Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4771
Revised: January 24, 2014
Accepted: March 5, 2014
Published online: April 28, 2014
Processing time: 178 Days and 19.7 Hours
AIM: To investigate whether mesenteric lymph from rats with severe intraperitoneal infection (SII) induces lung injury in healthy rats.
METHODS: Twenty adult male specific pathogen-free Wistar rats were divided into two groups. Animals in the SII group received intraperitoneal injection of Escherichia coli (E. coli) at a dose of 0.3 mL/100 g. Control rats underwent the same procedure, but were injected with normal saline rather than E. coli. We ligated and drained the mesenteric lymphatic vessels and collected the mesenteric lymph. Mesenteric lymph collected from SII or control rats was infused intravenously into male healthy rats at a rate of 1 mL/h for 4 h. At the end of the infusion, all rats were sacrificed. Lungs were removed and examined histologically, and wet-to-dry weight (W/D) ratio and myeloperoxidase (MPO) activity were determined. Enzyme-linked immunosorbent assay (ELISA) was performed to determine the levels of the proinflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-6. We performed Western blot to investigate the activation of Toll-like receptor (TLR)-4, and nuclear factor (NF)-κB p65.
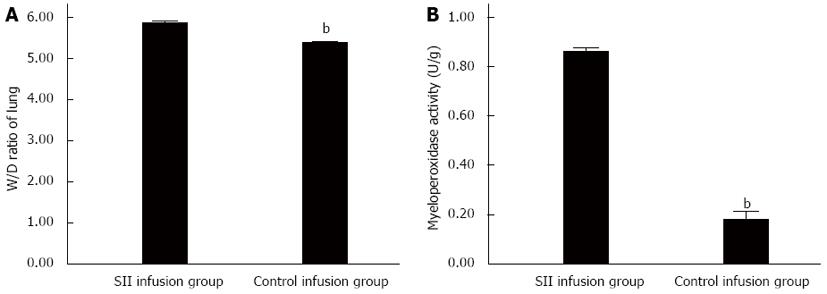
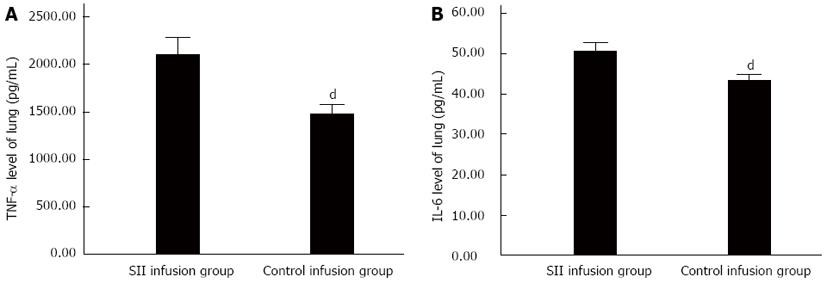
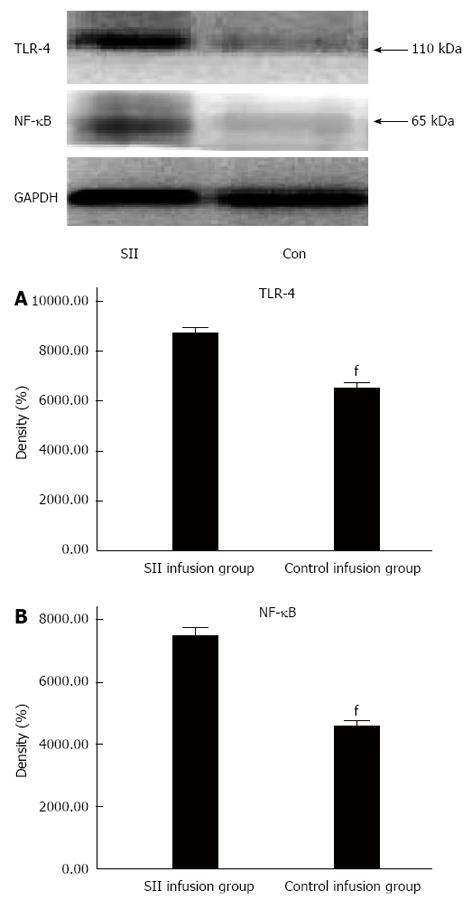
RESULTS: Compared with the control infusion group, there were obvious pathological changes in the SII group. The W/D ratio was significantly increased in the SII compared to control infusion group (5.86 ± 0.06 vs 5.37 ± 0.06, P < 0.01). MPO activity significantly increased in the SII infusion rats with a mean level of 0.86 ± 0.02 U/g compared to 0.18 ± 0.05 U/g in the control group (P < 0.01). The concentrations of TNF-α and IL-6 were significantly increased in the SII infusion group. The concentration of TNF-α was significantly increased in the SII infusion rats compared to control infusion rats (2104.46 ± 245.91 vs 1475.13 ± 137.82 pg/mL, P < 0.01). The concentration of IL-6 was significantly increased in the SII infusion rats with a mean level of 50.56 ± 2.85 pg/mL compared to 43.29 ± 2.02 pg/mL (P < 0.01). The expression levels of TLR-4 (7496.68 ± 376.43 vs 4589.02 ± 233.16, P < 0.01) and NF-κB (8722.19 ± 323.96 vs 6498.91 ± 338.76, P < 0.01) were significantly increased in the SII infusion group compared to the control infusion group. The infusion of SII lymph, but not control lymph, caused lung injury.
CONCLUSION: The results indicate that SII lymph is sufficient to induce acute lung injury.
Core tip: We previously speculated that the lymphatic pathway plays a leading role in the early lung injury caused by severe intraperitoneal infection (SII), and that the mesenteric lymph may be the original source of organ damage. Here, we infused mesenteric lymph from rats with SII into healthy rats to investigate the effect on lung tissues. We confirmed that the damage to the remote organ was caused via the mesenteric lymphatic pathway.
- Citation: Zhang YM, Zhang SK, Cui NQ. Intravenous infusion of mesenteric lymph from severe intraperitoneal infection rats causes lung injury in healthy rats. World J Gastroenterol 2014; 20(16): 4771-4777
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4771.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4771
For intensive care unit (ICU) patients, acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are the most common and life-threatening diseases[1]. In the field of abdominal surgery, severe intraperitoneal infection (SII) caused by some primary diseases, such as perforation peritonitis, severe acute pancreatitis, biliary tract infection, or celiac abscess, is known as the main cause of sepsis or multiple organ dysfunction syndrome (MODS). According to the intestinal lymphatic hypothesis of SIRS/MODS proposed by Deitch et al[2], early in intraperitoneal infection, endotoxin and endogenous inflammatory mediators can enter mesenteric lymphatic vessels and then the lacteal and systemic circulation via the thoracic duct.
Septic peritonitis induced by SII is a clinically relevant polymicrobial sepsis model in rodents[3-5]. Multiple Toll-like receptor (TLR)-dependent pathways are activated during sepsis[6]. Within the TLR family, TLR-4 appears to have a prominent role in the pathogenesis of microbial as well as sterile inflammatory states[7]. Endotoxin signaling is mainly via TLR-4. Endotoxin binds to TLR-4 and leads to activation of nuclear factor (NF)-κB to induce the production of proinflammatory cytokines[8].
We have previously studied endotoxin distribution in the viscera and body fluids in rats with intraperitoneal infection after translocation of endogenous endotoxin. The level of endotoxin in the thoracic duct lymph was significantly higher than that in the portal vein blood[9], and blocking the backflow of abdominal lymph can attenuate ALI in SII rats[10]. Thus, the lymphatic but not portal vein pathway is speculated to play the leading role in the early lung injury caused by SII, and at the same time, the lymph in the thoracic duct may be the original source of organ damage.
In the present study, we infused mesenteric lymph from rats with SII into healthy rats, and examined its effect on lung tissues. We aimed to confirm whether damage to the remote organ was caused via the mesenteric lymphatic pathway, and whether the lymph from SII rats was sufficient to cause lung injury.
Twenty adult male specific pathogen-free Wistar rats were purchased from the Chinese Academy of Military Medical Sciences [Animal license for SCXK (Army), 2009-003]. The animals (250-300 g) were maintained in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the experiments were approved by the Tianjin Nankai Hospital Animal Care and Use Committee.
This study aimed to test whether SII mesenteric lymph was sufficient to induce lung injury. Mesenteric lymph samples collected from both control and SII rats was infused into different healthy rats. In the initial experiment, lymph was collected from the SII or control group for 4 h after the end of the infection period. The collected lymph specimens were centrifuged at 2000 rpm at 4 °C for 10 min and stored at -80 °C. The collected SII and control lymph specimens were infused intravenously into rats at a rate of 1 mL/h for 4 h. The 20 rats were divided equally into the SII infusion and control infusion groups. The volume of lymph infusion was 0.35 mL/100 g, which was based on the fact that the total lymph was produced by the rats during the entire lymph collection period. At the end of the 4-h infusion, the rats were killed and lung tissues were harvested to assess injury.
After a 7-d acclimatization period, rats underwent mesenteric lymph duct cannulation, followed by SII or control infusion, as previously described. The SII group received intraperitoneal injection of Escherichia coli (E. coli) at a dose of 0.3 mL/100 g. Two hours after injection of E. coli, rats were anesthetized with 10% chloral hydrate (3 mL/kg). We surgically exposed the superior mesenteric artery and mesenteric lymphatic vessels, and ligated and drained the latter and collected the mesenteric lymph. Control group rats underwent the same procedure, but were injected with normal saline rather than E. coli. The body temperature was maintained above 36.3 °C with the use of heating pads or lamps as necessary. Mesenteric lymph was collected continuously into heparin-wetted sterile tubes that were placed on ice. The lymph samples were centrifuged for 10 min at 2000 rpm to remove all cellular components and stored at -80 °C until use. A total of 20 rats were used for lymph collection.
Male Wistar rats underwent laparotomy as well as internal jugular vein cannulation. The pooled SII or control group mesenteric lymph specimens were infused intravenously via the jugular vein catheter and microinjection pump at a rate of 1 mL/h for 4 h. At the end, the rats were killed and lung injury was assessed by the wet and dry weight (W/D) ratio of the lung, the levels of inflammatory cytokines, and lung myeloperoxidase (MPO; marker of pulmonary leuko sequestration).
The inferior lobe of the left lung was removed and weighed after gently wiping away the moisture on the surface. The specimen was placed in an 80 °C oven for 48 h and weighed to measure the dry weight. The W/D ratio was calculated for each group.
Frozen lung tissue was homogenized and processed for measurement of MPO activity. The MPO Activity Assay kit (Nanjing Jiancheng Bioengineering Institute, China) was used for MPO determination, according to the instructions provided with the kit.
Enzyme-linked immunosorbent assay (ELISA) was performed to determine the levels of the proinflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-6, using commercially available kits from RayBiotech Inc., Norcross, GA, United States. The optical density was measured on an ELISA plate scanner (KHB ST-360; Shanghai Danding Company, China) at 490 nm. The results were expressed as picograms of TNF-α or IL-6 per milliliter of lung tissue.
The lungs harvested from rats were immediately fixed in 10% buffered formalin. After fixation, the tissue samples were dehydrated and embedded in paraffin blocks. The sections were cut (4 μm thick) and stained with hematoxylin and eosin (HE). The slides were viewed under a standard light microscope.
Lung tissues immediately frozen in liquid nitrogen were disrupted using a homogenizer with RIPA cell lysis buffer (150 mmol NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 50 mmol Tris-HCl, pH = 7.5, and 2 mmol EDTA) containing a mixture of protease inhibitors (Roche, Switzerland). Protein samples were separated by 8%-15% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. After blocking with 5% skimmed milk for 2 h, the membrane was incubated overnight with a primary antibody at 4 °C. The membrane was then triple-washed with Tris-buffered saline containing 0.1% Tween 20 (TBS-T) and incubated with a secondary antibody for 2 h at room temperature. Horseradish-peroxidase-conjugated IgG in 5% skimmed milk (1:1000) was used as the secondary antibody. The target protein was detected using the ECL Western Blotting Analysis System (Santa Cruz Biotechnology, Santa Cruz, CA, United States) and X-ray film. The expression levels of TLR-4 (Cell Signaling Technology, Inc, United States), NF-κB (Cell Signaling Technology, Inc, United States), and GAPDH (Cell Signaling Technology, Inc, United States) were measured.
Mean ± SD values were calculated to summarize all outcome measurements. The t test was used to compare the means of two groups and P < 0.05 was considered significant. All statistical analyses were performed using SPSS version 17.0 (SPSS, Chicago, IL, United States).
To investigate the effect of mesenteric lymph of SII rats on lung edema, we measured lung W/D ratios. Lung W/D ratios were obviously increased in SII lymph-treated rats, compared with the control group (Figure 1A).
Neutrophil infiltration in lung tissue plays an important role in lung injury induced by SII lymph. The MPO assay is widely used to quantify the number of neutrophils in tissues and serves as an index of inflammation, because MPO is an enzyme that is released mainly from neutrophils. Compared with the control infusion group, the lung MPO activity was increased in the SII infusion group (Figure 1B). There is a growing body of evidence that involves polymorphonuclear leukocytes (PMNs) in the pathophysiological progress of SII. The increased MPO activity in the lung tissue after SII suggested activation of an inflammatory response.
To investigate the mechanism of the lung damage induced by mesenteric lymph from SII, we studied the levels of TNF-α and IL-6 in lung tissues. Compared with the control infusion group, lung tissue TNF-α and IL-6 levels increased significantly in the SII infusion group (Figure 2).
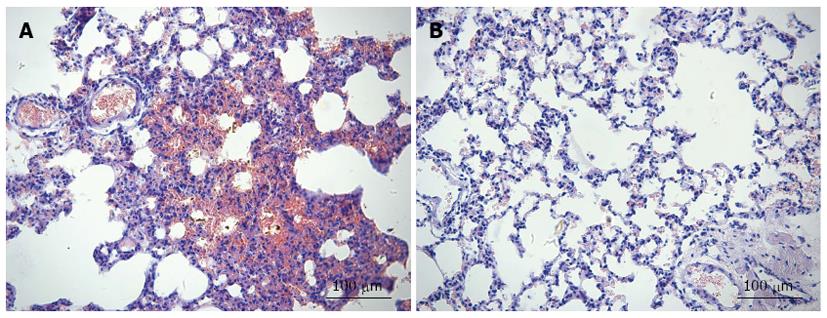
Lung injury is characterized by edema, hemorrhage and PMN infiltration. The control infusion group showed normal appearance of lung tissues. In the SII infusion group, the majority of the interstitial capillaries were congested with erythrocytes. The lung tissue also showed hemorrhage and edema between alveoli. Inflammatory cell infiltration increased septal thickness (Figure 3).
Activation of TLR-4 and NF-κB signaling pathways plays a key role in the regulation of inflammatory mediator production. Therefore, we performed Western blot to investigate the activation of TLR-4 and NF-κB p65. The expression of TLR-4 and NF-κB was significantly increased after injection of SII lymph, compared with the control group (Figure 4).
The role of gut and gut-derived factors in the pathogenesis of ARDS and MODS has been studied extensively and its understanding has evolved over the past two decades. Initially, it was believed that gut barrier failure during shock led to systemic inflammation and MODS by the translocation of bacteria and their products from the gut into the systemic circulation[11]. However, several studies, including that by Moore et al[12], in which neither bacteria nor endotoxin was found in the portal blood of severely injured trauma patients, raised doubts about the clinical relevance of bacterial translocation. Recently, new insights into the pathogenesis of gut-induced ALI and inflammation after systemic insults, such as traumatic hemorrhage or major thermal injury have emerged, leading to the gut-lymph hypothesis of MODS[2,13,14]. This hypothesis states that toxic products produced by the gut during shock are carried via the mesenteric lymph into the systemic circulation, resulting in multiple organ failure[15].
MPO exists in azurophilic granules of neutrophils and accounts for about 5% of the dry weight of cells. In ALI, neutrophils first retain in pulmonary capillaries, adhere to endothelial cells, leave the pulmonary vascular bed, and release a series of inflammatory factors, causing diffuse lung damage. MPO is a characteristic neutrophil aggregation enzyme, is a sign of neutrophil degranulation, and can be used to assess the degree of neutrophil infiltration. Bradley et al[16] showed a significant correlation between the activation of MPO and neutrophil count. Its increased activity in the tissue shows the number of PMNs and tissue damage. We used MPO as an index of evaluating lung inflammation. The experimental results show that the activity of lung tissue MPO increased after infusing mesenteric lymph of SII rats to healthy rats, which correlates with neutrophil chemotaxis and lung inflammatory response. Increased lung W/D ratio suggests lung tissue edema and increased pulmonary capillary permeability. Consistent with the pathological observations, lung tissue was damaged.
It has been found that, the process of sepsis in stress states, such as trauma and infection, causes enterogenic infection and multiple organ failure. However, ALI that occurs early and severely is often the direct cause of death in patients. ALI is a clinical syndrome characterized by pulmonary edema, and its severe phase is ARDS. Numerous studies have shown that Gram-negative bacillus endotoxin induces inflammatory cells such as neutrophils, mononuclear macrophages and endothelial cells to release a large number of cytokines and inflammatory mediators during the course of ARDS. TNF-α plays an important role in lung tissue damage and dysfunction in SII rats. TNF-α is a strong cytokine, and the amount of TNF-α is related to the degree of tissue damage. TNF-α can lead to injury of lung tissue and vascular endothelial cells, and significantly increase lung permeability, protein content of bronchial lavage fluid, and white blood cell count. TNF-α also inhibits the release of the alveolar surface-active substances, increases edema, and causes ARDS[17]. The level of IL-6 is often used as a sign of cytokine cascade activation, which reflects the correlation between host inflammatory response and disease severity. If the level of IL-6 continues to increase, it is often accompanied by complications and mortality[18]. It has been suggested that the level of IL-6 can serve as an indicator of the prognosis[19,20].
TNF-α and IL-6 are both proinflammatory cytokines, and high expression levels can promote the generation of vascular active substances, activate complement and neutrophils, promote neutrophils to cross the vascular endothelium and gather in tissue, and form a cascade effect of inflammation to increase tissue injury. We showed that the levels of TNF-α and IL-6 in lung tissue increased significantly, which indicated an inflammatory reaction in the lungs. Reducing macrophages and NF-κB nuclear translocation can partly mediate these inflammatory effects[21]. ALI or ARDS is characterized by neutrophil- and macrophage-mediated injury and release of inflammatory cytokines and proteases[22]. Tiesi et al[23] tested the hypothesis that gut-derived factors in mesenteric lymph are capable of simultaneously leading to an immune-mediated state as well as inducing systemic inflammation through a TLR-4-dependent pathway. TLR-4 stimulation can mediate ALI induced by SII, because TLR-4 induces the release of the inflammatory cytokines that are critical for the activation of powerful immune responses[24]. TLR-4 stimulation is related to TLR-4-TRIF-TRAF6-NF-κB signaling, and the severity of ALI is controlled by this key pathway[25]. NF-κB activation regulates expression of cytokines, such as TNF-α and IL-6, at the transcriptional level[26]. We found that infusion of mesenteric lymph of SII rats increased lung injury, for example, vascular leakage, neutrophil activation, inflammatory cytokine levels, and NF-κB expression enhancement.
The potential clinical significance of the gut lymph hypothesis is that systemic organ and cellular injury/dysfunction can be abrogated by limiting gut injury and/or by preventing or neutralizing the production of biologically active SII lymph. In recent years, several preclinical studies have tested the strategy and found that gut-directed or lymph neutralizing-directed resuscitation approaches could limit SII-induced lung injury[27,28] and systemic inflammation. Successful strategies have included: (1) ligation of mesenteric lymph ducts can attenuate lung injury caused by SII and reduce systemic inflammatory response and damage of other organs; and (2) in vitro experiments have shown that mesenteric lymph of sepsis can activate neutrophils and cause dysfunction of endothelial cells[29-31].
In the present study, we infused the mesenteric lymph of SII rats into healthy rats via the internal jugular vein, which could lead to ALI in healthy rats. This supports the intestinal lymphatic hypothesis of MODS. In addition, in the experiments, the amount of mesenteric lymph infused was equal to the drainage amount of mesenteric lymphatic vessels from SII rats. This dose can fully induce lung injury in healthy rats. This indicates that the mesenteric lymph of SII rats itself is sufficient to cause lung damage.
The mesenteric lymph of SII rats can cause lung injury, and it may be associated with some toxic agents contained in lymph. On the basis of previous trials, if we drain mesenteric lymph from SII patients, it will have a profound effect on ICU patients who have sepsis-induced ARDS or MODS.
The role of gut and gut-derived factors in the pathogenesis of acute respiratory distress syndrome (ARDS) and multiple organ dysfunction syndrome (MODS) has been studied extensively and its understanding has evolved over the past two decades. The authors investigated the pathway and biological foundation of lung injury caused by severe intraperitoneal infection (SII) to better understand the relationship between lung and gut disease.
To reveal the pathway and biological foundation of lung injury induced by intestinal barrier damage and SII.
The authors infused mesenteric lymph of SII rats into healthy rats and examined its effect on lung tissue. The authors confirmed damage to the remote organ caused via the mesenteric lymphatic pathway, and the hypothesis that lymph from SII is sufficient to cause lung injury.
Mesenteric lymph from SII patients could have a profound effect on intensive care unit patients who have sepsis-induced ARDS or MODS.
The lymphatic system is an important bypass of tissue fluid reflex to blood. The blind side of capillary lymphatic vessels starts from the tissue space, and merges into large lymphatic vessels gradually. Lymphatic vessels collect systemic lymph and flow into veins via the right lymphatic duct and thoracic duct.
A simple but important rat study of the role of mesenteric lymph in lung infection. The findings of the study are significant, and may have favorable clinical outcomes.
P- Reviewers: HowarthGS, Sinha R S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
| 1. | Azoulay E, Darmon M, Delclaux C, Fieux F, Bornstain C, Moreau D, Attalah H, Le Gall JR, Schlemmer B. Deterioration of previous acute lung injury during neutropenia recovery. Crit Care Med. 2002;30:781-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Deitch EA, Forsythe R, Anjaria D, Livingston DH, Lu Q, Xu DZ, Redl H. The role of lymph factors in lung injury, bone marrow suppression, and endothelial cell dysfunction in a primate model of trauma-hemorrhagic shock. Shock. 2004;22:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun. 1999;67:6603-6610. [PubMed] |
| 4. | Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 416] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 5. | Remick DG, Ward PA. Evaluation of endotoxin models for the study of sepsis. Shock. 2005;24 Suppl 1:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Chandra R, Federici S, Bishwas T, Németh ZH, Deitch EA, Thomas JA, Spolarics Z. IRAK1-dependent signaling mediates mortality in polymicrobial sepsis. Inflammation. 2013;36:1503-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1390] [Cited by in RCA: 1302] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 8. | Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 682] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 9. | Deitch EA. Gut lymph and lymphatics: a source of factors leading to organ injury and dysfunction. Ann N Y Acad Sci. 2010;1207 Suppl 1:E103-E111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Badami CD, Senthil M, Caputo FJ, Rupani BJ, Doucet D, Pisarenko V, Xu DZ, Lu Q, Feinman R, Deitch EA. Mesenteric lymph duct ligation improves survival in a lethal shock model. Shock. 2008;30:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Baker JW, Deitch EA, Li M, Berg RD, Specian RD. Hemorrhagic shock induces bacterial translocation from the gut. J Trauma. 1988;28:896-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 248] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Moore FA, Moore EE, Poggetti R, McAnena OJ, Peterson VM, Abernathy CM, Parsons PE. Gut bacterial translocation via the portal vein: a clinical perspective with major torso trauma. J Trauma. 1991;31:629-636; discussion 636-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 315] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 349] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 14. | Magnotti LJ, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph: a link between burn and lung injury. Arch Surg. 1999;134:1333-1340; discussion 1340-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Deitch EA. Bacterial translocation or lymphatic drainage of toxic products from the gut: what is important in human beings? Surgery. 2002;131:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2674] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 17. | Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1318] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 18. | Mastorakos G, Ilias I. Interleukin-6: a cytokine and/or a major modulator of the response to somatic stress. Ann N Y Acad Sci. 2006;1088:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Razavi HM, Wang L, Weicker S, Quinlan GJ, Mumby S, McCormack DG, Mehta S. Pulmonary oxidant stress in murine sepsis is due to inflammatory cell nitric oxide. Crit Care Med. 2005;33:1333-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Kaminski A, Kasch C, Zhang L, Kumar S, Sponholz C, Choi YH, Ma N, Liebold A, Ladilov Y, Steinhoff G. Endothelial nitric oxide synthase mediates protective effects of hypoxic preconditioning in lungs. Respir Physiol Neurobiol. 2007;155:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Shyamsundar M, McKeown ST, O’Kane CM, Craig TR, Brown V, Thickett DR, Matthay MA, Taggart CC, Backman JT, Elborn JS. Simvastatin decreases lipopolysaccharide-induced pulmonary inflammation in healthy volunteers. Am J Respir Crit Care Med. 2009;179:1107-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3976] [Cited by in RCA: 3860] [Article Influence: 154.4] [Reference Citation Analysis (0)] |
| 23. | Tiesi G, Reino D, Mason L, Palange D, Tomaio JN, Deitch EA. Early trauma-hemorrhage-induced splenic and thymic apoptosis is gut-mediated and toll-like receptor 4-dependent. Shock. 2013;39:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1860] [Cited by in RCA: 2430] [Article Influence: 142.9] [Reference Citation Analysis (0)] |
| 25. | Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 964] [Cited by in RCA: 1104] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 26. | Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect Dis. 2007;7:358-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 193] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 27. | Deitch EA, Adams C, Lu Q, Xu DZ. A time course study of the protective effect of mesenteric lymph duct ligation on hemorrhagic shock-induced pulmonary injury and the toxic effects of lymph from shocked rats on endothelial cell monolayer permeability. Surgery. 2001;129:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Sambol JT, Xu DZ, Adams CA, Magnotti LJ, Deitch EA. Mesenteric lymph duct ligation provides long term protection against hemorrhagic shock-induced lung injury. Shock. 2000;14:416-419; discussion 419-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Deitch EA, Adams CA, Lu Q, Xu DZ. Mesenteric lymph from rats subjected to trauma-hemorrhagic shock are injurious to rat pulmonary microvascular endothelial cells as well as human umbilical vein endothelial cells. Shock. 2001;16:290-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Caruso JM, Feketeova E, Dayal SD, Hauser CJ, Deitch EA. Factors in intestinal lymph after shock increase neutrophil adhesion molecule expression and pulmonary leukosequestration. J Trauma. 2003;55:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Upperman JS, Deitch EA, Guo W, Lu Q, Xu D. Post-hemorrhagic shock mesenteric lymph is cytotoxic to endothelial cells and activates neutrophils. Shock. 1998;10:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 3.4] [Reference Citation Analysis (0)] |