Published online Aug 21, 2014. doi: 10.3748/wjg.v20.i31.10895
Revised: April 10, 2014
Accepted: May 25, 2014
Published online: August 21, 2014
Processing time: 182 Days and 11.1 Hours
AIM: To evaluate cortisolemia by using conventional electrochemiluminescence immunoassay (ECLIA) method compared to liquid chromatography-tandem mass spectrometry (LC-MS/MS) method in active ulcerative colitis (UC) patients treated with oral prednisone (PD).
METHODS: Twenty patients (12 males) with acute relapse of UC started oral PD at a dose of 40 mg once a day, tapered of 10 mg every 2 wk. When a stable 2-wk daily dose of 30 mg was reached, blood samples for cortisol levels’ measurement were drawn in the morning in fasting conditions to determine circulating cortisol by LC-MS/MS and ECLIA assay.
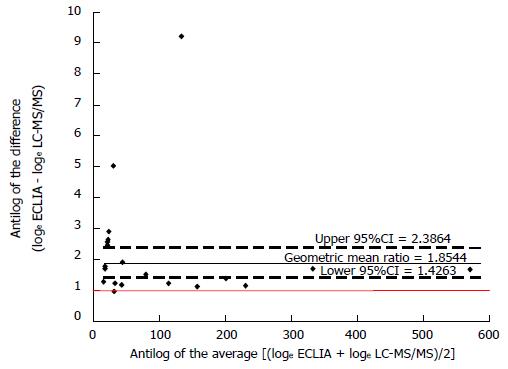
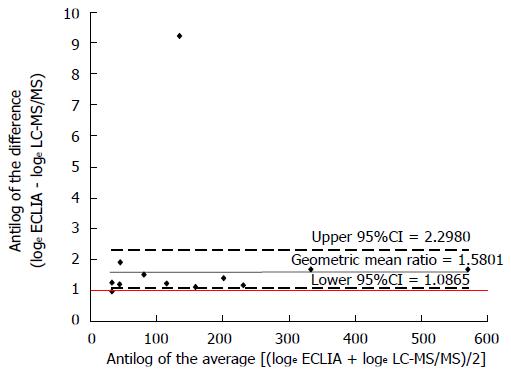
RESULTS: Median interquartile range cortisolemia with ECLIA and LC-MS/MS method was 54.1 (185.8) nmol/L and 32.1 (124.0) nmol/L, respectively (P < 0.001). The within-patient median differences between the two methods was 23.2 (40.6) nmol/L, with higher cortisol levels for the ECLIA method. The estimated geometric mean ratio between methods was 1.85 (95%CI: 2.39-1.43) considering all data or 1.58 (95%CI: 2.30-1.09) considering only data above the limit of quantification (n = 12). The 95%CIs of the geometric mean ratio between methods confirm a statistically significant difference.
CONCLUSION: Blood cortisol levels detected with ECLIA method seems to be higher than the ones measured by LC-MS/MS, indicating a possible overestimation of them in patients treated with PD. Therefore, the cortisol suppression in patients under treatment with oral PD should not be measured using ECLIA method.
Core tip: The determination of morning cortisol levels is used in clinical practice and as specific safety endpoint in various clinical trials. This study was designed to compare the efficacy of electrochemiluminescent assays (ECLIA) method and liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for measurement of cortisolemia in active ulcerative colitis (UC) patients treated with oral prednisone (PD). Blood cortisol levels detected with ECLIA method are higher than the ones measured by LC-MS/MS, indicating a possible overestimation of them in UC patients treated with PD. Therefore, the cortisol suppression in patients under treatment with oral PD should not be measured using ECLIA method.
- Citation: Manguso F, Bennato R, Lombardi G, Viola A, Riccio E, Cipolletta L. Electrochemiluminescence immunoassay method underestimates cortisol suppression in ulcerative colitis patients treated with oral prednisone. World J Gastroenterol 2014; 20(31): 10895-10899
- URL: https://www.wjgnet.com/1007-9327/full/v20/i31/10895.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i31.10895
Glucocorticosteroids (GS) are drugs of choice for the management of ulcerative colitis (UC) if symptoms of active colitis do not respond to mesalazine[1]. Patients undergoing repeated administrations of GS may experience a variety of adverse events (AEs), some of which of concerning clinical relevance[2]. Among them, morning cortisol levels below the lower limit of the normal range during treatment may represent the suppression of hypothalamic-pituitary-adrenal (HPA) axis, and is used as specific safety endpoint in the various clinical trials concerning the treatment with GS[3,4].
In the majority of earlier studies, cortisol levels in human biological samples has been determined by immunoassay methods, first radio-immunoassay (RIA), followed by enzyme-linked immunosorbent assay (ELISA), then automated electrochemiluminescent assays (ECLIA) and more recently by liquid chromatographic methods coupled with mass spectrometry detection (LC-MS/MS)[5]. Immunoassays can be affected by cross-reactivity from other steroids and the use of drugs containing GS can positively affect the methods[5,6], depending on the degree of the cross reactivity with the particular assay. Notably, prednisone (PD) and its metabolites are chemically similar to serum cortisol and may strongly interfere with cortisol measurements by immunoassay methods[6]. Analytical methods using chromatographic separation of cortisol from PD and its metabolites, such as LC-MS/MS, may avoid problems of interference[7].
Concentrations of cortisol may be overestimated by using conventional ECLIA method in samples from UC patients who are treated with PD. Therefore, the aim of the study was to compare the blood cortisol levels measured using ECLIA and a selective LC-MS/MS method in samples collected from patients with UC treated with oral PD.
In a prospective study (ELICA Study: ACTRN12610000425099) 20 patients of both sexes, aged between 18 and 70 years, with acute relapse of UC uncontrolled with mesalazine alone were included. All patients had an extension of the disease above the rectum and indication for systemic CS treatment. The exclusion criteria were proctitis, ongoing local (enema) or systemic treatment with CS within 3 mo, low compliance to medical treatment and presence of other diseases or treatments that are known to interfere with the evaluation of blood cortisol concentrations as a measure of HPA axis.
The study included a screening visit for study presentation and signature of informed consent, collection of demographic data, medical history and concomitant treatment, together with physical examination and vital signs, inclusion/exclusion criteria check and assignment of a patient identification number. The extension of UC was reported according to Montreal classification[8].
Following a minimum of 3-d run-in period, inclusion/exclusion criteria were confirmed and oral PD treatment (Deltacortene®, 5 and 25 mg tablets, Bruno Farmaceutici, Italy) was started at a dose of 40 mg once a day with a decalage of 10 mg every 2 wk. An appointment for blood sampling visit was given at the end of the first 4 wk of treatment, when a stable 2-wk daily dose of 30 mg was reached.
Blood sampling visit included the recording of steroid-related AEs and blood drawn of about 10-12 mL (about 8 mL in serum vials and about 2-4 mL in EDTA-coated vials) in fasting conditions at a 24-h distance from last PD dose (between 8 AM and 9 AM).
For serum preparation, blood samples were collected by direct venipuncture or via an indwelling cannula in the forearm into the serum vacutainer coagulating for 30 min, protected from light, and centrifuged for 10 min at 1300 g/room temperature. Afterwards, serum was transferred each 1.5 mL of the clear supernatant into two sample tubes using a disposable pipette. For plasma preparation, blood samples were collected into vacuum tubes containing EDTA as anticoagulant, and centrifuged within 15 min after collection. The plasma was separated in a centrifuge at 1500 g for 10 min. After each centrifugation, the supernatant (plasma) was dispensed in labeled polypropylene tubes, using Pasteur pipettes. The plasma was dispensed equally between the two polypropylene tubes (minimum of 1 mL of plasma per tube). Once collected, the plasma samples were immediately stored vertically below -20 °C.
Serum (from untreated blood) was used to determine circulating cortisol by ECLIA assay, and plasma (from EDTA-treated blood) was used to determine circulating cortisol by LC-MS/MS assay. One aliquot of serum and one aliquot of plasma were stored as backup for possible re-analysis. Specimens were stored at -20 °C at the investigational site until shipment to two different laboratories for cortisol assay. The analytical work was conducted in two specialized laboratories. Plasma specimens were shipped to the laboratory of the Mass Spectrometry Division at SGS Life Science Services, Wavre, Belgium, while serum specimens to the laboratories at INTERLAB GmbH, Munich, Germany. Biological samples were sent to the laboratories conducting the assays in insulated containers filled with a sufficient amount of coolant material. In all cases, cortisol concentrations were reported in units of nmol/L, and values < 171.1 nmol/L were considered below normal.
Serum cortisol was measured using the Cortisol assay (Roche Diagnostics) following the manufacturer’s instruction[9]. Serum cortisol concentrations were determined using an electrochemiluminescence immunoassay on a Roche Cobas analyzer. The lower and upper limits of measurements were 0.5 and 1750 nmol/L, respectively.
Plasma cortisol was measured by SGS Life Science Services with a validated LC-MS/MS method. The methodology and its validation are described in SGS Life Science Services’ validation report no. B090806 entitled ‘Validation of a LC-MS/MS method for the determination of cortisol in EDTA human plasma’. Cortisol levels were detected using an Api 4000 mass spectrometer (AB Sciex). Computer application programs used to acquire and derive data for this study included the validated Thermoelectron Corporation Watson version 6.4, and MDS Sciex Analyst® 1.4.1 version. The acceptance criteria for the carry-over, the calibration samples, the QCs as well as the criteria for re-assay, correspond to the Standard Operating Procedures in use in the laboratory conducting the assays. Seven samples, arranged in increasing sequence, were used for calibration, with the first concentration level corresponding to the lower limit of quantification (LLOQ). The lower and upper limits of measurements were 27.6 and 829 nmol/L, respectively. The precision of the method as characterized by the coefficient of variation (CV%) for plasma quality control samples ranged between 4.28% and 10.8%. The method proved to be selective against prednisone and its metabolites prednisolone and 16-α-hydroxyprednisolone.
Primary evaluation parameter was the difference between cortisol concentrations assayed with the two analytical methods.
The study was approved by the Local Ethics Committee of A. Cardarelli Hospital (Napoli, Italy) and all patients gave written informed consent prior to any study related procedures.
For descriptive analyses, continuous variables are presented as mean ± SD or median interquartile range (IQR) according to the Gaussian distribution. Wilcoxon signed ranks test was used to compare the two related cortisol samples, and differences between the two analytical methods were graphically showed according to Bland and Altman suggestions[10]. Values below LLOQ obtained with the LC-MS/MS method were numerically set at ½ LLOQ. Data were loge transformed and showed as simple scatterplot with the line of the equality represented. Moreover, in all patients and in those with data above the limit of quantification, the antilog of the difference between the two methods (loge ECLIA - loge LC-MS/MS) was plotted against the antilog of the average [(loge ECLIA + loge LC-MS/MS)/2] showing the geometric mean ratio with upper and lower 95%CI. Statistical analysis was performed using SAS 9.1.3 software (SAS Institute®, Cary, NC, United States).
The main demographic characteristics of the recruited subjects are reported in Table 1. At the end of the study period two patient (10%) presented with steroid related AEs not including cortisol levels below normal values. Higher values of cortisol levels were found for ECLIA than LC-MS/MS method (P < 0.001). Median (IQR) blood cortisol levels with ECLIA were 54.1 (185.8) nmol/L, while with LC-MS/MS assay 32.1 (124.0) nmol/L. The within-patient median differences between the two methods was 23.2 (40.6) nmol/L and the ECLIA/LC-MS/MS ratio 1.7 (1.3), reflecting higher cortisol levels measured with the ECLIA method. Mean percentage difference between the results obtained with the two methods was of 39%.
| Characteristics | Data |
| Age, yr | 37.9 ± 16.2 |
| Range | 20-70 |
| Male/females, n | 12/8 |
| Disease localization, n (%) | |
| Left colitis | 11 (55) |
| Pancolitis | 9 (45) |
| Years from diagnosis, median (IQR) | 4 (9) |
| Height, cm | 166.7 ± 7.7 |
| Weight, kg | 68.8 ± 15.2 |
| BMI | 24.7 ± 5.3 |
| SBP, mmHg | 113.9 ± 7.9 |
| DBP, mmHg | 71.3 ± 5.6 |
| HR, bpm | 76.5 ± 9.2 |
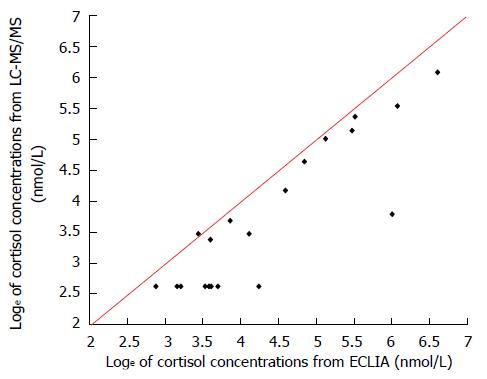
As showed in Figure 1, all values obtained with the ECLIA method were below the line of equality suggesting a systematic over-estimation with this method. The estimated geometric mean ratio between methods, based on differences of loge transformed data, was 1.85 (95%CI: 2.39-1.43) considering all data (Figure 2) or 1.58 (95%CI: 2.30-1.09) considering only data above the limit of quantification (n = 12) (Figure 3). The 95%CIs of the geometric mean ratio between methods confirm a statistically significant difference.
The risk of underestimation of the suppression of endogenous cortisol production in patients treated with prednisone, when conventional RIA methods are used to detect cortisol levels, is reported in asthmatic patients and is related to cross reactivity of prednisone and its metabolites with cortisol[6]. No data are reported in patients affected by UC.
We tested the degree of interferences of the ECLIA Cortisol assay method when analyzing samples collected from patients treated with prednisone, finding that cortisol levels measured using ECLIA method were 39% higher than the ones measured by the selective LC-MS/MS method. These data indicate an overestimation of cortisol levels and an underestimation of the HPA axis suppression using ECLIA method in patients with UC treated with PD. When assessing cortisol suppression by systemic corticosteroids, we believe that researchers and clinicians might not be aware of this problem or the extent of it. In large-scale clinical trials on UC patients treated with PD where HPA axis suppression is a safety endpoint, the results may be biased by the determination of serum cortisol levels with RIA or ECLIA methods. Selective analytical method against prednisone and its metabolites prednisolone and 16-α-hydroxyprednisolone LC-MS/MS should be used in this clinical setting to avoid the interference of PD in the determination of cortisol levels.
In conclusion, the use of ECLIA method to assess the effects of PD on cortisol suppression could be misleading and the use of LC-MS/MS evaluation should be preferred.
We thank Mrs. S. Amato for her nursing care.
The determination of morning cortisol levels during treatment with glucocorticosteroids (GS) is used in clinical practice and as specific safety endpoint in the various clinical trials concerning the treatment with GS. In samples from patients who have been treated with prednisone (PD), concentrations of cortisol may be overestimated by using conventional electrochemiluminescence immunoassay (ECLIA) method.
Data about the degree of possible differences between ECLIA and liquid chromatographic methods coupled with mass spectrometry detection (LC-MS/MS) methods in the determination of cortisol levels in ulcerative colitis (UC) patients in treatment with PD are lacking.
This is the first study comparing blood cortisol levels measured using ECLIA and a selective LC-MS/MS method in samples collected from patients with UC treated with oral PD.
Data obtained with the ECLIA method are higher than the ones measured by LC-MS/MS, indicating overestimation of cortisol levels in patients treated with PD. The authors conclude that the cortisol suppression in the presence of PD should not be assessed by ECLIA method.
Cortisol levels in human biological samples has been determined by immunoassay methods, first radio-immunoassay, followed by enzyme-linked immunosorbent assay, then automated ECLIA and more recently by LC-MS/MS.
For some scientific researches, which focus on the depression effect of glucocorticosteroid on hypothalamic-pituitary-adrenal axis in UC patients, this study may provide useful information.
P- Reviewer: Guo XZ, Wittmann T S- Editor: Nan J L- Editor: A E- Editor: Zhang DN
| 1. | Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, Allez M, D’Haens G, D’Hoore A, Mantzaris G, Novacek G. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 702] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 2. | Ford AC, Bernstein CN, Khan KJ, Abreu MT, Marshall JK, Talley NJ, Moayyedi P. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:590-599; quiz 600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Ardizzone S, Bianchi Porro G. Comparative tolerability of therapies for ulcerative colitis. Drug Saf. 2002;25:561-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Wallace I, Cunningham S, Lindsay J. The diagnosis and investigation of adrenal insufficiency in adults. Ann Clin Biochem. 2009;46:351-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Inder WJ, Dimeski G, Russell A. Measurement of salivary cortisol in 2012 - laboratory techniques and clinical indications. Clin Endocrinol (Oxf). 2012;77:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Meijer RJ, Postma DS, Kerstjens HA. Conventional RIA underestimates cortisol suppression in the presence of prednisolone. Thorax. 2002;57:374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Koal T, Schmiederer D, Pham-Tuan H, Röhring C, Rauh M. Standardized LC-MS/MS based steroid hormone profile-analysis. J Steroid Biochem Mol Biol. 2012;129:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 8. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [PubMed] |
| 9. | Roche Cobas E170/Elecsys Cortisol reagent, catalog number 11875116, data sheet 2008-02, V 13 English. . |
| 10. | Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3857] [Cited by in RCA: 5113] [Article Influence: 196.7] [Reference Citation Analysis (0)] |