head cancer based on preoperative imaging studies predicts that tumor may be considered unresectable due to occult hepatic metastases. However, further multi-center studies are needed on high D-dimers in pancreatic head cancer and preoperative clinical decision making and surgical treatment. If verified, D-dimer testing should be performed in all subjects with pancreatic cancer.
- Citation: Durczynski A, Kumor A, Hogendorf P, Szymanski D, Grzelak P, Strzelczyk J. Preoperative high level of D-dimers predicts unresectability of pancreatic head cancer. World J Gastroenterol 2014; 20(36): 13167-13171
- URL: https://www.wjgnet.com/1007-9327/full/v20/i36/13167.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i36.13167
The prognosis of pancreatic adenocarcinoma is poor, with the lowest overall long-term survival rate of any cancers[1]. Radical resection represents the only chance for a cure, but only 15% of all pancreatic tumors are indicated for surgery at presentation[2]. The accuracy of preoperative staging for the unresectability, including contrast enhanced computed tomography (CT) and magnetic resonance imaging is high, but prediction of resectability is much less accurate[3]. Undetected, occult hepatic metastases and incorrect estimation of vascular involvement, are mainly responsible for this inaccuracy. Therefore, about half of patients previously qualified for the radical surgery are found to be unsuitable for resection during surgical exploration[4]. Exploratory laparotomy for these patients does not provide a survival advantage, and can be associated with significant morbidity and mortality[5]. Recovery from unnecessary laparotomy further delays palliative systemic chemotherapy. Therefore, these patients would be more likely designated for less invasive procedures including endoscopic biliary or duodenal stenting[6].
Improved diagnostic tests for optimized preoperative evaluation of pancreatic cancer resectability are needed. The use of hemostatic factors may be of potential help, since patients with cancer are characterized by higher tendency to hypercoagulability. Pancreatic cancer is among the most common malignancies associated with this phenomenon. Therefore, increasing attention has been focused on plasma D-dimer level in patients with pancreatic cancer. D-dimers are specific final degradation products of cross-linked fibrin. Up to date, D-dimer levels have proved to play important role in tumor spread and distant metastases and showed better correlation with tumor stage and unfavorable prognosis in patients with pancreatic cancer[7,8] than the others clinically widely used classic tumor markers.
In our previous study, we showed that this correlation may be even stronger when portal blood D-dimers are assessed[9]. However, to date, no study, to our best knowledge, has correlated D-dimer levels with the resectability of pancreatic cancer.
The aim of this study was to assess the value of the levels of D-dimers in portal and peripheral blood, bile and urine in determining unresectability of pancreatic cancer, in patients who were qualified for radical surgery by preoperative radiographic studies.
This single center clinical trial study comprised patients with pancreatic head tumor (n = 64) and without detectable venous thrombosis, whose disease was deemed resectable by imaging studies, scheduled for pancreatoduodenectomy in the Department of General and Transplant Surgery of Medical University in Lodz, Poland. All our patients underwent spiral CT with intravenous contrast enhancement, since it remains the single most useful method for diagnosis of pancreatic adenocarcinoma. Computed tomography is the imaging modality of choice because it is widely available and the best validated tool for staging of pancreatic adenocarcinoma[10,11].
All patients provided written informed consent. Ethical approval for this study (Ethical Committee No RNN/367/12/KB) was provided by the Ethical Committee of Medical University of Lodz, Poland.
Resectability was defined as no evidence of nodal involvement, distant spread and no invasion of mesenteric vessels. Final decision of pancreatic cancer resectability was confirmed intraoperatively. Experienced pancreatic surgeon performed all surgeries. Surgery was performed through a right subcostal laparotomy. When tumor was found unresectable and radical surgical management (Whipple procedure) was not possible, triple by-pass or gastrojejunostomy (in patients with biliary stent inserted to common bile duct) were performed. Following the dissection of hepatoduodenal ligament, samples of portal blood and bile were taken, as described earlier[12,13]. Peripheral blood via central line and urine via Foley catheter were sampled. D-dimer levels (VIDAS D-Dimer Exclusion II, bioMérieux, France) were further measured. Pancreatic cancer was confirmed by pathological examination in all cases. Doppler ultrasonography was used for the exclusion of deep venous thrombosis.
To compare the differences in D-dimer levels between resectable and unresectable pancreatic cancer we applied non-parametric Mann-Whitney test. Receiver operating characteristics (ROC) curves were constructed. The sensitivity and specificity of tumor unresectability were calculated for optimal operating point. All statistical calculations were performed using SigmaPlot version 12.0 (Systat Software Inc., San Jose, CA) with the level of statistical significance P < 0.05.
Despite the fact that all patients were shown by CT staging to have resectable disease, at laparotomy only 29 (45.3%) tumors were found to be resectable. In all these patients the final pathological examination confirmed tumor-free margins of resection. Of the remaining group, 15 patients were diagnosed with liver metastases and 20 patients had locally unresectable tumor. Our analysis showed higher by 57.5% (P < 0.001) mean D-dimer values in peripheral and by 43.7% (P = 0.035) in portal blood of patients with unresectable when compared with resectable pancreatic cancer. Additionally, we did not observe such differences when analyzing D-dimer levels in bile and urine (Table 1). Analysis in subgroups revealed considerably high peripheral D-dimers level in patients with liver metastases (2470.7 ± 3028.8, P < 0.001), and moderately elevated in locally advanced tumors (904.2 ± 662.9, P = 0.013) when compared to resectable disease (630.9 ± 593.8).
| Samples | Resectable (n = 29) | Unresectable (n = 35) | P value | ||
| mean ± SD | Median | mean ± SD | Median | ||
| Peripheral blood D-dimer level (μg/L) | 630.9 ± 593.8 | 476.1 | 1726.4 ± 2283.9 | 1009.3 | < 0.001 |
| Bile D-dimer level (μg/L) | 2385.6 ± 3206 | 750 | 2745.6 ± 3697.7 | 870.2 | 0.947 |
| Urine D-dimer level (μg/L) | 154.5 ± 343.6 | 80.4 | 86.8 ± 64.9 | 55.7 | 0.398 |
| Portal blood D-dimer level (μg/L) | 2856.9 ± 3699.5 | 726.5 | 4106.9 ± 3965.1 | 2243.5 | 0.035 |
The area under ROC curve for peripheral blood was 0.78 (Figure 1A). This suggested that D-dimer level may have direct relation with pancreatic cancer resectability. When optimal cut-off value was accepted as 570.6 μg/L the sensitivity and specificity for assessment of tumor unresectability was 82.8% and 66.6%, respectively (Table 2).
| Sample | ROC curve area | Standard error | Optimal operating point | Sensitivity | Specificity |
| Peripheral blood | 0.78 | 0.05 | 570 | 82.6% | 66.6% |
| Portal blood | 0.65 | 0.07 | 604 | 44.8% | 85.7% |
| Bile | 0.50 | 0.08 | 810 | 60.7% | 56.5% |
| Urine | 0.43 | 0.08 | 130 | 17.8% | 91.3% |
Interestingly, the D-dimer level in peripheral blood of metastatic disease (n = 15) was significantly higher when compared to locally advanced (n = 20) pancreatic cancer (2470.7 ± 3028.81 vs 1168.2 ± 1345.4, respectively, P = 0.029). The area under ROC curve for this subgroup of patients was higher (ROC curve area was 0.87 vs 0.78 for all unresectable cases) with sensitivity of 86.6% and specificity 80% for cut-off value was 769.8 μg/L (Figure 1B).
Idiopathic venous thromboembolism as a paraneoplastic phenomenon, was first described by Trousseau in 1865 year (Trousseau syndrom). Pancreatic cancer is among the most common malignancies associated with hypercoagulability as thrombosis occurs in 50% of patients[14]. The initiation of coagulation is suggested to be the result of increased expression of tissue factor, that may play a central role in pancreatic cancer progression, as well[15]. Its overexpression was proved in pancreatic cancer cells[16], with subsequent fibrin biofilm formation surrounding cancer. Fibrin deposits may act as a scaffold for progressing tumor preventing the cancer cells from being killed by host immune system[16]. This results in increase of plasma D-dimers, that may be consider as a biomarker indicating malignant tumor. Up to date, high plasma D-dimers in patients with cancer and without detectable thrombosis has been proved to be associated with tumor staging and poor postoperative survival rate[7].
The current study found elevated concentration of D-dimers in all studied samples. However, ROC curves analysis showed that only peripheral blood D-dimer levels may be related with pancreatic cancer unresectability. Our study showed, that elevated peripheral D-dimers in patients with pancreatic head tumors found by radiologists to be potentially resectable, may indicate presence of occult metastases in the liver. Previous studies showed that almost one-fourth of all patients have hepatic micrometastases undetected preoperatively[17]. Negative laparotomy in this group of patients may be related to significant perioperative morbidity and decreased quality of life postoperatively[18]. Minimally invasive surgery may help avoid unnecessary diagnostic laparotomies. Laparoscopic staging with the addition of laparoscopic ultrasonogaphy enhances the ability to determine resectability of pancreatic tumors[19-25]. However, currently there is no data addressing factors found in preoperative studies, that may predict positive laparoscopy. We would suggest that patients with primary resectable pancreatic cancer and high peripheral D-dimer level without detectable thrombosis, should undergo diagnostic laparoscopy and/or laparoscopic ultrasound to assess resectability approaches. This specific group of patients may harbor occult metastases in the liver, undetectable by preoperative CT.
In our previous study in non-metastatic pancreatic cancer patients[12], we found a very high concentration of D-dimer levels in portal, but only moderately elevated values in peripheral blood. Therefore, we hypothesized that liver may act as a specific “levee” for D-dimers of portal blood draining directly pancreatic tumor. We cannot exclude that in metastatic pancreatic cancer, D-dimers by-pass the hepatic sieve and its concentration becomes very high in general circulation blood indicating tumor unresectability. Liver contribution in D-dimer clearance needs to be clarified. Interestingly, bile D-dimer levels were very high in all patients with pancreatic cancer (Table 1). Further studies are definitely needed to elucidate if elevated bile D-dimer levels can be considered as a biomarker of pancreatic cancer, likewise, CEA and CA 19-9 in patients with cholangiocarcinoma[26].
Renal excretion is the dominant D-dimers elimination pathway. The reasonable question is whether the high plasma D-dimer levels in unresectable cancer is due to increased production, or to reduced renal clearance of D-dimers from the circulation. The authors measured random urine D-dimer levels. Surprisingly, urinary D-dimer levels were lower in patients with unresectable pancreatic cancer when compared with resectable cases (Table 1). Despite the fact that baseline renal function was comparable in both our groups, our results may suggest that very high D-dimer levels recorded in unresectable cases, may be at least partly due to slower renal clearance. This raises a question of whether a ratio of plasma and urine D-dimer level is more informative then an isolated measurement. Further studies need to be carried out to find out kinetics of D-dimers in pancreatic cancer patients.
In conclusion, we propose that D-dimer testing should be done in clinical setting in all patients with pancreatic cancer. Elevated preoperative D-dimers may predict unresectability of pancreatic head tumor. Peripheral blood D-dimers is of minor relevance in determining local advancement of pancreatic cancer, however, it may be effectively used in the diagnostic algorithm for occult hepatic metastases exclusion before surgery.
Preoperative prediction of pancreatic head cancer resectability remains inaccurate. The use of hemostatic factors may be of potential help, since D-dimers correlate with tumor stage.
The present study demonstrates that preoperative serum D-dimers can be used for the prediction of resectability in patients with pancreatic head adenocarcinoma, which may enable a simple and cost-effective exclusion of such patients who are unlikely to benefit from surgery.
This is the first paper worldwide which highly precisely describe D-dimers in portal and peripheral blood, bile and urine in determining unresectability of pancreatic cancer, in patients who are qualified for radical surgery by preoperative radiographic studies.
The study results suggest that D-dimer testing is to be done in clinical setting, in all subjects with pancreatic cancer. Peripheral blood D-dimers may be effectively used in the diagnostic algorithm for hepatic metastases exclusion before surgery. In patients with resectable pancreatic cancer and high peripheral blood D-dimers diagnostic laparoscopy should be first line therapy.
Such a simple and cheap lab test, like D-dimer test is very useful to decide by operability in case of pancreatic cancer. If hepatic micrometastases occur the life expectancy is lower so the major operation (i.e., Whipple) should be avoided. This test could be one of the part of triage in decision.
P- Reviewer: Furka A, Noguera J, Romani A, Sonzogn A, Symeonidis NG, Xu JM S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8283] [Cited by in RCA: 8225] [Article Influence: 483.8] [Reference Citation Analysis (0)] |
| 2. | Moon HJ, An JY, Heo JS, Choi SH, Joh JW, Kim YI. Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas. 2006;32:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Morgan DE, Waggoner CN, Canon CL, Lockhart ME, Fineberg NS, Posey JA, Vickers SM. Resectability of pancreatic adenocarcinoma in patients with locally advanced disease downstaged by preoperative therapy: a challenge for MDCT. AJR Am J Roentgenol. 2010;194:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Chiang KC, Yeh CN, Ueng SH, Hsu JT, Yeh TS, Jan YY, Hwang TL, Chen MF. Clinicodemographic aspect of resectable pancreatic cancer and prognostic factors for resectable cancer. World J Surg Oncol. 2012;10:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Mann O, Strate T, Schneider C, Yekebas EF, Izbicki JR. Surgery for advanced and metastatic pancreatic cancer--current state and perspectives. Anticancer Res. 2006;26:681-686. [PubMed] |
| 6. | Mutignani M, Tringali A, Shah SG, Perri V, Familiari P, Iacopini F, Spada C, Costamagna G. Combined endoscopic stent insertion in malignant biliary and duodenal obstruction. Endoscopy. 2007;39:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Ay C, Dunkler D, Pirker R, Thaler J, Quehenberger P, Wagner O, Zielinski C, Pabinger I. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 244] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 8. | Oya M, Akiyama Y, Okuyama T, Ishikawa H. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol. 2001;31:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Nagy Z, Horváth O, Kádas J, Valtinyi D, László L, Kopper B, Blaskó G. D-dimer as a potential prognostic marker. Pathol Oncol Res. 2012;18:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Bipat S, Phoa SS, van Delden OM, Bossuyt PM, Gouma DJ, Laméris JS, Stoker J. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma: a meta-analysis. J Comput Assist Tomogr. 2005;29:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Wong JC, Raman S. Surgical resectability of pancreatic adenocarcinoma: CTA. Abdom Imaging. 2010;35:471-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Durczyński A, Szymański D, Nowicki M, Hogendorf P, Poznańska G, Strzelczyk J. Very high concentration of D-dimers in portal blood in patients with pancreatic cancer. Pol Przegl Chir. 2012;84:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Hogendorf P, Durczyński A, Kumor A, Strzelczyk J. Prostaglandin E2 (PGE2) in portal blood in patients with pancreatic tumor--a single institution series. J Invest Surg. 2012;25:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004;5:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, Key NS, Barcel DA, Scheithauer W, Kornek G. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 748] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 17. | Jimenez RE, Warshaw AL, Rattner DW, Willett CG, McGrath D, Fernandez-del Castillo C. Impact of laparoscopic staging in the treatment of pancreatic cancer. Arch Surg. 2000;135:409-14; discussion 414-5. [PubMed] |
| 18. | Camacho D, Reichenbach D, Duerr GD, Venema TL, Sweeney JF, Fisher WE. Value of laparoscopy in the staging of pancreatic cancer. JOP. 2005;6:552-561. [PubMed] |
| 19. | Muniraj T, Barve P. Laparoscopic staging and surgical treatment of pancreatic cancer. N Am J Med Sci. 2013;5:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Pisters PW, Lee JE, Vauthey JN, Charnsangavej C, Evans DB. Laparoscopy in the staging of pancreatic cancer. Br J Surg. 2001;88:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Vollmer CM, Drebin JA, Middleton WD, Teefey SA, Linehan DC, Soper NJ, Eagon CJ, Strasberg SM. Utility of staging laparoscopy in subsets of peripancreatic and biliary malignancies. Ann Surg. 2002;235:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Callery MP, Strasberg SM, Doherty GM, Soper NJ, Norton JA. Staging laparoscopy with laparoscopic ultrasonography: optimizing resectability in hepatobiliary and pancreatic malignancy. J Am Coll Surg. 1997;185:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Appel BL, Tolat P, Evans DB, Tsai S. Current staging systems for pancreatic cancer. Cancer J. 2012;18:539-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Maemura K, Shinchi H, Mataki Y, Kurahara H, Hayashi T, Kuwahata T, Sakoda M, Ueno S, Takao S, Natsugoe S. Advanced staging laparoscopy using single-incision approach for unresectable pancreatic cancer. Surg Laparosc Endosc Percutan Tech. 2011;21:e301-e305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Gaujoux S, Allen PJ. Role of staging laparoscopy in peri-pancreatic and hepatobiliary malignancy. World J Gastrointest Surg. 2010;2:283-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Alvaro D. Serum and bile biomarkers for cholangiocarcinoma. Curr Opin Gastroenterol. 2009;25:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |