Published online Aug 21, 2014. doi: 10.3748/wjg.v20.i31.10813
Revised: January 11, 2014
Accepted: April 30, 2014
Published online: August 21, 2014
Processing time: 300 Days and 0.9 Hours
Pancreatic cancer continues to be a leading cause of cancer-related death worldwide and there is an urgent need to develop novel diagnostic and therapeutic strategies to reduce the mortality of patients with this disease. In pancreatic cancer, some tight junction proteins, including claudins, are abnormally regulated and therefore are promising molecular targets for diagnosis, prognosis and therapy. Claudin-4 and -18 are overexpressed in human pancreatic cancer and its precursor lesions. Claudin-4 is a high affinity receptor of Clostridium perfringens enterotoxin (CPE). The cytotoxic effects of CPE and monoclonal antibodies against claudin-4 are useful as novel therapeutic tools for pancreatic cancer. Claudin-18 could be a putative marker and therapeutic target with prognostic implications for patients with pancreatic cancer. Claudin-1, -7, tricellulin and marvelD3 are involved in epithelial to mesenchymal transition (EMT) of pancreatic cancer cells and thus might be useful as biomarkers during disease. Protein kinase C is closely related to EMT of pancreatic cancer and regulates tight junctions of normal human pancreatic duct epithelial cells and the cancer cells. This review focuses on the regulation of tight junctions via protein kinase C during EMT in human pancreatic cancer for the purpose of developing new diagnostic and therapeutic modalities for pancreatic cancer.
Core tip: There is an urgent need to develop novel diagnostic and therapeutic strategies to reduce the mortality of pancreatic cancer patients. In pancreatic cancer, some tight junction proteins, including claudins, are abnormally regulated and thus are promising molecular targets for Clostridium perfringens enterotoxin and monoclonal antibodies. Protein kinase C is closely related to epithelial to mesenchymal transition (EMT) of this cancer and regulates tight junctions of normal human pancreatic duct epithelial (HPDE) cells and pancreatic cancer cells. This review focuses on the regulation of tight junctions via protein kinase C during EMT in human pancreatic cancer compared to normal HPDE cells.
- Citation: Kyuno D, Yamaguchi H, Ito T, Kono T, Kimura Y, Imamura M, Konno T, Hirata K, Sawada N, Kojima T. Targeting tight junctions during epithelial to mesenchymal transition in human pancreatic cancer. World J Gastroenterol 2014; 20(31): 10813-10824
- URL: https://www.wjgnet.com/1007-9327/full/v20/i31/10813.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i31.10813
Pancreatic cancer continues to be a leading cause of cancer-related death worldwide due to late detection, lack of therapeutic targets and ineffective therapies. At the time of diagnosis, few patients with pancreatic cancer present with localized disease amenable to surgical resection, while the remaining patients present with locally advanced or distant metastasis. It exhibits the poorest prognosis of all solid tumors with a 5-year survival rate < 5% and a median survival of 3-6 mo after diagnosis[1]. Thus, there is an urgent need to develop novel diagnostic and therapeutic strategies to reduce the mortality of these patients.
Transition of a cancer cell from an epithelial to mesenchymal morphology leads to increased migratory and invasive properties, and thus facilitates the initiation of metastasis in pancreatic cancer[2,3]. The epithelial to mesenchymal transition (EMT) is characterized by a loss of cell-cell contact and apicobasal polarity. The hallmarks of EMT in vitro and in vivo include the upregulation of mesenchymal markers, the downregulation of epithelial cell adhesion molecules including tight junction proteins, and dysfunction of the tight junction fence[4,5]. EMT is accompanied by loss of occludin and claudins as well as E-cadherin via the Snail family[6-9]. The transcription factor Snail, which has high to moderate expression in 78% of pancreatic ductal adenocarcinoma specimens, appears to promote metastasis and chemoresistance in pancreatic cancer[10,11]. The activation of protein kinase C (PKC) is known to be involved in EMT in various type of cancer including pancreatic cancer. The PKC activator 12-O-tetradecanoylphorbol 13-acetate (TPA) induces EMT in human prostate cancer cells[12] and pancreatic cancer cell line HPAC[13]. Expression of PKCα and PKCδ closely contributes to EMT in colon cancer cells[14,15]. Transforming growth factor-β1 (TGF-β1), which promotes EMT in pancreatic cancer cells[16], induces PKCα in poorly differentiated pancreatic cancer cell line BXPC-3[17].
In several human cancers, including pancreatic cancer, some tight junction proteins are abnormally regulated and therefore promising molecular targets for diagnosis and therapy[18,19]. The current review will focus on the roles of tight junction proteins, including claudins, and PKC signaling with regard to the potential applicability for diagnosis, prognosis and the therapy during EMT in pancreatic cancer.
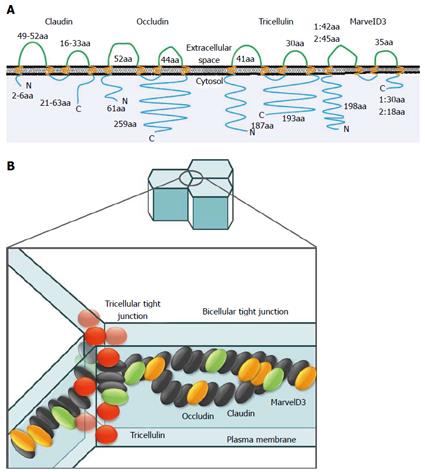
Epithelial cells including pancreatic epithelial cells are bordered by two functionally and biochemically different membranes[20]. This integrity is maintained by intercellular junctional complexes, such as tight junctions, adherent junctions, and desmosomes[21]. Tight junctions are the most apical components of intercellular junctional complexes in epithelial and endothelial cells. They separate the apical and basolateral cell surface domains, maintaining cell polarity (termed the “fence” function), and selectively control solute and water flow through the paracellular space (termed the “barrier” function)[22-25]. They also participate in signal transduction mechanisms that regulate epithelial cell proliferation, gene expression, differentiation and morphogenesis[26]. The tight junction is formed by integral membrane proteins and peripheral membrane proteins. The integral membrane proteins are claudins[27,28], occludin[29], tricellulin[30], marvelD3[31] and junctional adhesion molecules[32] (Figure 1). Peripheral membrane proteins include the scaffold PDZ-expression proteins zonula occludens (ZO)-1, ZO-2, ZO-3, multi-PDZ domain protein-1, membrane-associated guanylate kinase with inverted orientation-1 (MAGI)-1, MAGI-2, MAGI-3, cell polarity molecules atypical PKC isotype-specific interacting protein/PAR-3, PAR-6, PALS-1, and PALS-1-associated tight junction, as well as the non-PDZ-expressing proteins cingulin, symplekin, ZONAB, GEF-H1, aPKC, PP2A, Rab3b, Rab13, PTEN, and 7H6[21,33,34]. These tight junction proteins are regulated by various cytokines and growth factors via distinct signal transduction pathways including PKC[35,36].
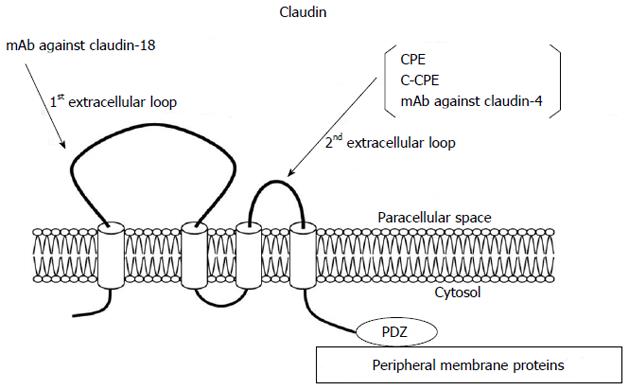
The claudin family, which consists of at least 27 members, is solely responsible for forming tight junction strands and has four transmembrane domains and two extracellular loops[21,37] (Figure 2). The first extracellular loop is the coreceptor of hepatitis C virus[38] and influences the paracellular charge selectivity[39], and the second extracellular loop is the receptor of Clostridium perfringens enterotoxin (CPE)[40].
Both occludin and tricellulin (marvelD2) contain the tetra-spanning MARVEL (MAL and related proteins for vesicle trafficking and membrane link) domain that is present in proteins involved in membrane apposition and concentrated in cholesterol-rich microdomains[41]. The novel tight junction protein marvelD3 contains a conserved MARVEL domain like occludin and tricellulin[31,42].
In general, cancer cells lose their specific functions and polarity with a decrease in the development of tight junctions. It is thought that the loss of tight junction functions in part leads to invasion and metastasis of cancer cells[43].
Tight junction proteins are dysregulated during carcinogenesis and EMT. Expression of some claudin family members is significantly altered by epigenetic regulation in human cancer[44-46].
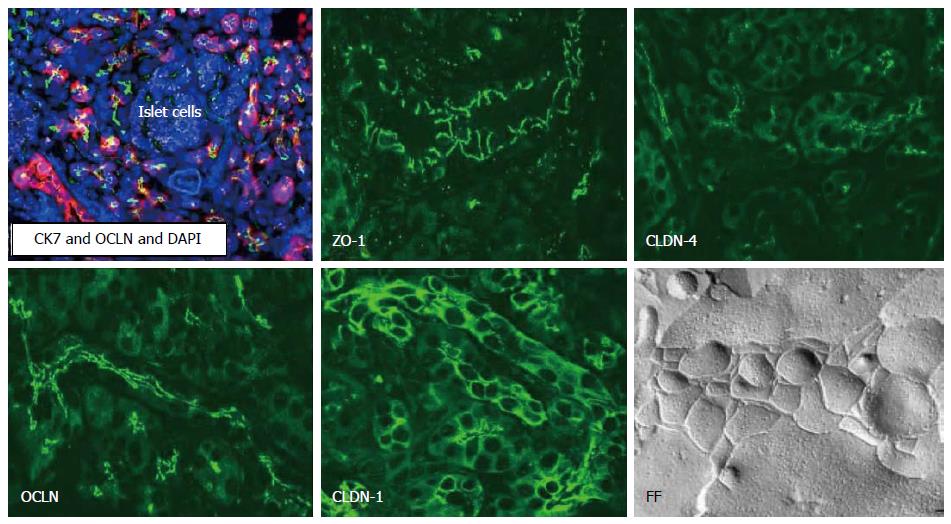
Several tight junction proteins are expressed in a tissue-specific and organ-specific manner[47-49]. Normal ductal and acinar structures of the pancreas express claudin-1, -2, -3, -4, and -7, whereas endocrine cells within the islets of Langerhans express claudin-3 and -7 (Figure 3)[50,51]. Pancreatic duct cells deliver the enzymes produced by acinar cells into duodenum and secrete a HCO3--rich fluid to neutralize gastric acid from the stomach[52]. Tight junctions of the pancreatic duct form the pancreatic ductal barrier. Freeze-fracture analysis of the pancreatic duct reveals that tight junctions contained a parallel array of three to five continuous sealing strands and the pancreatic enzymes cannot leak out from the lumen into the intercellular spaces (Figure 3)[53,54]. Tight junctions of the pancreatic duct are also regulators of physiologic secretion of the pancreas. Pancreatic ductal tight junctions, which is leaky and has the function of selective permeability, may play a role of channels of Na+ and HCO3-via paracellular pathway[55,56].
The tight junctions of pancreatic duct epithelial cells and exocrine cells are dynamic structures that can be disrupted by various external stimuli including ductal hypertension[57,58]. The disruption of pancreatic duct tight junctions is an early event in different types of pancreatitis[59-64]. Although dysfunction of tight junctions in pancreatic duct is observed by various pathological conditions, the regulatory mechanisms of tight junctions remain unknown even in normal human pancreatic duct epithelial (HPDE) cells.
The tight junction protein expression pattern varies between normal pancreatic tissue and pancreatic cancer. Claudin-1, -4, -7 and -18 are positive in pancreatic adenocarcinoma, whereas endocrine tumors are negative for claudin-1 and -4. Claudin-3 and -7 proteins are detected in endocrine tumors, whereas claudin-13 is negative in ductal adenocarcinoma[18,50,51]. Claudin-1, -2 and -4 are detected in exocrine tumors[65]. In borderline cystic tumors the level of claudin-1, -4 and -7 protein expression is between that of benign and malignant tumors[65]. This supports the sequential development theory regarding mucinous cystic tumors.
Liver metastasis of pancreatic cancer is strongly positive for claudin-4, weakly positive for claudin-1, and negative or faintly positive for claudin-7[66]. It is interesting that claudin-3 is positive in liver metastasis of pancreatic cancer whereas claudin-3 staining is not detected in primary pancreatic cancer[50,66].
A study investigating ZO-1 in pancreatic cancer showed that expression of ZO-1 was increased in pancreatic adenocarcinoma samples in comparison with normal samples[67]. In pancreatic cancer cells, ZO-1 protein translocalizes from apical and apicolateral areas to the cytoplasm and nucleus, and translocation of ZO-1 is involved in the induction of invasion through epidermal growth factor receptor (EGFR) activation[68].
We established human telomerase reverse transcriptase-transfected HPDE cells as models of normal pancreatic duct epithelial cells[51]. The hTERT-HPDE cells are positive for HPDE cell markers such as CK7, CK19 and carbonic anhydrase isozyme 2 and express epithelial tight junction molecules claudin-1, -4, -7 and -18, occludin, tricellulin, marvelD3, JAM-A, ZO-1, and ZO-2[51]. The expression patterns of tight junction molecules in the hTERT-HPDE cells are similar to those of pancreatic tissues in vivo[51].
Claudin-1 is expressed in various types of epithelial cells, and plays an important role in epithelial cell polarity and cancer invasion and metastasis[69-72]. However, its role remains controversial far in various cancers. In pancreatic cancer, claudin-1 expression is responsible for tumor necrosis factor α-dependent cell growth signals that lead to apoptosis and the inhibition of cell proliferation[73]. Claudin-1 is localized at the cell membranes of normal pancreatic ducts and well-differentiated pancreatic carcinoma, whereas in poorly differentiated pancreatic carcinoma it is weakly detected in cytoplasm[74].
EMT is associated with the simultaneous repression of the genes encoding E-cadherin, claudins and occludin[8]. The transcription factors Snail and Slug, which play a central role in EMT, bind to the E-box motifs present in the claudin-1 promoter and have a critical negative regulatory role in malignant cancer cell lines that express low levels of the claudin-1 transcript[8,75]. Treatment with TGF-β1 induces EMT in pancreatic cancer cells and TGF-β upregulates Snail and downregulates claudin-1, -4 and occludin in PANC-1 cells[74]. Taken together, this indicates that claudin-1 may be a potential biomarker for the development of pancreatic cancer. Thus further investigation of the significance of claudin-1 in pancreatic cancer cells and normal pancreatic duct epithelial cells is required.
DNA microarray, immunohistochemical, and quantitative real-time reverse transcription-polymerase chain reaction analyses have provided evidence that claudin-4 is upregulated in pancreatic cancer tissues[76]. Furthermore, claudin-4 is also overexpressed in pancreatic intraepithelial neoplasia (PanIN), intraductal papillary neoplasia (IPMN), and mucinous cystic neoplasia (MCN), and is correlated with the histological tumor grade in both IPMN and MCN[77,78]. On the other hand, overexpression of claudin-4 decreases the invasiveness and metastatic potential of pancreatic cancer cells in vitro[19]. Patients with high expression of claudin-4 mRNA and protein survive longer than those with low claudin-4 expression[79].
Claudin-4 is also a high-affinity receptor of CPE[80]. The 35-kDa polypeptide CPE causes food poisoning in humans, binds to its claudin receptor, and then causes changes in membrane permeability via formation of a complex on the plasma membrane followed by the induction of apoptosis[81]. Full-length CPE with a direct cytotoxic effect and the COOH-terminal receptor-binding domain of CPE (C-CPE) without a cytotoxic effect are employed as selective treatment and drug delivery systems against claudin-4 expressing pancreatic tumors[82,83].
CPE induces an acute dose-dependent cytotoxic effect in claudin-4-expressing nude mouse xenografts of PANC-1, which is a poorly differentiated pancreatic cancer cell line[82,84]. In the pancreatic cell lines PANC-1, BXPC-3, HPAF-II and HPAC, claudin-4 is found not only at the apicalmost regions but also at basolateral membranes[85]. When these pancreatic cancer cell lines are treated with CPE, it induces dose-dependent cytotoxic effects in all of them[85]. Furthermore, in HPAC cells, the cytotoxicity of CPE is significantly decreased by knockdown of claudin-4 by siRNAs[85].
In hTERT-HPDE cells cultured with 10% FBS, claudin-4 is localized at the apicalmost regions, which are tight junction areas[85]. When hTERT-HPDE cells cultured with 10% FBS in which the expression of claudin-4 protein is as high as in pancreatic cell lines in Western blotting, are treated with CPE, cytotoxicity is not observed even at high concentrations of CPE[85]. These findings suggest that, in pancreatic cancer cells, CPE binds to the free second extracellular loop of claudin-4 outside of tight junctions and that, in normal HPDE cells, it cannot bind to that of claudin-4 in tight junction areas.
The functional domains of CPE can be separated into a receptor-binding region (C-terminal of CPE, C-CPE) and cytotoxic region (N-terminal of CPE). C-CPE is a C-terminal fragment composed of the CPE amino acids 184 to 319[80]. The receptor binding region of CPE has been reported to be in the C-terminal 30 residues (amino acids 290 to 319) of CPE[86].
C-CPE is a nontoxic molecule that disrupts the tight junction barrier function and enhances cellular absorption[87]. It enhances the effectiveness of clinically relevant anticancer agents such as Taxol and carboplatin against cancer cells[88]. In our study, when HPAC cells were treated with C-CPE, the barrier function was markedly decreased at a nontoxic concentration of C-CPE and recovered in the absence of C-CPE (personal data). C-CPE may enhance the effectiveness of clinically relevant chemotherapies in pancreatic cancer.
The development of molecular imaging approaches using tissue- and cell-specific tracers plays a crucial role to improve early diagnosis and therapy in cancer. Claudin-4 is utilized as a target for imaging of pancreatic cancer. Non-cadmium-based quantum dots bioconjugated to claudin-4 monoclonal antibodies are used as highly efficient, nontoxic optical probes for imaging live pancreatic cancer cells in vivo and in vitro[89]. C-CPE labelled with a cyanine dye with novel optical imaging methods, 2D planar fluorescence reflectance imaging technology and 3D fluorescence-mediated tomography, enables noninvasive visualization of claudin-4 positive pancreatic cancer and its precursor lesions[90]. Furthermore, it is thought that C-CPE can be used as a carrier for other bacterial toxins to claudin-4-positive cancer cells. A claudin-4-targeting antitumor molecule that consisted of C-CPE fused to protein synthesis inhibitory factor derived from Pseudomonas aeruginosa exotoxin or diphtheria toxin fragment A (DTA) were especially toxic to claudin-4 positive cancer cells in vivo and in vitro[83,91,92].
Claudin-7 is expressed in various types of epithelial cells and directly interacts with EpCAM, forming a complex with CD44 variant isoforms and tetraspanins outside of tight junction areas[93,94]. Furthermore, EpCAM is one of the surface markers in pancreatic cancer stem cells[95], and claudin-7 regulates the EpCAM-mediated functions in tumor progression such as proliferation, migration, and anti-apoptosis[96,97]. Claudin-7 supports tumorigenic features of EpCAM by provoking EpCAM cleavage and its cotranscription factor activity, and is directly engaged in motility and resistance to apoptosis in rat pancreatic cancer[98].
In human pancreatic ductal adenocarcinoma, there is a gradual decline in membrane-bound expression of claudin-7 immunoreactivity in parallel with the degree of tumor differentiation[99]. Claudin-7 expression also appears to be inversely associated with the gland size in tumors, with large neoplastic glands displaying more frequent claudin-7 positivity than smaller glands[99]. There is no association between claudin-7 and tumor size, the presence of nodal metastases or survival of the patients, indicating that while expression of claudin-7 is related to differentiation of ductal pancreatic adenocarcinoma it does not influence tumor progression[99].
In a human pancreatic cancer cell line and hTERT-HPDE cells, ELF3 is associated with claudin-7[51]. ELF3 belongs to the ELF (E74-like factor) subfamily of the ETS transcription factors, but it is distinguished from most ETS family members by its expression pattern, which is specific in epithelial tissues of the lung, liver, kidney, pancreas, prostate, small intestine, and colon mucosa[100]. ELF3 controls intestinal epithelial differentiation[101]. It is reported that the expression of claudin-7 in epithelial structures in synovial sarcoma is regulated by ELF3[102]. Thus, the expression of claudin-7 and its regulation via ELF3 may be important as potential therapeutic targets for pancreatic cancer.
In pancreatic cancer, claudin-18 is as highly expressed as claudin-4[18]. Claudin-18 has two alternatively spliced variants, claudin-18a1 and claudin-18a2, which are highly expressed in the lung and stomach, respectively[103]. Claudin-18a2 is activated in a wide range of human malignant tumors, including gastric, esophageal, pancreatic, lung, and ovarian cancers, and can be specifically targeted by monoclonal antibodies against the first extracellular loop[44]. Claudin-18 is highly expressed in PanIN, IPMN, MCN, pancreatic duct carcinoma, and metastases of pancreatic cancer, and serves as a diagnostic marker[18,78,99,104-106]. Neuroendocrine neoplasia is found positive with low rates[105]. Thus, claudin-18 could be useful as a putative marker and therapeutic target for neoplasia of the pancreas. Furthermore, because claudin-18 expression is most pronounced in well-differentiated pancreatic cancers, and patients with high expression of claudin-18 survive longer than those with low claudin-18 expression[18], its expression level may also have prognostic implications for patients with pancreatic cancer.
Tricellulin was identified as the first marker of the tricellular tight junction, which formed at the meeting points of three cells[30]. It is required for the maintenance of the transepithelial barrier and expressed in both the normal pancreatic duct and pancreatic cancer[30,107,108]. It is one of three members of the tight junction-associated MARVEL protein family. The other two members are occludin and marvelD3[31,42]. Occludin and tricellulin are present at bicellular and tricellular tight junctions, respectively, whereas marvelD3 is present at both sites[31,42]. Both normal and neoplastic pancreatic exocrine tissues express tricellulin, whereas no expression is seen in normal or neoplastic endocrine cells[108]. Tricellulin expression in pancreatic ductal adenocarcinomas shows a significant negative correlation with the degree of differentiation[108].
Tricellulin expression in tricellular tight junctions is strongly regulated together with the barrier function via the c-Jun N-terminal kinase (JNK) transduction pathway[109]. Activation of JNK promotes the development of various tumors[110-112]. Furthermore, JNK inhibitors decrease the growth of human and murine pancreatic cancers in vitro and in vivo[113]. Tricellulin expression and the barrier function are upregulated together with the activity of phospho-JNK by treatment with the JNK activator anisomycin in HPAC cells[109]. In hTERT-HPDE cells, tricellulin expression is significantly increased by all JNK activators, similar to the response in HPAC cells[109]. JNK may be involved in the regulation of tight junctions, including tricellulin expression and the barrier function in normal pancreatic duct epithelial cells, and may be a potential therapeutic target for pancreatic cancer.
MarvelD3, the novel tight junction protein, is transcriptionally downregulated in poorly differentiated pancreatic cancer cells, whereas it is maintained in well-differentiated human pancreatic cancer cells and normal pancreatic duct epithelial cells[114]. Furthermore, marvelD3 is transcriptionally downregulated in Snail-induced EMT during the progression of pancreatic cancer[114]. Therefore, marvelD3 could be a new marker during pancreatic cancer progression. However, little is known about the detailed role of marvelD3 in epithelial tight junctions and how it is regulated in various types of cells, including normal pancreatic duct epithelial cells and pancreatic cancer cells.
PKC belongs to the family of serine-threonine kinases and regulates various cellular functions[115]. It has been shown to induce both assembly and disassembly of tight junctions depending on the cell type and conditions of activation[116-118]. At least 12 different isozymes of PKC are known and can be subdivided into three classes (classic or conventional, novel and atypical isozymes) according to their responsiveness to activators[119,120]. The levels of PKCα, PKCβ1, PKCδ and PKCι are higher in pancreatic cancer, whereas that of PKCε is higher in normal tissue[121,122]. In pancreatic cancer, tumorigenicity is directly related to PKCα expression, as demonstrated by decreased survival when it is overexpressed[123]. The increased level of PKCα is also associated with pancreatic cancer cell proliferation[124].
Tight junction proteins are regulated by various cytokines and growth factors via distinct signal transduction pathways including PKC[35,36]. In various cancer cells, the regulation of tight junctions via PKC pathway is reported. The assembly of ZO-1 and occludin is involved in PKC-dependent signaling in gastric cancer cells[125]. The activation of c-Abl-PKCδ signaling pathway is critically required for the claudin-1-induced acquisition of the malignant phenotype in human liver cells[72]. PKC activation causes an increase in claudin-1 transcription and claudin-1 appears to contribute to cell invasion in human melanoma cells[126]. PKCε activation regulates an α5 integrin-ZO-1 complex and correlates with invasion and unfavorable prognosis in lung cancer cells[127].
We have previously reported that the regulation of tight junctions in normal human pancreatic duct epithelial cells and pancreatic cancer cells is closely associated with PKC and PKC-induced transcriptional factors[13,51,74,104,109,128]. To confirm whether the PKC signal pathway was closely associated with the regulation of tight junctions, hTERT-HPDE cells and pancreatic cancer cells were treated with the PKC activator TPA and the specific PKC isoform inhibitors. Treatment with TPA enhanced expression of claudin-1, -4, -7, and -18, occludin, JAM-A and ZO-1, -2[51]. The upregulation of claudin-4 by TPA was prevented by a PKCα inhibitor and the upregulation of claudin-7, occludin, ZO-1 and ZO-2 was prevented by a PKCδ inhibitor[51]. In HPAC cells, tricellulin was in part regulated via PKCδ and PKCε pathways[109], and the expression of claudin-18 and localization of claudin-4 and occludin were in part regulated via a PKCα pathway[13,104,128]. Claudin-18 mRNA and protein, indicated to be claudin-18a2, were markedly induced by TPA in well- and moderately differentiated human pancreatic cancer cell lines HPAF-II and HPAC and hTERT-HPDE cells[104]. The upregulation of claudin-18 by TPA in human pancreatic cancer cell lines was prevented by inhibitors of PKCδ, PKCα and PKCε, whereas the upregulation of claudin-18 by TPA in hTERT-HPDE cells was prevented by inhibitors of PKCδ, PKCα and PKCθ[104].
On the other hand, a PKCα inhibitor enhances sensitivity of HPAC cells to CPE by preventing mislocalization of claudin-4[13], and prevents downregulation of claudin-1 during EMT of pancreatic cancer cells[74]. The TGF-β-PKCα-PTEN cascade is a key pathway for pancreatic cancer cells to proliferate and metastasize[129]. The PKC may be a useful target for pancreatic cancer therapy[119] and PKCα inhibitors may be potential therapeutic agents against the malignancy of human pancreatic cancer cells[130]. Further study of the tight junctions of normal HPDE cells and pancreatic cancer cells via PKC pathways including isoforms is important for not only physiological regulation of tight junction molecules but also for therapeutic targeting of pancreatic cancer cells. In addition to PKC pathway, other signaling pathways including Ras/ERK1/2, Smad/STAT3, Notch, Wnt and Src are closely related to EMT of pancreatic cancer[131-135]. However, the regulation of tight junctions in normal pancreatic duct and pancreatic cancer via these signal pathways remain unknown.
The signaling pathways including PKC regulate tight junctions during EMT in pancreatic cancer. By using hTERT-HPDE cells, we found that the expression of tight junction proteins in normal HPDE cells was regulated by various factors. For developing new diagnostic and therapeutic modalities via tight junction molecules in pancreatic cancer, it is necessary to investigate the profile and the regulation of tight junctions in normal HPDE cells as well as pancreatic cancer cells.
P- Reviewer: Servin AL, Zhang L S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8283] [Cited by in RCA: 8226] [Article Influence: 483.9] [Reference Citation Analysis (0)] |
| 2. | Karamitopoulou E. Tumor budding cells, cancer stem cells and epithelial-mesenchymal transition-type cells in pancreatic cancer. Front Oncol. 2012;2:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1667] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 4. | Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031-1049. [PubMed] |
| 5. | Lee DB, Huang E, Ward HJ. Tight junction biology and kidney dysfunction. Am J Physiol Renal Physiol. 2006;290:F20-F34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, García De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2076] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 7. | Cano CE, Motoo Y, Iovanna JL. Epithelial-to-mesenchymal transition in pancreatic adenocarcinoma. ScientificWorldJournal. 2010;10:1947-1957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116:1959-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 514] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 9. | Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1272] [Cited by in RCA: 1330] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 10. | Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13:4769-4776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 337] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 11. | Yin T, Wang C, Liu T, Zhao G, Zha Y, Yang M. Expression of snail in pancreatic cancer promotes metastasis and chemoresistance. J Surg Res. 2007;141:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | He H, Davidson AJ, Wu D, Marshall FF, Chung LW, Zhau HE, He D, Wang R. Phorbol ester phorbol-12-myristate-13-acetate induces epithelial to mesenchymal transition in human prostate cancer ARCaPE cells. Prostate. 2010;70:1119-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Kyuno D, Kojima T, Ito T, Yamaguchi H, Tsujiwaki M, Takasawa A, Murata M, Tanaka S, Hirata K, Sawada N. Protein kinase Cα inhibitor enhances the sensitivity of human pancreatic cancer HPAC cells to Clostridium perfringens enterotoxin via claudin-4. Cell Tissue Res. 2011;346:369-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Ghoul A, Serova M, Astorgues-Xerri L, Bieche I, Bousquet G, Varna M, Vidaud M, Phillips E, Weill S, Benhadji KA. Epithelial-to-mesenchymal transition and resistance to ingenol 3-angelate, a novel protein kinase C modulator, in colon cancer cells. Cancer Res. 2009;69:4260-4269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Masur K, Lang K, Niggemann B, Zanker KS, Entschladen F. High PKC alpha and low E-cadherin expression contribute to high migratory activity of colon carcinoma cells. Mol Biol Cell. 2001;12:1973-1982. [PubMed] |
| 16. | Ellenrieder V, Hendler SF, Boeck W, Seufferlein T, Menke A, Ruhland C, Adler G, Gress TM. Transforming growth factor beta1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res. 2001;61:4222-4228. [PubMed] |
| 17. | Chen Y, Yu G, Yu D, Zhu M. PKCalpha-induced drug resistance in pancreatic cancer cells is associated with transforming growth factor-beta1. J Exp Clin Cancer Res. 2010;29:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Karanjawala ZE, Illei PB, Ashfaq R, Infante JR, Murphy K, Pandey A, Schulick R, Winter J, Sharma R, Maitra A. New markers of pancreatic cancer identified through differential gene expression analyses: claudin 18 and annexin A8. Am J Surg Pathol. 2008;32:188-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Michl P, Barth C, Buchholz M, Lerch MM, Rolke M, Holzmann KH, Menke A, Fensterer H, Giehl K, Löhr M. Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res. 2003;63:6265-6271. [PubMed] |
| 20. | Furuse M. Knockout animals and natural mutations as experimental and diagnostic tool for studying tight junction functions in vivo. Biochim Biophys Acta. 2009;1788:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1851] [Cited by in RCA: 1903] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 22. | Cereijido M, Valdés J, Shoshani L, Contreras RG. Role of tight junctions in establishing and maintaining cell polarity. Annu Rev Physiol. 1998;60:161-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 190] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Gumbiner BM. Breaking through the tight junction barrier. J Cell Biol. 1993;123:1631-1633. [PubMed] |
| 24. | Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992;262:L647-L661. [PubMed] |
| 25. | van Meer G, Gumbiner B, Simons K. The tight junction does not allow lipid molecules to diffuse from one epithelial cell to the next. Nature. 1986;322:639-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 127] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 650] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 27. | Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539-1550. [PubMed] |
| 28. | Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA. 1999;96:511-516. [PubMed] |
| 29. | Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777-1788. [PubMed] |
| 30. | Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939-945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 553] [Cited by in RCA: 601] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 31. | Steed E, Rodrigues NT, Balda MS, Matter K. Identification of MarvelD3 as a tight junction-associated transmembrane protein of the occludin family. BMC Cell Biol. 2009;10:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 32. | Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, Parkos CA. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113:2363-2374. [PubMed] |
| 33. | Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213-C1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1008] [Cited by in RCA: 1043] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 34. | Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, Chiba H. Tight junctions and human diseases. Med Electron Microsc. 2003;36:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 255] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 35. | González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 594] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 36. | Kojima T, Murata M, Yamamoto T, Lan M, Imamura M, Son S, Takano K, Yamaguchi H, Ito T, Tanaka S. Tight junction proteins and signal transduction pathways in hepatocytes. Histol Histopathol. 2009;24:1463-1472. [PubMed] |
| 37. | Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 406] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 38. | Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wölk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 943] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 39. | Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 865] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 40. | Fujita K, Katahira J, Horiguchi Y, Sonoda N, Furuse M, Tsukita S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett. 2000;476:258-261. [PubMed] |
| 41. | Sánchez-Pulido L, Martín-Belmonte F, Valencia A, Alonso MA. MARVEL: a conserved domain involved in membrane apposition events. Trends Biochem Sci. 2002;27:599-601. [PubMed] |
| 42. | Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21:1200-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 43. | Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta. 2009;1788:872-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 360] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 44. | Sahin U, Koslowski M, Dhaene K, Usener D, Brandenburg G, Seitz G, Huber C, Türeci O. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14:7624-7634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 301] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 45. | Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003;22:2021-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 341] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 46. | Honda H, Pazin MJ, Ji H, Wernyj RP, Morin PJ. Crucial roles of Sp1 and epigenetic modifications in the regulation of the CLDN4 promoter in ovarian cancer cells. J Biol Chem. 2006;281:21433-21444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 47. | Lal-Nag M, Morin PJ. The claudins. Genome Biol. 2009;10:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 48. | González-Mariscal L, Lechuga S, Garay E. Role of tight junctions in cell proliferation and cancer. Prog Histochem Cytochem. 2007;42:1-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 49. | Singh AB, Sharma A, Dhawan P. Claudin family of proteins and cancer: an overview. J Oncol. 2010;2010:541957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 50. | Borka K, Kaliszky P, Szabó E, Lotz G, Kupcsulik P, Schaff Z, Kiss A. Claudin expression in pancreatic endocrine tumors as compared with ductal adenocarcinomas. Virchows Arch. 2007;450:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Yamaguchi H, Kojima T, Ito T, Kimura Y, Imamura M, Son S, Koizumi J, Murata M, Nagayama M, Nobuoka T. Transcriptional control of tight junction proteins via a protein kinase C signal pathway in human telomerase reverse transcriptase-transfected human pancreatic duct epithelial cells. Am J Pathol. 2010;177:698-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Grapin-Botton A. Ductal cells of the pancreas. Int J Biochem Cell Biol. 2005;37:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Tsukiyama K. Ultrastructure of intercellular junctions in the rat exocrine pancreas stimulated by pancreozymin. Arch Histol Jpn. 1979;42:141-152. [PubMed] |
| 54. | Madden ME, Sarras MP. The pancreatic ductal system of the rat: cell diversity, ultrastructure, and innervation. Pancreas. 1989;4:472-485. [PubMed] |
| 55. | Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375-412. [PubMed] |
| 56. | Greenwell JR. The selective permeability of the pancreatic duct of the cat to monovalent ions. Pflugers Arch. 1977;367:265-270. [PubMed] |
| 57. | Akao S, Oya M, Akiyama H, Ishikawa H. The tight junction of pancreatic exocrine cells is a morphometrically dynamic structure altered by intraductal hypertension. J Gastroenterol. 2000;35:758-767. [PubMed] |
| 58. | Akao S, Kiumi F. The tight junction of main pancreatic duct epithelial cells is a morphometrically dynamic structure altered by intraductal hypertension. Med Electron Microsc. 2002;35:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Harvey MH, Wedgwood KR, Austin JA, Reber HA. Pancreatic duct pressure, duct permeability and acute pancreatitis. Br J Surg. 1989;76:859-862. [PubMed] |
| 60. | Arendt T, Rogos R. Pancreatic exocrine secretion in acute experimental pancreatitis. Gastroenterology. 1991;101:276-278. [PubMed] |
| 61. | Fallon MB, Gorelick FS, Anderson JM, Mennone A, Saluja A, Steer ML. Effect of cerulein hyperstimulation on the paracellular barrier of rat exocrine pancreas. Gastroenterology. 1995;108:1863-1872. [PubMed] |
| 62. | Schmitt M, Klonowski-Stumpe H, Eckert M, Lüthen R, Häussinger D. Disruption of paracellular sealing is an early event in acute caerulein-pancreatitis. Pancreas. 2004;28:181-190. [PubMed] |
| 63. | Coskun T, Bozoklu S, Ozenç A, Ozdemir A. Effect of hydrogen peroxide on permeability of the main pancreatic duct and morphology of the pancreas. Am J Surg. 1998;176:53-58. [PubMed] |
| 64. | Rotoli BM, Orlandini G, Guizzardi S, Uggeri J, Dall’Asta V, Gazzola GC, Bussolati O, Gatti R. Ethanol increases the paracellular permeability of monolayers of CAPAN-1 pancreatic duct cells. J Mol Histol. 2004;35:355-362. [PubMed] |
| 65. | Borka K. [Claudin expression in different pancreatic cancers and its significance in differential diagnostics]. Magy Onkol. 2009;53:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Holczbauer Á, Gyöngyösi B, Lotz G, Szijártó A, Kupcsulik P, Schaff Z, Kiss A. Distinct claudin expression profiles of hepatocellular carcinoma and metastatic colorectal and pancreatic carcinomas. J Histochem Cytochem. 2013;61:294-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 67. | Kleeff J, Shi X, Bode HP, Hoover K, Shrikhande S, Bryant PJ, Korc M, Büchler MW, Friess H. Altered expression and localization of the tight junction protein ZO-1 in primary and metastatic pancreatic cancer. Pancreas. 2001;23:259-265. [PubMed] |
| 68. | Takai E, Tan X, Tamori Y, Hirota M, Egami H, Ogawa M. Correlation of translocation of tight junction protein Zonula occludens-1 and activation of epidermal growth factor receptor in the regulation of invasion of pancreatic cancer cells. Int J Oncol. 2005;27:645-651. [PubMed] |
| 69. | Kojima T, Takano K, Yamamoto T, Murata M, Son S, Imamura M, Yamaguchi H, Osanai M, Chiba H, Himi T. Transforming growth factor-beta induces epithelial to mesenchymal transition by down-regulation of claudin-1 expression and the fence function in adult rat hepatocytes. Liver Int. 2008;28:534-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Oku N, Sasabe E, Ueta E, Yamamoto T, Osaki T. Tight junction protein claudin-1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin-5 gamma2 chain via matrix metalloproteinase (MMP)-2 and membrane-type MMP-1. Cancer Res. 2006;66:5251-5257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 71. | Singh AB, Sharma A, Smith JJ, Krishnan M, Chen X, Eschrich S, Washington MK, Yeatman TJ, Beauchamp RD, Dhawan P. Claudin-1 up-regulates the repressor ZEB-1 to inhibit E-cadherin expression in colon cancer cells. Gastroenterology. 2011;141:2140-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 72. | Yoon CH, Kim MJ, Park MJ, Park IC, Hwang SG, An S, Choi YH, Yoon G, Lee SJ. Claudin-1 acts through c-Abl-protein kinase Cdelta (PKCdelta) signaling and has a causal role in the acquisition of invasive capacity in human liver cells. J Biol Chem. 2010;285:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 73. | Kondo J, Sato F, Kusumi T, Liu Y, Motonari O, Sato T, Kijima H. Claudin-1 expression is induced by tumor necrosis factor-alpha in human pancreatic cancer cells. Int J Mol Med. 2008;22:645-649. [PubMed] |
| 74. | Kyuno D, Kojima T, Yamaguchi H, Ito T, Kimura Y, Imamura M, Takasawa A, Murata M, Tanaka S, Hirata K. Protein kinase Cα inhibitor protects against downregulation of claudin-1 during epithelial-mesenchymal transition of pancreatic cancer. Carcinogenesis. 2013;34:1232-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 75. | Martínez-Estrada OM, Cullerés A, Soriano FX, Peinado H, Bolós V, Martínez FO, Reina M, Cano A, Fabre M, Vilaró S. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem J. 2006;394:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 231] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 76. | Neesse A, Griesmann H, Gress TM, Michl P. Claudin-4 as therapeutic target in cancer. Arch Biochem Biophys. 2012;524:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Sato N, Maehara N, Goggins M. Gene expression profiling of tumor-stromal interactions between pancreatic cancer cells and stromal fibroblasts. Cancer Res. 2004;64:6950-6956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 78. | Lee JH, Kim KS, Kim TJ, Hong SP, Song SY, Chung JB, Park SW. Immunohistochemical analysis of claudin expression in pancreatic cystic tumors. Oncol Rep. 2011;25:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Tsutsumi K, Sato N, Tanabe R, Mizumoto K, Morimatsu K, Kayashima T, Fujita H, Ohuchida K, Ohtsuka T, Takahata S. Claudin-4 expression predicts survival in pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2012;19 Suppl 3:S491-S499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem. 1997;272:26652-26658. [PubMed] |
| 81. | McClane BA, Chakrabarti G. New insights into the cytotoxic mechanisms of Clostridium perfringens enterotoxin. Anaerobe. 2004;10:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 82. | Michl P, Buchholz M, Rolke M, Kunsch S, Löhr M, McClane B, Tsukita S, Leder G, Adler G, Gress TM. Claudin-4: a new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin. Gastroenterology. 2001;121:678-684. [PubMed] |
| 83. | Saeki R, Kondoh M, Kakutani H, Tsunoda S, Mochizuki Y, Hamakubo T, Tsutsumi Y, Horiguchi Y, Yagi K. A novel tumor-targeted therapy using a claudin-4-targeting molecule. Mol Pharmacol. 2009;76:918-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Deer EL, González-Hernández J, Coursen JD, Shea JE, Ngatia J, Scaife CL, Firpo MA, Mulvihill SJ. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 2010;39:425-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 751] [Cited by in RCA: 728] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 85. | Yamaguchi H, Kojima T, Ito T, Kyuno D, Kimura Y, Imamura M, Hirata K, Sawada N. Effects of Clostridium perfringens enterotoxin via claudin-4 on normal human pancreatic duct epithelial cells and cancer cells. Cell Mol Biol Lett. 2011;16:385-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Hanna PC, Mietzner TA, Schoolnik GK, McClane BA. Localization of the receptor-binding region of Clostridium perfringens enterotoxin utilizing cloned toxin fragments and synthetic peptides. The 30 C-terminal amino acids define a functional binding region. J Biol Chem. 1991;266:11037-11043. [PubMed] |
| 87. | Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, Tsukita S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. J Cell Biol. 1999;147:195-204. [PubMed] |
| 88. | Gao Z, Xu X, McClane B, Zeng Q, Litkouhi B, Welch WR, Berkowitz RS, Mok SC, Garner EI. C terminus of Clostridium perfringens enterotoxin downregulates CLDN4 and sensitizes ovarian cancer cells to Taxol and Carboplatin. Clin Cancer Res. 2011;17:1065-1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 89. | Yong KT. Anti-claudin-4-conjugated highly luminescent nanoparticles as biological labels for pancreatic cancer sensing. Methods Mol Biol. 2011;762:427-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 90. | Neesse A, Hahnenkamp A, Griesmann H, Buchholz M, Hahn SA, Maghnouj A, Fendrich V, Ring J, Sipos B, Tuveson DA. Claudin-4-targeted optical imaging detects pancreatic cancer and its precursor lesions. Gut. 2013;62:1034-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 91. | Saeki R, Kondoh M, Kakutani H, Matsuhisa K, Takahashi A, Suzuki H, Kakamu Y, Watari A, Yagi K. A claudin-targeting molecule as an inhibitor of tumor metastasis. J Pharmacol Exp Ther. 2010;334:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 92. | Kakutani H, Kondoh M, Saeki R, Fujii M, Watanabe Y, Mizuguchi H, Yagi K. Claudin-4-targeting of diphtheria toxin fragment A using a C-terminal fragment of Clostridium perfringens enterotoxin. Eur J Pharm Biopharm. 2010;75:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 93. | Ladwein M, Pape UF, Schmidt DS, Schnölzer M, Fiedler S, Langbein L, Franke WW, Moldenhauer G, Zöller M. The cell-cell adhesion molecule EpCAM interacts directly with the tight junction protein claudin-7. Exp Cell Res. 2005;309:345-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 94. | Kuhn S, Koch M, Nübel T, Ladwein M, Antolovic D, Klingbeil P, Hildebrand D, Moldenhauer G, Langbein L, Franke WW. A complex of EpCAM, claudin-7, CD44 variant isoforms, and tetraspanins promotes colorectal cancer progression. Mol Cancer Res. 2007;5:553-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 197] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 95. | Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J Clin Oncol. 2008;26:2806-2812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 285] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 96. | Nübel T, Preobraschenski J, Tuncay H, Weiss T, Kuhn S, Ladwein M, Langbein L, Zöller M. Claudin-7 regulates EpCAM-mediated functions in tumor progression. Mol Cancer Res. 2009;7:285-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 97. | Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69:5627-5629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 415] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 98. | Thuma F, Zöller M. EpCAM-associated claudin-7 supports lymphatic spread and drug resistance in rat pancreatic cancer. Int J Cancer. 2013;133:855-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 99. | Soini Y, Takasawa A, Eskelinen M, Juvonen P, Kärjä V, Hasegawa T, Murata M, Tanaka S, Kojima T, Sawada N. Expression of claudins 7 and 18 in pancreatic ductal adenocarcinoma: association with features of differentiation. J Clin Pathol. 2012;65:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Tymms MJ, Ng AY, Thomas RS, Schutte BC, Zhou J, Eyre HJ, Sutherland GR, Seth A, Rosenberg M, Papas T. A novel epithelial-expressed ETS gene, ELF3: human and murine cDNA sequences, murine genomic organization, human mapping to 1q32.2 and expression in tissues and cancer. Oncogene. 1997;15:2449-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 101. | Jedlicka P, Gutierrez-Hartmann A. Ets transcription factors in intestinal morphogenesis, homeostasis and disease. Histol Histopathol. 2008;23:1417-1424. [PubMed] |
| 102. | Kohno Y, Okamoto T, Ishibe T, Nagayama S, Shima Y, Nishijo K, Shibata KR, Fukiage K, Otsuka S, Uejima D. Expression of claudin7 is tightly associated with epithelial structures in synovial sarcomas and regulated by an Ets family transcription factor, ELF3. J Biol Chem. 2006;281:38941-38950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 103. | Yano K, Imaeda T, Niimi T. Transcriptional activation of the human claudin-18 gene promoter through two AP-1 motifs in PMA-stimulated MKN45 gastric cancer cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G336-G343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 104. | Ito T, Kojima T, Yamaguchi H, Kyuno D, Kimura Y, Imamura M, Takasawa A, Murata M, Tanaka S, Hirata K. Transcriptional regulation of claudin-18 via specific protein kinase C signaling pathways and modification of DNA methylation in human pancreatic cancer cells. J Cell Biochem. 2011;112:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 105. | Wöll S, Schlitter AM, Dhaene K, Roller M, Esposito I, Sahin U, Türeci Ö. Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms. Int J Cancer. 2014;134:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 106. | Tanaka M, Shibahara J, Fukushima N, Shinozaki A, Umeda M, Ishikawa S, Kokudo N, Fukayama M. Claudin-18 is an early-stage marker of pancreatic carcinogenesis. J Histochem Cytochem. 2011;59:942-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 107. | Krug SM, Amasheh S, Richter JF, Milatz S, Günzel D, Westphal JK, Huber O, Schulzke JD, Fromm M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell. 2009;20:3713-3724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 108. | Korompay A, Borka K, Lotz G, Somorácz A, Törzsök P, Erdélyi-Belle B, Kenessey I, Baranyai Z, Zsoldos F, Kupcsulik P. Tricellulin expression in normal and neoplastic human pancreas. Histopathology. 2012;60:E76-E86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 109. | Kojima T, Fuchimoto J, Yamaguchi H, Ito T, Takasawa A, Ninomiya T, Kikuchi S, Ogasawara N, Ohkuni T, Masaki T. c-Jun N-terminal kinase is largely involved in the regulation of tricellular tight junctions via tricellulin in human pancreatic duct epithelial cells. J Cell Physiol. 2010;225:720-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 110. | Sancho R, Nateri AS, de Vinuesa AG, Aguilera C, Nye E, Spencer-Dene B, Behrens A. JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J. 2009;28:1843-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 111. | Cui J, Han SY, Wang C, Su W, Harshyne L, Holgado-Madruga M, Wong AJ. c-Jun NH(2)-terminal kinase 2alpha2 promotes the tumorigenicity of human glioblastoma cells. Cancer Res. 2006;66:10024-10031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 112. | Yang YM, Bost F, Charbono W, Dean N, McKay R, Rhim JS, Depatie C, Mercola D. C-Jun NH(2)-terminal kinase mediates proliferation and tumor growth of human prostate carcinoma. Clin Cancer Res. 2003;9:391-401. [PubMed] |
| 113. | Takahashi R, Hirata Y, Sakitani K, Nakata W, Kinoshita H, Hayakawa Y, Nakagawa H, Sakamoto K, Hikiba Y, Ijichi H. Therapeutic effect of c-Jun N-terminal kinase inhibition on pancreatic cancer. Cancer Sci. 2013;104:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | Kojima T, Takasawa A, Kyuno D, Ito T, Yamaguchi H, Hirata K, Tsujiwaki M, Murata M, Tanaka S, Sawada N. Downregulation of tight junction-associated MARVEL protein marvelD3 during epithelial-mesenchymal transition in human pancreatic cancer cells. Exp Cell Res. 2011;317:2288-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 115. | Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer. 2007;7:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 116. | Andreeva AY, Piontek J, Blasig IE, Utepbergenov DI. Assembly of tight junction is regulated by the antagonism of conventional and novel protein kinase C isoforms. Int J Biochem Cell Biol. 2006;38:222-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 117. | Ellis B, Schneeberger EE, Rabito CA. Cellular variability in the development of tight junctions after activation of protein kinase C. Am J Physiol. 1992;263:F293-F300. [PubMed] |
| 118. | Sjö A, Magnusson KE, Peterson KH. Distinct effects of protein kinase C on the barrier function at different developmental stages. Biosci Rep. 2003;23:87-102. [PubMed] |
| 119. | Ali AS, Ali S, El-Rayes BF, Philip PA, Sarkar FH. Exploitation of protein kinase C: a useful target for cancer therapy. Cancer Treat Rev. 2009;35:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 120. | Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161-167. [PubMed] |
| 121. | El-Rayes BF, Ali S, Philip PA, Sarkar FH. Protein kinase C: a target for therapy in pancreatic cancer. Pancreas. 2008;36:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 122. | Scotti ML, Bamlet WR, Smyrk TC, Fields AP, Murray NR. Protein kinase Ciota is required for pancreatic cancer cell transformed growth and tumorigenesis. Cancer Res. 2010;70:2064-2074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 123. | Denham DW, Franz MG, Denham W, Zervos EE, Gower WR, Rosemurgy AS, Norman J. Directed antisense therapy confirms the role of protein kinase C-alpha in the tumorigenicity of pancreatic cancer. Surgery. 1998;124:218-223; discussion 223-224. [PubMed] |
| 124. | Zhang X, Wen J, Aletta JM, Rubin RP. Inhibition of expression of PKC-alpha by antisense mRNA is associated with diminished cell growth and inhibition of amylase secretion by AR4-2J cells. Exp Cell Res. 1997;233:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 125. | Yoshida K, Kanaoka S, Takai T, Uezato T, Miura N, Kajimura M, Hishida A. EGF rapidly translocates tight junction proteins from the cytoplasm to the cell-cell contact via protein kinase C activation in TMK-1 gastric cancer cells. Exp Cell Res. 2005;309:397-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 126. | Leotlela PD, Wade MS, Duray PH, Rhode MJ, Brown HF, Rosenthal DT, Dissanayake SK, Earley R, Indig FE, Nickoloff BJ. Claudin-1 overexpression in melanoma is regulated by PKC and contributes to melanoma cell motility. Oncogene. 2007;26:3846-3856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 127. | Tuomi S, Mai A, Nevo J, Laine JO, Vilkki V, Ohman TJ, Gahmberg CG, Parker PJ, Ivaska J. PKCepsilon regulation of an alpha5 integrin-ZO-1 complex controls lamellae formation in migrating cancer cells. Sci Signal. 2009;2:ra32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 128. | Kojima T, Sawada N. Regulation of tight junctions in human normal pancreatic duct epithelial cells and cancer cells. Ann N Y Acad Sci. 2012;1257:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 129. | Chow JY, Dong H, Quach KT, Van Nguyen PN, Chen K, Carethers JM. TGF-beta mediates PTEN suppression and cell motility through calcium-dependent PKC-alpha activation in pancreatic cancer cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G899-G905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 130. | Konopatskaya O, Poole AW. Protein kinase Calpha: disease regulator and therapeutic target. Trends Pharmacol Sci. 2010;31:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 131. | Zhao S, Venkatasubbarao K, Lazor JW, Sperry J, Jin C, Cao L, Freeman JW. Inhibition of STAT3 Tyr705 phosphorylation by Smad4 suppresses transforming growth factor beta-mediated invasion and metastasis in pancreatic cancer cells. Cancer Res. 2008;68:4221-4228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 132. | Javle MM, Gibbs JF, Iwata KK, Pak Y, Rutledge P, Yu J, Black JD, Tan D, Khoury T. Epithelial-mesenchymal transition (EMT) and activated extracellular signal-regulated kinase (p-Erk) in surgically resected pancreatic cancer. Ann Surg Oncol. 2007;14:3527-3533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 133. | Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J. 2011;30:770-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 134. | Wang L, Heidt DG, Lee CJ, Yang H, Logsdon CD, Zhang L, Fearon ER, Ljungman M, Simeone DM. Oncogenic function of ATDC in pancreatic cancer through Wnt pathway activation and beta-catenin stabilization. Cancer Cell. 2009;15:207-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 135. | Nagathihalli NS, Merchant NB. Src-mediated regulation of E-cadherin and EMT in pancreatic cancer. Front Biosci (Landmark Ed). 2012;17:2059-2069. [PubMed] |