Published online Jan 14, 2014. doi: 10.3748/wjg.v20.i2.498
Revised: September 15, 2013
Accepted: October 17, 2013
Published online: January 14, 2014
Processing time: 219 Days and 4.4 Hours
AIM: To generate a Gpr128 gene knockout mouse model and to investigate its phenotypes and the biological function of the Gpr128 gene.
METHODS: Bacterial artificial chromosome-retrieval methods were used for constructing the targeting vector. Using homologous recombination and microinjection technology, a Gpr128 knockout mouse model on a mixed 129/BL6 background was generated. The mice were genotyped by polymerase chain reaction (PCR) analysis of tail DNA and fed a standard laboratory chow diet. Animals of both sexes were used, and the phenotypes were assessed by histological, biochemical, molecular and physiological analyses. Semi-quantitative reverse transcription-PCR and Northern blotting were used to determine the tissue distribution of Gpr128 mRNA. Beginning at the age of 4 wk, body weights were recorded every 4 wk. Food, feces, blood and organ samples were collected to analyze food consumption, fecal quantity, organ weight and constituents of the blood and plasma. A Trendelenburg preparation was utilized to examine intestinal motility in wild-type (WT) and Gpr128-/- mice at the age of 8 and 32 wk.
RESULTS: Gpr128 mRNA was highly and exclusively detected in the intestinal tissues. Targeted deletion of Gpr128 in adult mice resulted in reduced body weight gain, and mutant mice exhibited an increased frequency of peristaltic contraction and slow wave potential of the small intestine. The Gpr128+/+ mice gained more weight on average than the Gpr128-/- mice since 24 wk, being 30.81 ± 2.84 g and 25.74 ± 4.50 g, respectively (n = 10, P < 0.01). The frequency of small intestinal peristaltic contraction was increased in Gpr128-/- mice. At the age of 8 wk, the frequency of peristalsis with an intraluminal pressure of 3 cmH2O was 6.6 ± 2.3 peristalsis/15 min in Gpr128-/- intestine (n = 5) vs 2.6 ± 1.7 peristalsis/15 min in WT intestine (n = 5, P < 0.05). At the age of 32 wk, the frequency of peristaltic contraction with an intraluminal pressure of 2 and 3 cmH2O was 4.6 ± 2.3 and 3.1 ± 0.8 peristalsis/15 min in WT mice (n = 8), whereas in Gpr128-/- mice (n = 8) the frequency of contraction was 8.3 ± 3.0 and 7.4 ± 3.1 peristalsis/15 min, respectively (2 cmH2O: P < 0.05 vs WT; 3 cmH2O: P < 0.01 vs WT). The frequency of slow wave potential in Gpr128-/- intestine (35.8 ± 4.3, 36.4 ± 4.2 and 37.1 ± 4.8/min with an intraluminal pressure of 1, 2 and 3 cmH2O, n = 8) was also higher than in WT intestine (30.6 ± 4.2, 31.4 ± 3.9 and 31.9 ± 4.5/min, n = 8, P < 0.05).
CONCLUSION: We have generated a mouse model with a targeted deletion of Gpr128 and found reduced body weight and increased intestinal contraction frequency in this animal model.
Core tip: The Adhesion family is the second largest subfamily of the G-protein-coupled receptors (GPCR). The physiological function of the orphan Adhesion-GPCR Gpr128 is unknown. In the present study, we generated Gpr128 knockout mice and confirmed the selective expression of Gpr128 in the intestinal tissues. Phenotypic analysis revealed that targeted deletion of Gpr128 in the mouse resulted in reduced body weight gain and increased frequency of peristaltic contraction and slow wave potential in the small intestine. The physiological roles of Gpr128 in the gastrointestinal tract and its potential as a therapeutic target for obesity and nutritional disorders warrant further investigation.
-
Citation: Ni YY, Chen Y, Lu SY, Sun BY, Wang F, Wu XL, Dang SY, Zhang GH, Zhang HX, Kuang Y, Fei J, Gu MM, Rong WF, Wang ZG. Deletion of
Gpr128 results in weight loss and increased intestinal contraction frequency. World J Gastroenterol 2014; 20(2): 498-508 - URL: https://www.wjgnet.com/1007-9327/full/v20/i2/498.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i2.498
G protein-coupled receptors (GPCRs) constitute one of the largest protein families in humans[1,2] and play important roles in the transduction of intercellular signals across the plasma membrane via different G-proteins[3,4]. GPCRs respond to a large variety of extracellular signals including small molecules such as Ca2+, hormones, peptides, chemokines and other factors as well as sensory stimuli such as vision, smell, taste and neuronal transmission in response to photons[5]. Due to their extremely diverse roles in biological processes, GPCRs represent important molecular targets for biomedical research and drug discovery[6].
The adhesion family of GPCRs (Adhesion-GPCRs) is the second largest subfamily of GPCRs, with over 30 members found in mammals[7,8]. These proteins are characterized by the dual presence of a secretin-like seven-transmembrane (7TM) domain and a long cell adhesion-like N-terminal domain, which typically consists of a functional GPCR proteolytic site domain (GPS domain) and one or more conserved domains[9,10]. Generally, the long N-termini bind various proteins that promote cell-to-cell and cell-to-matrix interactions[11]. However, some Adhesion-GPCRs were found to have a GPS domain but to lack the conserved domains. HE6 and GPR56 are two such members for which no N-terminal conserved domains have been identified, although they have both been shown to have adhesive properties. HE6 attachment appeared to be required for the maturation of germ cells because mutation of this receptor resulted in male infertility in mice[12]. Mutations in GPR56 have been shown to be associated with cortical malformation of the human brain[13,14] and to participate in tumor cell adhesion[15,16].
GPR128 is an orphan receptor of the Adhesion-GPCR family uncovered during BLASTP searches of the Celera database in 2003. GPR128 is phylogenetically related to HE6 and GPR56 and lacks the conserved N-termini domains apart from the GPS domain[17]. The mouse Gpr128 shares 69.9% homology with human GPR128 and contains 16 exons.
GPCRs are expressed in virtually all tissue types in the body[18]. However, some GPCRs are expressed in specific tissues and therefore are important targets for drug discovery[19]. The tissue distribution of GPR128, as derived from the EST data or analysed by real-time quantitative polymerase chain reaction (RT-qPCR), shows specific patterns in human and mouse gastrointestinal tissue[20,21]. However, until the commencement of this study, there was little information regarding the ligand or the physiological function of GPR128 in mammals. Using PCR, Northern blotting and immunofluorescence staining, we show that Gpr128 might be exclusively expressed in mouse intestine tissue. To study the role of Gpr128 in the intestine, we generated mice with a targeted deletion of Gpr128. We found that Gpr128 knockout mice exhibited less body weight gain and an increase in intestinal contraction frequency compared with their wild-type (WT) counterparts.
The 129/Sv bacterial artificial chromosome (BAC) clone bMQ-239c21 was provided by the Sanger Institute. BAC-retrieval methods were used for constructing the targeting vector[22,23].
The sequence, including the GPS domain and a portion of the 7TM domain, was retrieved from the BAC clone using a retrieval vector containing two homologous arms.
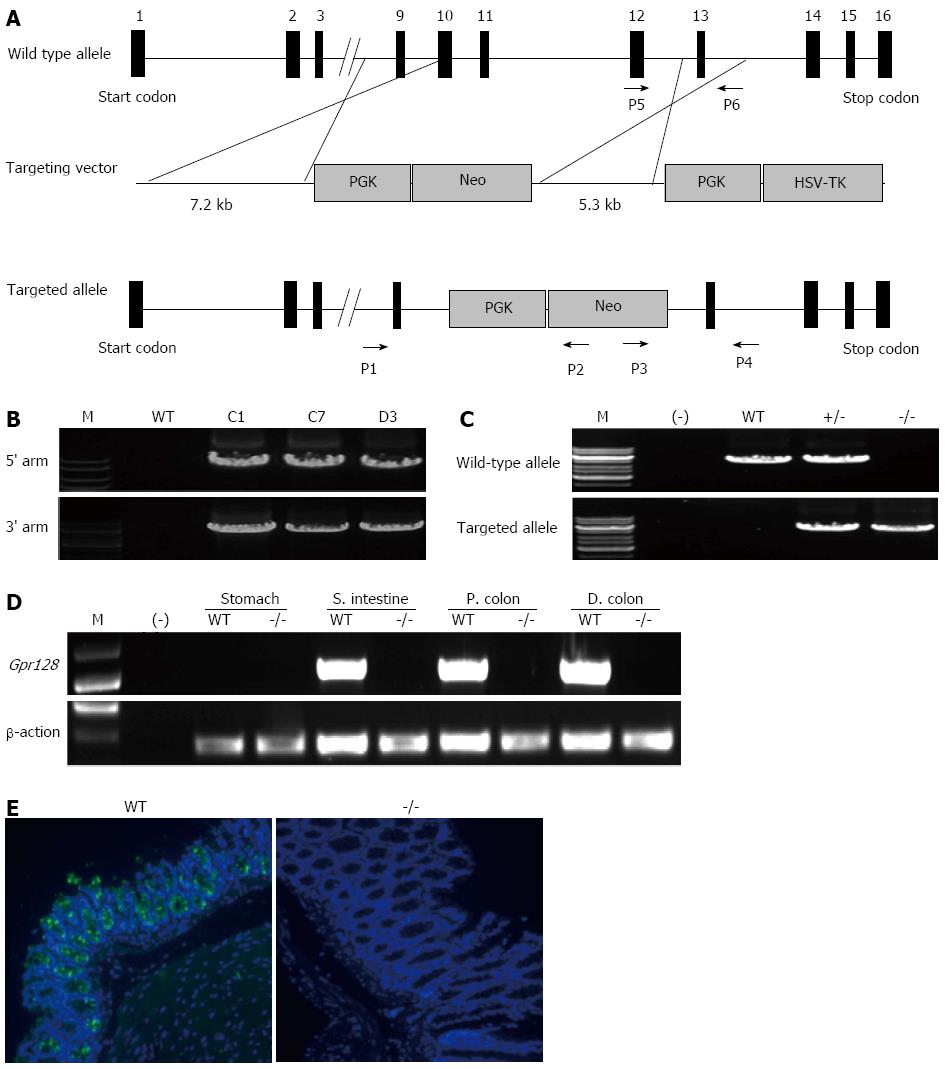
A targeting vector was constructed by replacing the mouse Gpr128 genomic fragment (8.4 kb) covering exons 10-12 with the 1.9-kb phosphoglycerate kinase-neomycin resistance (PGK-Neo) cassette for positive selection and was laid with an external herpes simplex virus-1-thymidine kinase cassette for negative selection[24]. Additionally, this deletion causes an out-of-frame reading frame shift and thereby generates a loss-of-function allele.
The targeting vector contained 7.1 kb of homologous DNA upstream of the PGK-Neo cassette and 5.3 kb of homologous DNA downstream of the cassette as homologous recombination arms. After linearization, the targeting vector was electroporated into embryonic stem (ES) cells derived from 129/Sv G418- and GANC-resistant clones were selected using two pairs of PCR primers. The sequences of the primers used for identifying the recombinant clones are as follows: 5’-CCATAGGAAGAATAATATCAACCAATC-3’ (forward primer P1), 5’-CTGAGCCCAGAAAGCGAAGGA-3’ (reverse primer P2), 5’-ACAAAAGCAAAACAAGGTCTGGAAAG-3’ (forward primer P3) and 5’-CCTCCCCCGT GCCTTCCTTGAC-3’ (reverse primer P4).
Chimeric male mice were generated by injecting the recombinant ES cell clone into C57BL/6 blastocysts, which were subsequently implanted into pseudopregnant female recipient mice. Germ line transmission was monitored by a coat color marker. Heterozygous mice were generated by crossing chimeras with WT 129/Sv female mice and selected for sib mating to create WT (Gpr128+/+), heterozygous (Gpr128+/-) and homozygous mice (Gpr128-/-) for further experiments.
The mice were genotyped by PCR analysis of tail DNA using two primer pairs, which allows the amplification of WT and targeted alleles. The forward primer P3 and reverse primer P4 were used to amplify the 3’ targeted allele, which yields a 5.7 kb band. The sequences of the primers used to amplify the WT allele are as follows: 5’-TCTTCATCTCATTAGTTGGATGGGGTA-3’ (forward primer P5) and 5’-ACAAAAGCAAAACAAGGTCTGGAAAG-3’ (reverse primer P6). The length of the WT allele is 5.4 kb.
All experiments involving animals were conducted under protocols approved by Institutional Animal Care and Use Committee of Shanghai Research Center for Model Organisms (Approval ID: 2010-0017), and the care of animals was in accord with the institution’s guidelines.
The mice were anesthetized with ketamine and xylazine diluted in 0.9% saline, and all efforts were made to minimize animal suffering. Total RNA was extracted from adult mouse tissues using Trizol reagent (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions. For RT-PCR analysis, total RNA was treated with RNase-free DNase I (Promega, Fitchburg, Wisconsin, United States) and quantitated. A 1-μg sample of total RNA was reverse-transcribed to cDNA with an RNA PCR kit (Takara, Dalian, Liaoning, China) according to the standard protocol. A fragment of Gpr128 was amplified (25 cycles) with forward primer R1 (5’-GATTCCAACTTCATTACTCTG-3’) and reverse primer R2 (5’-GGTCCATATCTGCCCACTG-3’). β-actin was amplified as a control. As shown in Figure 1D, the specific Gpr128 fragment from WT mice was amplified with forward primer R3 (5’-AACCACAAACTTT TCCAATCAA-3’) and reverse primer R4 (5’-CCACT CAGGGCATAAATAC TCC-3’).
Total RNA was extracted from adult mouse tissues using Trizol reagent (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions. Northern blotting was performed as described in the manual provided by the manufacturer (Northern Max-Gly; Ambion Inc., Carlsbad, CA, United States). A 1-μg aliquot was removed from each mRNA sample from adult WT mice for analysis. The probe used for Gpr128 was a 715-bp DNA fragment prepared from mouse intestine cDNA using the PCR forward primer N1 (5’-AGAGTCGACAGACAGACCACTGAAGGGAAG-3’) and reverse primer N2 (5’-TGGCA TCAAAATCTGACTC-3’). Probe DNA (25 ng) was labeled with [a32P]-dATP using a Random Primer Labeling Kit (NEBlot Kit, NEB, Beverly, MA, United States) and subsequently purified by gel filtration.
All mice used in this study were on a mixed 129/BL6 background. The mouse colony was maintained in a temperature- and humidity-controlled room with a 12:12-h light-dark cycle, and the mice were fed a standard laboratory chow diet with free access to water. The animals were maintained by crossing heterozygous progeny.
Beginning at the age of 4 wk, body weights were recorded every 4 wk. Animals of both sexes were used, but littermates were matched by gender.
The intestines of WT and Gpr128-/- mice at 8 wk of age were collected and fixed with 10% formalin for sectioning followed by hematoxylin and eosin (HE) staining. Sections (6 mm) were cut and stained with HE according to standard procedures. For immunofluorescence analysis, paraffin-embedded sections were deparaffinized with xylene and treated with gradually decreasing concentrations of ethanol. The sections were blocked for 1 h in 5% bovine serum followed by staining overnight at 37 °C with goat anti-GPR128 antibodies (sc-48208, Santa-Cruz Biotechnology Inc., Santa-Cruz, CA, United States) for human and mouse tissues and finally incubated with fluorescent-conjugated secondary antibody for 30 min. Finally, the slides were rinsed with PBS and mounted with VECTASHIELD mounting medium (H-1200, Vector Laboratories Inc., Burlingame, CA, United States).
At week 16 of the experimental diet period, the mice were individually caged and given preweighed food for 5 d. During this period, the amount of food consumed was determined, and feces were quantitatively collected over a 24 h period. The results are expressed as grams of food consumed and feces excreted per day.
After the 32 wk experimental feeding period, the mice were fasted for 16 h and subsequently anesthetized with ketamine and xylazine diluted in 0.9% saline. Blood was removed by cardiac puncture into tubes containing 1 mmol/L EDTA. White adipose (epididymal and uterine fat pads) and brown adipose (intrascapular) tissue as well as the heart, liver, spleen, lungs, and kidneys were removed, and the wet weight of each was recorded.
Blood samples were collected for complete blood counts including white blood cells, red blood cells, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, platelets, white-small cell rate, white-middle cell rate, and white-large cell rate using an automated hematology analyzer (Poch-100ivd, Sysmex, Kobe, Japan). Plasma was obtained by low-speed centrifugation of the blood samples for measurement of albumin/globulin, globulin, low-density lipoprotein cholesterol, albumin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, urea nitrogen, creatinine, glucose, high-density lipoprotein cholesterol, lactate dehydrogenase, total cholesterol, triglycerides and total protein using an automated chemistry analyzer (CHEMIX-180; Sysmex, Kobe, Japan).
Male and female mice at 8 and 32 wk of age were sacrificed. A Trendelenburg preparation was utilized to examine intestinal motility in WT and Gpr128-/- mice. Briefly, the jejunum was removed and placed in pre-oxygenated Kreb’s Ringer solution at room temperature. A segment of the jejunum (6 cm long) was placed into an organ bath and was superfused with oxygenated Krebs solution at 37 °C. Both ends of the jejunum were catheterized. The proximal tube was connected to a syringe cylinder (for altering the resting intraluminal pressure) and a pressure transducer via a three-way stopcock. A glass micropipette (tip diameter approximately 50 μm) was placed on the intestinal wall to record the slow waves through gentle suction. The peristalsis and slow waves were fed into a computer through the Micro1401 interface (Cambridge Electronic Design, United Kingdom) and analyzed using the Spike2 program (CED, United Kingdom). The preparation was allowed to stabilize for at least 40 min before the experiments were started.
The data are presented as the mean ± SD. Differences between groups were determined by the 2-tailed Student t test. P values less than 0.05 were considered significant.
To investigate the potential roles of Gpr128 in mice, we generated a targeted disruption of the mouse Gpr128 gene in ES cells by homologous recombination. In the targeting vector, 3 exons (10, 11 and 12), which encode the GPS domain and a portion of 7TM domain, were replaced with a PGK cassette followed by the neomycin resistance gene (Figure 1A). After electroporating ES cells with the linearized targeting vector under positive-negative selection, we identified three targeted ES clones by PCR (Figure 1B). Two of these clones were microinjected into C57BL/6 blastocysts to obtain chimeras. Mice heterozygous for Gpr128 showed normal development and were fertile, indicating that the targeted locus does not have detrimental dominant activity.
The genotypes of the offspring were analyzed by PCR to identify WT (+/+), heterozygous (+/-), and homozygous (-/-) mice. Amplification of the WT and targeted alleles produced bands of 5.4 and 5.7 kb, respectively (Figure 1C). As expected, the ratio of phenotypes was in accord with Mendelian frequency, indicating that there was no increased embryonic mortality in the mutant animals. Semi-quantitative RT-PCR and immunofluorescence staining demonstrated that Gpr128 was not detected in the intestine of homozygous mice (Figure 1D and E), indicating that we have successfully established a Gpr128 disruption mouse model.
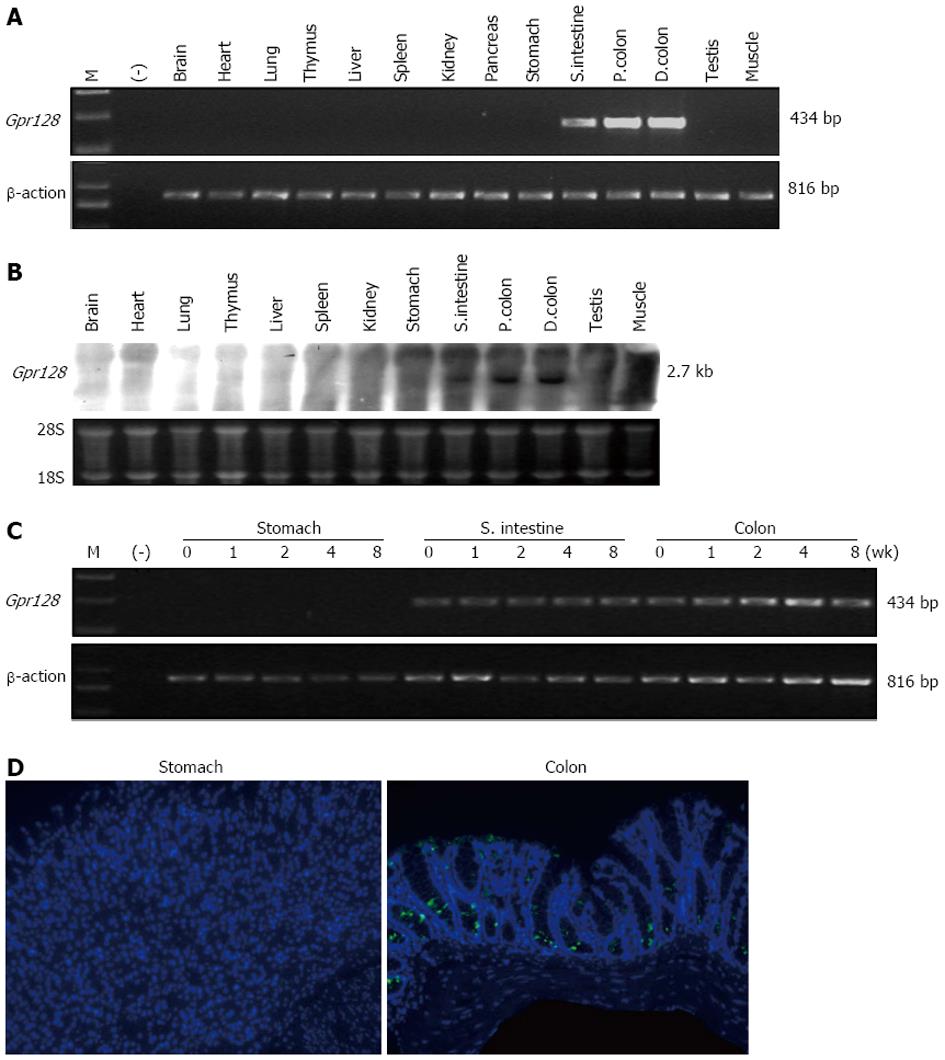
We investigated the expression pattern of the WT Gpr128 gene in adult mouse tissues by semi-quantitative RT-PCR, Northern blotting and immunofluorescence staining. Gpr128 mRNA was highly and exclusively detected in the intestine (Figure 2A, B and D). RT-PCR was then performed to determine the presence of Gpr128 mRNA throughout the digestive tract and at different postnatal development stages. Gpr128 expression was detected prominently in the small intestine and colon from postnatal day 0 through 8 wk (Figure 2C). The distribution of Gpr128 protein in the mouse intestine was then analyzed by immunofluorescence staining. We found that the Gpr128 protein was confined to the mucosa. As shown in Figure 2D, Gpr128 expression was restricted to epithelial cells.
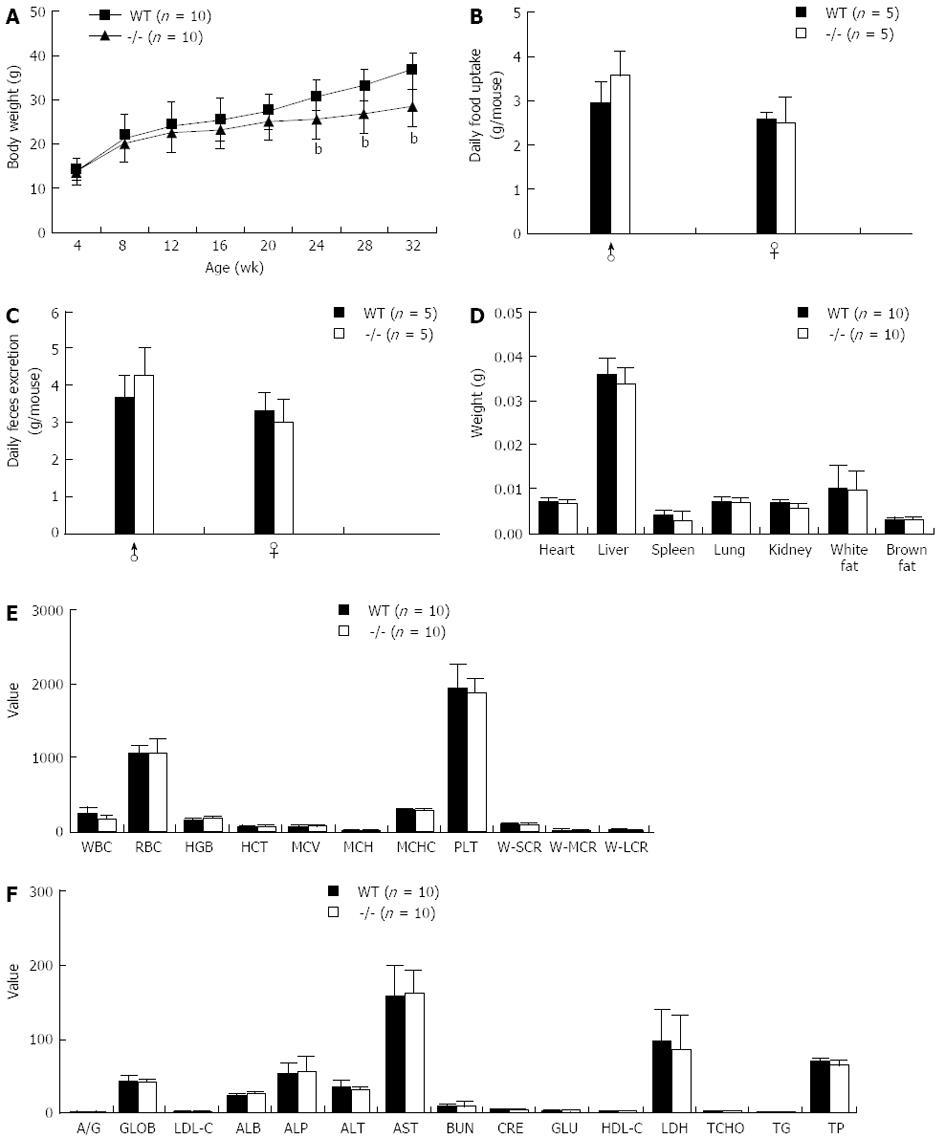
Mice lacking the Gpr128 gene (Gpr128-/-) grew normally and displayed normal reproductive functions on a standard mouse chow diet. We found no differences between Gpr128+/+ and Gpr128-/- mice with respect to food intake or fecal excretion (Figure 3B and C). However, Gpr128-/- mice gained less weight on average than their Gpr128+/+ littermates by 24 wk of age. The body weights of WT and Gpr128-/- mice were 30.81 ± 2.84 and 25.74 ± 4.50 g, respectively (Figure 3A, n = 10, P < 0.01). When separated by sex, both male and female Gpr128-/- mice gained less weight than their WT counterparts (data not shown). The decreased weight gain in Gpr128-/- mice persisted at 28 and 32 wk (26.69 ± 4.29 and 28.46 ± 4.42 g vs 33.15 ± 3.20 and 36.75 ± 4.18 g in Gpr128+/+ mice, n = 10, P < 0.01, Figure 3A).
To account for the differences in body weight gain between the Gpr128+/+ and Gpr128-/- mice, various tissues were removed and weighed. There were no differences in the epididymal and uterine fat pads, brown fat, or liver weights between male and female Gpr128+/+ and Gpr128-/- mice (Figure 3D). There were also no differences in heart, spleen, lung, and kidney weights between the Gpr128+/+ and Gpr128-/- mice (Figure 3D).
The cell counts and biochemical parameters of the blood of Gpr128-/- mice were not different from those of the WT mice (Figure 3E and F). Furthermore, there were no overt differences in the gross morphology or histology (HE staining) of the GI tract between the Gpr128-/- and the WT mice (data not shown).
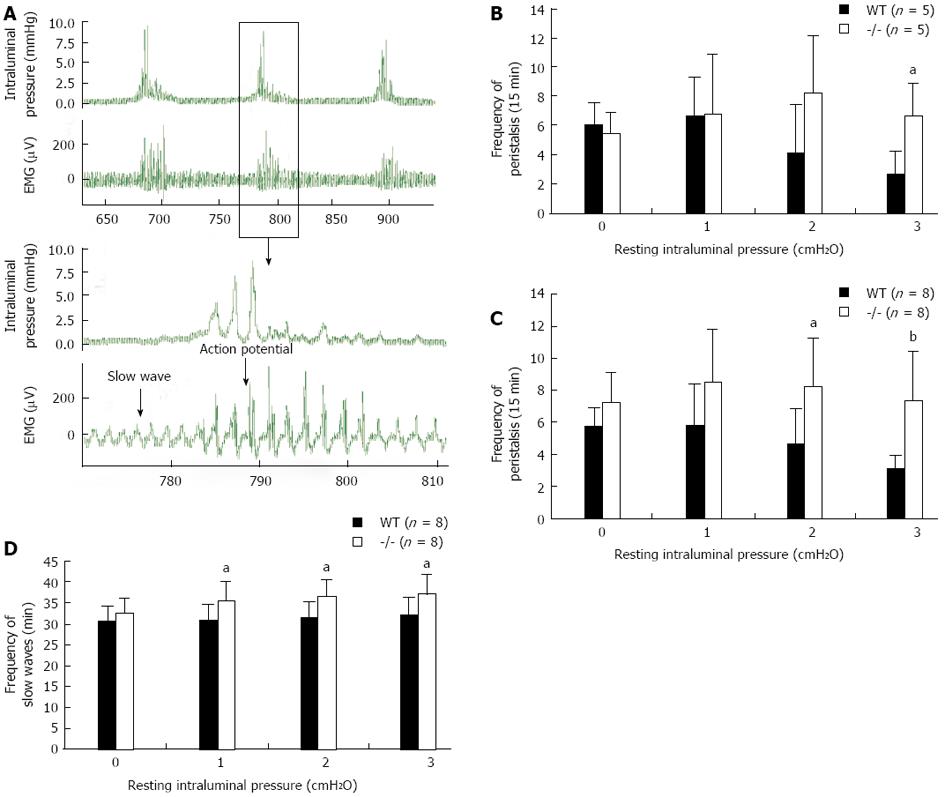
Using a Trendelenburg model, we analyzed the peristalsis and the slow waves of the small intestine (jejunum) in WT and Gpr128-/- mice (Figure 4A). The amplitudes of peristaltic movement at resting intraluminal pressures of 0, 1, 2 and 3 cmH2O were not different between WT and Gpr128-/- mice (data not shown). The frequency of peristaltic contraction was increased in Gpr128-/- mice since 8 wk when the resting intraluminal pressure increased. The frequency of peristalsis was higher in Gpr128-/- mice than in WT mice when the resting intraluminal pressure was 3 cmH2O (6.6 ± 2.3 peristalsis/15 min in Gpr128-/- intestine vs 2.6 ± 1.7 peristalsis/15 min in WT intestine, n = 5, P < 0.05, Figure 4B). At the age of 32 wk, the frequency of peristalsis was higher in Gpr128-/- mice than in WT mice when the resting intraluminal pressure was 2 or 3 cmH2O (8.3 ± 3.0 and 7.4 ± 3.1 peristalsis/15 min in Gpr128-/- intestine vs 4.6 ± 2.3 and 3.1 ± 0.8 peristalsis/15 min in WT intestine, n = 8, 2 cmH2O: P < 0.05, 3 cmH2O: P < 0.01, Figure 4C) and the frequency of slow waves was also higher in Gpr128-/- intestine compared with WT intestine (30.6 ± 4.2, 31.4 ± 3.9, and 31.9 ± 4.5/min and 35.8 ± 4.3, 36.4 ± 4.2, and 37.1 ± 4.8/min in normal and Gpr128-/- mice, respectively, n = 8, P < 0.05, Figure 4D).
Here, we describe the first genetic analysis of Gpr128 function in a mammalian model. A targeted mutation of GPR128 causes a deletion of part of the 7TM region (Figure 1A) and is presumably a null allele. Residual WT transcripts could not be detected in the intestines of mutant mice (Figure 1D and E).
GPR128 is an orphan GPCR, the physiological function of which is unknown. To explore the role of Gpr128, we first examined its expression profile in different tissues. We found that Gpr128 mRNA expression is exclusively confined to the small intestine and colon. Through immunofluorescence staining, Gpr128 immunoreactivity was detected in the mucosa of the intestine and was found to be restricted to epithelial cells.
The cell count and biochemical parameters of Gpr128-/- mice were not different from those of their WT counterparts, indicating that Gpr128 is not essential for the maintenance of homeostasis.
A major finding in the Gpr128-/- mice was the lower body weight gain compared with the WT littermates by 24 wk of age when the animals were maintained on a standard laboratory rodent chow diet. Additionally, there were no significant differences in the weights of epididymal or uterine fat pads, brown fat, or the liver between WT and Gpr128-/- mice. These data suggest that the observed weight difference between the mice was not due to reduced adiposity in the Gpr128 knockout mice.
A number of factors may potentially participate in the regulation of energy balance and weight gain, including gastric emptying[25], gastrointestinal motility[26] as well as gastrointestinal peptides such as ghrelin and cholecystokinin. The release of these two hormones is known to be regulated by ingestion and their action may in turn regulate gastrointestinal function and food intake[29,30]. However, given that Gpr128-/- and WT mice consumed equivalent amounts of chow, the excretion of feces was similar in the two groups and Gpr128 was confined to the intestinal tissue, we tested the potential differences in intestinal motility between Gpr128-/- and WT mice. The frequency of peristaltic movement and slow waves were found to be increased in Gpr128-/- intestine compared with WT intestine. Despite similar levels of chow consumption, Gpr41-/- mice colonized with the model fermentative community are significantly leaner and lighter than their WT littermates because their increased intestinal motility reduces the time required to harvest energy from the diet[31]. Whether the increase in gut motility accounts for the lower weight gain in Gpr128-/- mice awaits further investigation. Because peristalsis is known to be regulated by the enteric nerve plexus[32], whereas the slow waves are known to originate from the interstitial cells of Cajal[33], further studies should be conducted to examine their development and function in Gpr128-/- mice. Given the epithelial localization of Gpr128 within the gut, it will also be important to explore its role in the regulation of intestinal secretion and absorption.
In summary, the present study shows that Gpr128 is expressed exclusively in the small and large intestine, and Gpr128 deficiency resulted in a decrease in body weight gain and an increase in intestinal motility. The potential for Gpr128 as a novel therapeutic target for obesity and nutritional disorders is worth exploring.
The Adhesion family is the second largest subfamily of G-protein-coupled receptors (GPCR) which is one of the largest superfamilies of cell-surface receptors. Family members are characterized by the dual presence of a secretin-like seven-transmembrane domain and a long cell adhesion-like N-terminus that typically contains one functional GPCR proteolytic site domain domain; however, the function of most of these receptors is still not understood.
An orphan receptor of the Adhesion-GPCR GPR128 was identified during BLASTP searches of the Celera database in 2003. The tissue distribution of GPR128 derived from the EST data shows specific pattern in humans and mice. The physiological function of GPR128 in mammals is still unknown.
In this study, the authors generated a targeted deletion of Gpr128 mouse model to explore the biological function of Gpr128. Furthermore, they found that Gpr128 is exclusively expressed in mouse intestinal tissue. Finally, we showed that the targeted deletion of the orphan adhesion-GPCR Gpr128 resulted in reduced body weight gain and increased intestinal contraction frequency in mice.
The present findings regarding the activities of Gpr128 in mouse intestinal cells showed for the first time that Gpr128 is a regulator of host energy balance and may help explain the biological functions of Gpr128 in the intestine. Future studies are needed to identify the ligands of Gpr128 which are often the key to determining the functional role, and to determine the mechanism by which Gpr128 regulates intestinal contraction frequency. Gpr128 may be a potential drug target and may be useful for the development of novel therapies for obesity and nutritional disorders.
GPCRs constitute one of the largest protein families in humans. GPCRs receive extracellular signals and transmit them into cells via an intracellular signaling pathway that employs different G-proteins. The GPCR family has attracted significant attention from researchers due to its important role in drug discovery.
After the generation of a Gpr128 gene knockout mouse model and the investigation of its phenotypes and the biological function of Gpr128, the authors found that the deletion of Gpr128 in mice resulted in weight loss and increased intestinal contraction frequency. The authors attempted to demonstrate the relationship between weight loss and intestinal motility. Overall, this study fits nicely within the scope of the journal. The data are generally clean and could potentially uncover the physiological roles of Gpr128, which is of value to the field.
P- Reviewers: Han JY, Nakajima N, Tu Y S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W. Initial sequencing and analysis of the human genome. Nature. 2001;409:860-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16054] [Cited by in RCA: 15083] [Article Influence: 628.5] [Reference Citation Analysis (0)] |
| 2. | Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, Yao A, Ye J, Zhan M, Zhang W, Zhang H, Zhao Q, Zheng L, Zhong F, Zhong W, Zhu S, Zhao S, Gilbert D, Baumhueter S, Spier G, Carter C, Cravchik A, Woodage T, Ali F, An H, Awe A, Baldwin D, Baden H, Barnstead M, Barrow I, Beeson K, Busam D, Carver A, Center A, Cheng ML, Curry L, Danaher S, Davenport L, Desilets R, Dietz S, Dodson K, Doup L, Ferriera S, Garg N, Gluecksmann A, Hart B, Haynes J, Haynes C, Heiner C, Hladun S, Hostin D, Houck J, Howland T, Ibegwam C, Johnson J, Kalush F, Kline L, Koduru S, Love A, Mann F, May D, McCawley S, McIntosh T, McMullen I, Moy M, Moy L, Murphy B, Nelson K, Pfannkoch C, Pratts E, Puri V, Qureshi H, Reardon M, Rodriguez R, Rogers YH, Romblad D, Ruhfel B, Scott R, Sitter C, Smallwood M, Stewart E, Strong R, Suh E, Thomas R, Tint NN, Tse S, Vech C, Wang G, Wetter J, Williams S, Williams M, Windsor S, Winn-Deen E, Wolfe K, Zaveri J, Zaveri K, Abril JF, Guigó R, Campbell MJ, Sjolander KV, Karlak B, Kejariwal A, Mi H, Lazareva B, Hatton T, Narechania A, Diemer K, Muruganujan A, Guo N, Sato S, Bafna V, Istrail S, Lippert R, Schwartz R, Walenz B, Yooseph S, Allen D, Basu A, Baxendale J, Blick L, Caminha M, Carnes-Stine J, Caulk P, Chiang YH, Coyne M, Dahlke C, Mays A, Dombroski M, Donnelly M, Ely D, Esparham S, Fosler C, Gire H, Glanowski S, Glasser K, Glodek A, Gorokhov M, Graham K, Gropman B, Harris M, Heil J, Henderson S, Hoover J, Jennings D, Jordan C, Jordan J, Kasha J, Kagan L, Kraft C, Levitsky A, Lewis M, Liu X, Lopez J, Ma D, Majoros W, McDaniel J, Murphy S, Newman M, Nguyen T, Nguyen N, Nodell M, Pan S, Peck J, Peterson M, Rowe W, Sanders R, Scott J, Simpson M, Smith T, Sprague A, Stockwell T, Turner R, Venter E, Wang M, Wen M, Wu D, Wu M, Xia A, Zandieh A, Zhu X. The sequence of the human genome. Science. 2001;291:1304-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9175] [Cited by in RCA: 7859] [Article Influence: 327.5] [Reference Citation Analysis (0)] |
| 3. | Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164 Suppl 1:S1-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 712] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 4. | Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1840] [Cited by in RCA: 1788] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 5. | Latek D, Modzelewska A, Trzaskowski B, Palczewski K, Filipek S. G protein-coupled receptors--recent advances. Acta Biochim Pol. 2012;59:515-529. [PubMed] |
| 6. | Drews J. Drug discovery: a historical perspective. Science. 2000;287:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1915] [Cited by in RCA: 1669] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 7. | Bjarnadóttir TK, Fredriksson R, Schiöth HB. The adhesion GPCRs: a unique family of G protein-coupled receptors with important roles in both central and peripheral tissues. Cell Mol Life Sci. 2007;64:2104-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Foord SM, Jupe S, Holbrook J. Bioinformatics and type II G-protein-coupled receptors. Biochem Soc Trans. 2002;30:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Huang YS, Chiang NY, Hu CH, Hsiao CC, Cheng KF, Tsai WP, Yona S, Stacey M, Gordon S, Chang GW. Activation of myeloid cell-specific adhesion class G protein-coupled receptor EMR2 via ligation-induced translocation and interaction of receptor subunits in lipid raft microdomains. Mol Cell Biol. 2012;32:1408-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Yona S, Lin HH, Siu WO, Gordon S, Stacey M. Adhesion-GPCRs: emerging roles for novel receptors. Trends Biochem Sci. 2008;33:491-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Davies B, Baumann C, Kirchhoff C, Ivell R, Nubbemeyer R, Habenicht UF, Theuring F, Gottwald U. Targeted deletion of the epididymal receptor HE6 results in fluid dysregulation and male infertility. Mol Cell Biol. 2004;24:8642-8648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Piao X, Hill RS, Bodell A, Chang BS, Basel-Vanagaite L, Straussberg R, Dobyns WB, Qasrawi B, Winter RM, Innes AM. G protein-coupled receptor-dependent development of human frontal cortex. Science. 2004;303:2033-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 408] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 14. | Luo R, Jeong SJ, Jin Z, Strokes N, Li S, Piao X. G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc Natl Acad Sci USA. 2011;108:12925-12930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 15. | Shashidhar S, Lorente G, Nagavarapu U, Nelson A, Kuo J, Cummins J, Nikolich K, Urfer R, Foehr ED. GPR56 is a GPCR that is overexpressed in gliomas and functions in tumor cell adhesion. Oncogene. 2005;24:1673-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Ke N, Sundaram R, Liu G, Chionis J, Fan W, Rogers C, Awad T, Grifman M, Yu D, Wong-Staal F. Orphan G protein-coupled receptor GPR56 plays a role in cell transformation and tumorigenesis involving the cell adhesion pathway. Mol Cancer Ther. 2007;6:1840-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Fredriksson R, Gloriam DE, Höglund PJ, Lagerström MC, Schiöth HB. There exist at least 30 human G-protein-coupled receptors with long Ser/Thr-rich N-termini. Biochem Biophys Res Commun. 2003;301:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Fredriksson R, Schiöth HB. The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol Pharmacol. 2005;67:1414-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 424] [Reference Citation Analysis (0)] |
| 19. | Insel PA, Snead A, Murray F, Zhang L, Yokouchi H, Katakia T, Kwon O, Dimucci D, Wilderman A. GPCR expression in tissues and cells: are the optimal receptors being used as drug targets? Br J Pharmacol. 2012;165:1613-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Haitina T, Olsson F, Stephansson O, Alsiö J, Roman E, Ebendal T, Schiöth HB, Fredriksson R. Expression profile of the entire family of Adhesion G protein-coupled receptors in mouse and rat. BMC Neurosci. 2008;9:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Badiali L, Cedernaes J, Olszewski PK, Nylander O, Vergoni AV, Schiöth HB. Adhesion GPCRs are widely expressed throughout the subsections of the gastrointestinal tract. BMC Gastroenterol. 2012;12:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 981] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 23. | Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 892] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 24. | Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424-8428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1799] [Cited by in RCA: 1832] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 25. | Cunningham KM, Daly J, Horowitz M, Read NW. Gastrointestinal adaptation to diets of differing fat composition in human volunteers. Gut. 1991;32:483-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 149] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Boyd KA, O’Donovan DG, Doran S, Wishart J, Chapman IM, Horowitz M, Feinle C. High-fat diet effects on gut motility, hormone, and appetite responses to duodenal lipid in healthy men. Am J Physiol Gastrointest Liver Physiol. 2003;284:G188-G196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Lee HM, Wang G, Englander EW, Kojima M, Greeley GH. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology. 2002;143:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Verhulst PJ, Depoortere I. Ghrelin’s second life: from appetite stimulator to glucose regulator. World J Gastroenterol. 2012;18:3183-3195. [PubMed] |
| 29. | French SJ, Murray B, Rumsey RD, Fadzlin R, Read NW. Adaptation to high-fat diets: effects on eating behaviour and plasma cholecystokinin. Br J Nutr. 1995;73:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Stengel A, Taché Y. Interaction between gastric and upper small intestinal hormones in the regulation of hunger and satiety: ghrelin and cholecystokinin take the central stage. Curr Protein Pept Sci. 2011;12:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767-16772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1270] [Cited by in RCA: 1149] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 32. | Costa M, Brookes SJ, Hennig GW. Anatomy and physiology of the enteric nervous system. Gut. 2000;47 Suppl 4:iv15-iv19; discussion iv26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Huizinga JD, Zarate N, Farrugia G. Physiology, injury, and recovery of interstitial cells of Cajal: basic and clinical science. Gastroenterology. 2009;137:1548-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |