Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4607
Revised: February 10, 2014
Accepted: March 6, 2014
Published online: April 28, 2014
Processing time: 152 Days and 22.3 Hours
Helicobacter pylori (H. pylori) is one of the most common human bacterial pathogens, and infection causes a wide array of gastric disorders, including simple gastritis, peptic ulcers and gastric malignancies. Gastrointestinal inflammation caused by H. pylori can influence the absorption of glucose and lipids, which are also abnormal in diabetes mellitus. Type 2 diabetes mellitus (T2DM), formerly known as non-insulin-dependent diabetes mellitus or adult-onset diabetes, is a metabolic disorder that is characterized by high levels of blood glucose resulting from insulin resistance and relative insulin deficiency. It is an emerging pandemic and is rapidly becoming a serious threat to public health. Emerging data now indicate a strong relationship between H. pylori infection and the incidence of T2DM. The mechanisms underlying the pathogenesis of diabetes are complex, involving insulin resistance, chronic inflammation, insulin secretion deficiency as a result of pancreas β-cell dysfunction, glucotoxicity, and lipotoxicity. H. pylori infection is known to be involved in the pathogenesis of insulin resistance, and the growing awareness of its role in diabetes is important for the early detection of glucose dysregulation and prevention of T2DM in high-risk communities. This review probes the possible relationship between H. pylori and diabetes according to epidemiological surveys and discusses putative mechanisms underlying this correlation.
Core tip: A growing body of evidence suggests that Helicobacter pylori (H. pylori) infection is associated with diabetes, and may cause insulin resistance and chronic inflammation that contribute to the disease. H. pylori-induced gastritis can also potentially affect the secretion of gastric-related hormones and inflammatory cytokines. However, the relationship between H. pylori infection and diabetes is still under debate and further studies are warranted to define their association in more detail, and to characterize the corresponding mechanisms and mediators.
-
Citation: He C, Yang Z, Lu NH.
Helicobacter pylori infection and diabetes: Is it a myth or fact? World J Gastroenterol 2014; 20(16): 4607-4617 - URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4607.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4607
Helicobacter pylori (H. pylori) is a gram-negative, spiral-shaped pathogenic bacterium that specifically colonizes the gastric epithelium causing chronic gastritis, peptic ulcer disease, and/or gastric malignancy[1,2]. H. pylori is mainly acquired in childhood by the fecal-oral, oral-oral or gastro-oral route[3], and has been recognized as a worldwide public health problem that is more prevalent in developing countries. The infection induces an acute polymorphonuclear infiltration in the gastric mucosa, which is gradually replaced by an immunologically-mediated, chronic, predominantly mononuclear cellular infiltration[4]. The mononuclear infiltration is characterized by the local production and systemic diffusion of pro-inflammatory cytokines[5] that can affect remote tissues and organic systems[6]. As a result, an increased prevalence of extra-digestive diseases has been reported in those with evidence of H. pylori infection in recent years, including ischemic heart disease[7], autoimmune thyroid diseases[8], sideropenic anemia[9], idiopathic thrombocytopenic purpura[10], neurologic diseases[11-13], and hepatobiliary diseases[14-17]. Indeed, this bacterium produces a low-grade inflammatory state, induces molecular mimicry mechanisms, and interferes with the absorbance of nutrients and drugs, possibly influencing the occurrence and/or evolution of many diseases[18].
Type 2 diabetes mellitus (T2DM) is an emerging pandemic, responsible for an estimated 3.8 million adult deaths worldwide[19]. The pathogenesis of T2DM is complex, with risk factors associated with lifestyle (e.g., diet, obesity, physical activity), genetic background, and socioeconomic factors[20,21]. In T2DM, the pancreas can no longer produce enough insulin to overcome the cellular loss of sensitivity, resulting in the accumulation of sugar in the bloodstream[22]. Identification of treatable causes of this disease will aid in the development of strategies to delay or prevent its onset or slow its progression. Recent evidence implicates the pathological involvement of inflammation in T2DM, which is an important process induced by H. pylori infection[23]. This review focuses on the possible relationship between H. pylori and diabetes as well as the potential mechanisms and mediators concerning this correlation.
The link between H. pylori infection and diabetes remains controversial, as some studies indicate a higher prevalence of infection in diabetic patients[24-26], while others report no difference[27-29]. The relationship between H. pylori and diabetes mellitus was first explored in 1989 by Simon et al[30] who found that the prevalence of H. pylori infection in patients with diabetes mellitus was significantly higher than in asymptomatic controls (62% vs 21%). However, the test used for detecting H. pylori was only a rapid urease test, and their comparison did not adjust for age, which is a major confounding factor. Additional supportive data have come from groups in the Netherlands[31], Italy[32], Turkey[26], and Africa[33]. Recently, a meta-analysis conducted by Zhou et al[34] involved 14080 patients from 41 studies with a total H. pylori infection rate of 42.29%. The odds ratio (OR) for H. pylori infection was increased to 1.33 among patients with diabetes, especially in patients with T2DM (OR = 1.76). The first demonstration that H. pylori infection leads to an increased incidence of diabetes was in a study by Jeon et al[35] using a prospective cohort of 782 Latino individuals > 60 years of age. Participants, whose diabetic status was not known at the initiation of the study, had serum assayed twice yearly for a decade for antibodies to H. pylori, herpes simplex virus 1, varicella zoster virus, cytomegalovirus, and Toxoplasma gondii. During the course of the study, 144 individuals developed diabetes (presumably type 2), and individuals who were initially seropositive for H. pylori were found to be more than two times more likely to develop diabetes than those who were seronegative, even after adjusting for age, sex, education, and covariates such as smoking, body mass index (BMI), blood pressure, and lipids. In contrast, antibodies to the other infectious agents were not associated with an increased risk for the development of diabetes.
Levels of glycated hemoglobin (HbA1c), which result from the non-enzymatic glycosylation of hemoglobin and reflect the integrated blood glucose levels during the preceding 3-4 mo, can be used to diagnose prediabetes and diabetes and to predict diabetes prevalence and incidence[36-38]. A study performed by Chen and Blaser has provided new insight into the association between the seroprevalence of H. pylori infection and the mean levels of HbA1c in two large national surveys: the National Health and Nutrition Examination Survey (NHANES) III and the NHANES 1999-2000[39]. Their report showed that H. pylori seropositivity, and H. pylori cagA positivity in particular, was associated with higher mean HbA1c levels, an association that persisted after excluding individuals with a history of diabetes mellitus and controlling for potential confounders. The association was evident mainly in adults over 18 years of age. They also showed a synergistic effect of H. pylori and BMI on increased levels of HbA1c, indicating a role of H. pylori in impaired glucose tolerance in adults that may be potentiated by a higher BMI level. Similar results were reported in a recent study by Hsieh et al[40] showing long-term H. pylori infection was significantly associated with high levels of HbA1c, decreased insulin secretion, and a higher prevalence of T2DM in Taiwanese patients. Taken together, these results suggest that proper screening of H. pylori infection combined with regular monitoring of blood glucose and HbA1c levels may be effective for early detection of glucose dysregulation and prevention of T2DM.
In contrast, other studies have found no association between H. pylori infection and diabetes[27-29,41,42]. In a large, well-designed study by Xia et al[42], the seroprevalence of H. pylori infection was not significantly different in patients with diabetes mellitus compared to nondiabetic controls. In another study conducted in Nigeria, Oluyemi et al[43] found no significant difference in H. pylori prevalence between T2DM patients and controls, which is consistent with the results from various other regions of the world, including Italy[44], China[28], Turkey[45] and Romania[29]. The discrepancies reported concerning the association of H. pylori and diabetes are likely due to inconsistencies in the methods used to define H. pylori positivity and diabetic status, the limited sample sizes, and adjustments for potential confounders such as age and socioeconomic status[42]. In addition, the accuracy of self-reported data on medical history depends on the subjects’ knowledge and understanding of the relevant information, their ability to recall, and their willingness to report[46], which also may change over time.
Although there is no concrete evidence demonstrating that H. pylori plays a role in diabetes, the possibility for a causal relationship is an intriguing issue deserving discussion. There are several lines of evidence to implicate increased susceptibility to infection in diabetic patients. Firstly, a diabetes-induced impairment of cellular and humoral immunity may enhance an individual’s sensitivity to H. pylori infection[47]. Secondly, diabetes-induced reduction of gastrointestinal motility and acid secretion may promote pathogen colonization and infection rate in the gut[35]. Thirdly, altered glucose metabolism may produce chemical changes in the gastric mucosa that promote H. pylori colonization[48]. Finally, individuals with diabetes are more frequently exposed to pathogens than their healthy counterparts as they regularly attend hospital settings[49]. However, there are also indications that H. pylori infection may contribute to the development of diabetes. Whereas insulin insensitivity is an early phenomenon, pancreatic β-cell function declines gradually over time before the onset of clinical hyperglycemia, the result of many factors that can be influenced by infection, such as insulin resistance (IR), glucotoxicity, lipotoxicity, β-cell dysfunction, chronic inflammation, and genetic and epigenetic factors[23,50].
A growing body of evidence has linked H. pylori infection to IR[51-54], which is defined by a state where insulin can no longer effectively induce glucose disposal in skeletal muscle or suppress endogenous glucose production in the liver[55]. Insulin resistance and abnormal insulin secretion are central to the development of T2DM, and most studies support the view that IR precedes defects in insulin secretion[56]. The first direct evidence for an association between chronic H. pylori infection and IR came from a study by Aydemir et al[53] showing higher homeostatic model assessment-estimated insulin resistance (HOMA-IR) scores in H. pylori-positive individuals. Furthermore, a Japanese study in 2009 that included a large population of 1107 asymptomatic subjects also showed that H. pylori significantly and independently contributed to IR[52].
A recent systematic review of evidence for the association between H. pylori infection and quantitative indexes of IR shows a trend toward a positive association between H. pylori infection and IR, independent of several confounders[51]. However, Gillum et al[57] maintain that there are no consistent associations between H. pylori infection and diabetic prevalence or variables of the IR syndrome in American men 40-74 years of age. Furthermore, Park et al[58] reported that metabolic and inflammatory parameters, including blood sugar, lipid profiles, IR, white blood cell count, and C-reactive protein (CRP) levels, were not changed after H. pylori eradication. It is important to note that H. pylori infection was not determined in all studies by histologic detection of organisms in mucosal biopsy specimens, which is considered the diagnostic gold standard. Although the diagnostic utility of serum H. pylori-specific IgG antibodies is well established[59], the inclusion of false-positive or false-negative detections is unavoidable. As anti-H. pylori IgGs can be detected even after eradication, it is difficult to determine whether H. pylori merely initiates, or chronic active H. pylori infection is required to promote, IR[60]. However, serologic tests are widely available, noninvasive, and inexpensive, and thus suitable for screening and large epidemiologic studies. As IR can develop in the presence of inflammation[61] or as a result of alterations in counter-regulatory hormones that affect insulin[62], H. pylori may thus promote IR by inducing chronic inflammation and affecting insulin-regulating gastrointestinal hormones[53].
It is commonly believed that the chronic inflammation induced by H. pylori infection is strongly linked to the pathogenesis of T2DM, which is associated with a general activation of the innate immune system, and a chronic, cytokine-mediated state of low-grade inflammation. Many tissues are affected by pro-inflammatory cytokines, which cause recognizable features of T2DM[63]. Inflammation of the adipose tissue is considered a key factor in the pathogenesis of IR, and β-cell autoinflammation mediated by interleukin (IL)-1β impairs insulin secretion in T2DM. This inflammation is characterized by an increased infiltration of bone marrow-derived macrophages and increased expression of chemokines and cytokines such as IL-1β[64], CRP and IL-6[65], as well as tumor necrosis factor (TNF)[66-69]. These and other macrophage-secreted factors exert paracrine effects that result in the activation of serine kinases such as c-jun N-terminal kinases (c-JNK) and the inhibitor of nuclear factor kappa B kinase β, which phosphorylate insulin receptor substrate proteins and create a state of IR in adipose tissue[70].
Some epidemiological studies have suggested that pathogen burden is a risk factor for the inflammation that leads to IR[71,72]. Colonization of the gastric epithelium by H. pylori brings about active chronic inflammation by infiltrating gastric submucosal neutrophils and monocytes, which can lead to gastric mucosal damage and epithelial remodeling[73]. The host immune response to H. pylori infection is complex and involves upregulation of several proinflammatory cytokines, such as CRP[74-76], IL-6, and TNF-α[77], which are implicated in IR and the development of diabetes[78]. Human CRP is primarily synthesized by hepatocytes and regulated by inflammatory cytokines (mostly TNF-α and IL-6), and levels of high-sensitive CRP (hsCRP) have been the main focus of investigation for diabetes risk. Of 11 prospective studies, seven reported a significant positive association between hsCRP levels and diabetes risk[65,79-84], and four studies found no association[85-88]. However, it is not known whether hsCRP itself directly influences IR or diabetes. IL-6 is produced in a variety of tissues, including activated leukocytes, adipocytes, and endothelial cells[89,90]. Approximately 25% of in vivo systemic IL-6 originates from subcutaneous adipose tissue[89] and is thought to modify adipocyte glucose and lipid metabolism and body weight[91-93]. Pradhan et al[65] state that elevated levels of IL-6 predict the development of T2DM and further support a possible role for inflammation in diabetogenesis, an idea also supported by Spranger et al[69]. Increased production of TNF-α in adipose tissue may be a critical mechanism by which fat cells induce peripheral IR[94], by the indirect increase in free fatty acid oxidation, stimulation of insulin counter-regulatory hormones or cytokines (e.g., IL-6 and CRP), impairment of endothelial function, or direct inhibitory effects on glucose transporter protein GLUT4, insulin receptor substrates, or glucose-stimulated insulin release by pancreatic β-cells[84]. Furthermore, H. pylori in the gut microbiota leads to increased production of lipopolysaccharide, a constituent of the bacterial cell wall, which also activates innate inflammatory processes[95]. Concentrations of circulating lipopolysaccharide are higher in obese patients with T2DM than in non-diabetic, lean individuals and correlate with the degree of IR[96].
Despite the evidence implicating a link between H. pylori infection and inflammation that predisposes individuals to T2DM, there are some contradictory data. A study by Jeon et al[35] failed to find any significant association between levels of inflammatory mediators (CRP and IL-6) and H. pylori infection or T2DM. Studies by Danesh et al[97] and Ridker et al[98] also found no significant association. Therefore, more investigation is needed to determine whether inflammation triggered by H. pylori infection contributes to the development of T2DM.
H. pylori-induced gastritis can potentially affect the secretion of gastric-related hormones such as leptin and ghrelin[99,100], as well as gastrin and somatostatin[101], which may influence a predisposition to diabetes. Gastrin increases food-related and glucose-stimulated insulin release[102,103], and somatostatin regulates pancreatic insulin secretion and inhibits insulin release[104,105]. Patients with H. pylori infections could therefore have altered insulin release, as they have elevated basal and stimulated serum concentrations of gastrin and decreased somatostatin[101,106]. The regulation of leptin and ghrelin, which are produced in the stomach and are involved in energy homeostasis[107,108], affects obesity, insulin sensitivity, and glucose homeostasis[109,110]. Increasing evidence indicates H. pylori can influence the production of leptin and ghrelin, and thus could promote obesity and the development of diabetes[100,111-114]. Ghrelin decreases energy expenditure and promotes weight gain[115], whereas leptin, which is expressed mainly by adipocytes, reduces food intake and increases energy expenditure[116]. H. pylori infection has been shown to impair ghrelin production[117,118] and enhance the production of leptin[119]. Low ghrelin levels are associated with elevated fasting insulin concentrations, IR, and T2DM[120]. Leptin has also been implicated in the development of IR[121], and elevated levels correlate with IR in lean men[122] and patients with T2DM[123]. Elevation of leptin levels is likely deleterious to human islet function, as a clinical study revealed that improved pancreatic β-cell function was independently associated with the decreased leptin and increased adiponectin levels in obese women after standardized weight reduction[124]. There is evidence that in addition to mitigating the effects of insulin through phosphorylation of Ser-318 of insulin receptor substrate 1[125], high levels of leptin may also impair glucose-stimulated insulin secretion and induce apoptosis of β cells in human islets via activation of c-JNK[126]. However, a study by Brown et al[127] indicated that leptin has a protective role on pancreatic β cell function, showing that leptin could prevent apoptosis of pancreatic β cells through modulation of the Bcl protein family.
Decreased insulin secretion is one of the major pathophysiological defects in T2DM. The progression from normal glucose tolerance to prediabetes and T2DM is characterized by continuing defects in β-cell function[128]. A study by So et al[129] found that H. pylori titer could independently predict abnormal pancreatic β-cell function in Chinese men. Additionally, Rahman et al[130] also described a positive association between H. pylori infection and impaired insulin secretion. The insulin-producing pancreatic β-cells are especially susceptible to damage by inflammation and oxidative stress[131], therefore it is plausible that inflammation caused by H. pylori infection results in deficits in insulin secretion. Furthermore, it was reported in a study by Hsieh et al[40] that patients with H. pylori infection were more likely to have had impaired insulin secretion at a young age, which may increase the risk for T2DM.
Accumulating evidence indicates that cytokines play important roles in β-cell failure, as chronic exposure to IL-1β, TNF-α, and IFN-γ inhibits insulin secretion and induces apoptosis of β cells[132,133]. In addition, H. pylori vacuolating cytotoxin stimulates mitochondrial-dependent apoptosis in diabetic patients through downregulation of anti-apoptotic Bcl-2, upregulation of pro-apoptotic Bax, and increased activation of caspase-9 and -3[134]. Despite these studies, more studies are needed to elucidate the role of H. pylori infection in insulin secretion and the incidence of T2DM.
There are limited and conflicting data regarding the effect of H. pylori eradication on glucose metabolism and insulin sensitivity[58,135-137]. However, it may be beneficial for patients at risk of diabetes to be checked for the presence of H. pylori infection, as a report by Zojaji et al[136] showed that H. pylori treatment can improve the mean HbA1c and the metabolic abnormalities in patients with T2DM. Additionally, Gen et al[137] demonstrated that successful H. pylori eradication significantly decreased fasting insulin and HOMA-IR levels. Other studies focused on the effects of eradication on H. pylori-stimulated inflammatory cytokines. Some reports indicate that CRP levels are decreased after H. pylori eradication, suggesting a beneficial effect on low-grade inflammation[33,137]. However, there are also reports showing no effect of H. pylori eradication on mean HOMA-IR and CRP levels[58] or HbA1c levels[135]. Recently, Vafaeimanesh et al[138] found that in patients with T2DM, the mean decrease in HbA1c and fasting plasma glucose levels in eradicated cases was similar to non-eradicated subjects three and six months after treatment.
Many additional factors are likely involved in the relationship between H. pylori infection and diabetes. For example, lifestyle is a critical factor affecting both chronic H. pylori infection and T2DM, as it has been shown that older subjects with a low-risk lifestyle are less likely to develop T2DM[139]. Gastroduodenal conditions resulting from H. pylori infection could delay gastric emptying, which has been postulated to cause mismatch between the onset of insulin action and the absorption of carbohydrates in insulin-dependent children with diabetes[140,141]. However, it has also been suggested that delayed gastric emptying is a potential advantage, rather than a disadvantage, in relation to glycemic control in T2DM patients not treated with insulin[142], and others maintain that H. pylori infection does not affect the rate of gastric emptying in diabetic patients[143]. H. pylori infection has also been implicated in platelet activation and aggregation, increases in pro-atherogenic factors such as homocysteine, production of reactive oxygen species, and increases in lipid peroxides[144].
There is now solid evidence that obesity is the main etiological cause of T2DM, with new, controlled, clinical trials showing that a weight loss of as little as 5% is sufficient to prevent most obese subjects with impaired glucose tolerance from developing the disease[145]. However, there is no clear evidence linking obesity and H. pylori infection. According to some studies, obesity[146] and/or a high BMI[112] may be associated with an increased incidence of H. pylori colonization, likely resulting from reduced gastric motility. A study by Cohen et al[147] demonstrated that adults infected with H. pylori had higher BMI levels, even if asymptomatic, and further suggested that H. pylori therapy may lead to weight loss and improve diabetic control. In contrast, other studies showed no association between H. pylori seropositivity or CagA antibody status and BMI[148,149], or even an inverse relationship between morbid obesity and H. pylori seropositivity[150]. Nevertheless, there are data demonstrating that H. pylori eradication significantly increases the incidence of obesity in patients with peptic ulcer disease, as it increases BMI[151,152], and/or enhances the appetite of asymptomatic patients by elevating plasma ghrelin[113] and reducing leptin[153] levels.
Disturbances in the production and clearance of plasma lipoproteins are among the metabolic abnormalities that commonly accompany diabetes. Moreover, dyslipidemia may foster the development of diabetes[154]. High concentrations of plasma triglyceride and low-density lipoprotein cholesterol (LDL-c), along with low concentration of high-density lipoprotein cholesterol (HDL-c), are attributed mostly to IR and insulin deficiency[155,156]. H. pylori infection may induce dyslipidemia, as it leads to elevated plasma levels of total cholesterol[157,158], LDL-c[158] and triglyceride concentrations[159] and decreased levels of HDL-c[160,161]. It was postulated that chronic H. pylori infection may promote atherogenic lipid profiles through the action of pro-inflammatory cytokines, such as IL-6, interferon-α and TNF-α, which activate adipose tissue lipoprotein lipase, stimulate hepatic fatty acid synthesis and influence lipolysis[158,162]. However, as not all studies found significant changes in plasma levels of total cholesterol, triglycerides, and LDL-c with H. pylori infection[163,164], further studies are needed to verify this association.
Although there have been indications that T2DM may predispose an individual to H. pylori infection[35,47-49], this seems unlikely, considering the age at which the disease is typically acquired. A model of age-related pleiotropy, or life-course perspective, with respect to H. pylori colonization has been proposed by Atherton et al[165]. The potential benefits of H. pylori occur predominantly earlier in life, including reduced risks for asthma[166,167], tuberculosis reactivation[168], childhood diarrhea[169], and gastroesophageal reflux disease[170-172]. However, among older individuals, H. pylori can promote adverse health effects, such as peptic ulcer disease, gastric cancer, and perhaps increased glucose intolerance.
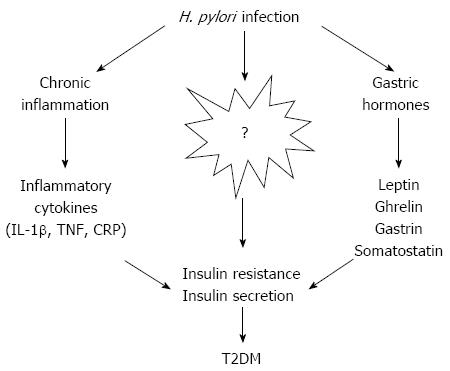
Since the discovery of H. pylori, a variety of epidemiological studies, therapeutic trials, and case reports have evaluated the direct or indirect involvement of this bacterium in the pathogenesis of various extragastric disorders. Although no current data provide concrete evidence that H. pylori plays a role in diabetes mellitus, the possibility cannot be ruled out. The evidence concerning an association between H. pylori infection and IR, chronic inflammation, the secretion of gastric-related hormones, and insulin secretion deficiency implicate H. pylori in a predisposition to diabetes (Figure 1). However, the pathophysiology of T2DM is complex, and many other factors could contribute to this process after H. pylori infection, such as lifestyle, changes in gastric emptying, dyslipidemia and so on. Diabetes mellitus is a multifaceted and multistep disease that is unlikely to result from a single cause, though risk factors that deserve attention include gastrointestinal infections and the composition of intestinal microbiota. Larger prospective studies investigating the impact of H. pylori infection on diabetes and corresponding mediating factors are warranted. Meanwhile, large interventional studies are urgently needed to evaluate the long-term benefit of H. pylori eradication for prevention and progression of diabetes. Evidence supporting an etiological role of H. pylori in the development of T2DM would indicate that preventive measures, such as increased hygiene and treatments using antibiotics and proton pump inhibitor combinations, should be explored as targets of intervention in high-risk communities.
P- Reviewer: Marzuillo P S- Editor: Gou SX L- Editor: Logan S E- Editor: Wang CH
| 1. | Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175-1176. [PubMed] |
| 2. | Parsonnet J. Helicobacter pylori and gastric cancer. Gastroenterol Clin North Am. 1993;22:89-104. [PubMed] |
| 3. | Malaty HM. Epidemiology of Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007;21:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Graham DY, Osato MS, Olson CA, Zhang J, Figura N. Effect of H. pylori infection and CagA status on leukocyte counts and liver function tests: extra-gastric manifestations of H. pylori infection. Helicobacter. 1998;3:174-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Perri F, Clemente R, Festa V, De Ambrosio CC, Quitadamo M, Fusillo M, Grossi E, Andriulli A. Serum tumour necrosis factor-alpha is increased in patients with Helicobacter pylori infection and CagA antibodies. Ital J Gastroenterol Hepatol. 1999;31:290-294. [PubMed] |
| 6. | Patel P, Mendall MA, Khulusi S, Northfield TC, Strachan DP. Helicobacter pylori infection in childhood: risk factors and effect on growth. BMJ. 1994;309:1119-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 168] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Eskandarian R, Ghorbani R, Shiyasi M, Momeni B, Hajifathalian K, Madani M. Prognostic role of Helicobacter pylori infection in acute coronary syndrome: a prospective cohort study. Cardiovasc J Afr. 2012;23:131-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Shi WJ, Liu W, Zhou XY, Ye F, Zhang GX. Associations of Helicobacter pylori infection and cytotoxin-associated gene A status with autoimmune thyroid diseases: a meta-analysis. Thyroid. 2013;23:1294-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Monzón H, Forné M, Esteve M, Rosinach M, Loras C, Espinós JC, Viver JM, Salas A, Fernández-Bañares F. Helicobacter pylori infection as a cause of iron deficiency anaemia of unknown origin. World J Gastroenterol. 2013;19:4166-4171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 10. | Payandeh M, Sohrabi N, Zare ME, Kansestani AN, Hashemian AH. Platelet Count Response to Helicobacter pylori Eradication in Iranian Patients with Idiopathic Thrombocytopenic Purpura. Mediterr J Hematol Infect Dis. 2012;4:e2012056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Asadi-Pooya AA, Dehghani SM, Petramfar P, Emami M, Mahmoodi M. Helicobacter pylori infection in patients with epilepsy. Seizure. 2012;21:21-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Nielsen HH, Qiu J, Friis S, Wermuth L, Ritz B. Treatment for Helicobacter pylori infection and risk of Parkinson’s disease in Denmark. Eur J Neurol. 2012;19:864-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Roubaud-Baudron C, Krolak-Salmon P, Quadrio I, Mégraud F, Salles N. Impact of chronic Helicobacter pylori infection on Alzheimer’s disease: preliminary results. Neurobiol Aging. 2012;33:1009.e11-1009.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Mishra RR, Tewari M, Shukla HS. Association of Helicobacter pylori infection with inflammatory cytokine expression in patients with gallbladder cancer. Indian J Gastroenterol. 2013;32:232-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Boonyanugomol W, Chomvarin C, Sripa B, Bhudhisawasdi V, Khuntikeo N, Hahnvajanawong C, Chamsuwan A. Helicobacter pylori in Thai patients with cholangiocarcinoma and its association with biliary inflammation and proliferation. HPB (Oxford). 2012;14:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Krüttgen A, Horz HP, Weber-Heynemann J, Vucur M, Trautwein C, Haase G, Luedde T, Roderburg C. Study on the association of Helicobacter species with viral hepatitis-induced hepatocellular carcinoma. Gut Microbes. 2012;3:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Polyzos SA, Kountouras J, Papatheodorou A, Patsiaoura K, Katsiki E, Zafeiriadou E, Zavos C, Anastasiadou K, Terpos E. Helicobacter pylori infection in patients with nonalcoholic fatty liver disease. Metabolism. 2013;62:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Gasbarrini A, Franceschi F, Cammarota G, Pola P, Gasbarrini G. Vascular and immunological disorders associated with Helicobacter pylori infection. Ital J Gastroenterol Hepatol. 1998;30:115-118. [PubMed] |
| 19. | van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17 Suppl 1:S3-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 464] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 20. | Qi L, Hu FB, Hu G. Genes, environment, and interactions in prevention of type 2 diabetes: a focus on physical activity and lifestyle changes. Curr Mol Med. 2008;8:519-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol. 2011;40:804-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 647] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 22. | Papamichael KX, Papaioannou G, Karga H, Roussos A, Mantzaris GJ. Helicobacter pylori infection and endocrine disorders: is there a link? World J Gastroenterol. 2009;15:2701-2707. [PubMed] |
| 23. | Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2178] [Cited by in RCA: 2624] [Article Influence: 187.4] [Reference Citation Analysis (0)] |
| 24. | Devrajani BR, Shah SZ, Soomro AA, Devrajani T. Type 2 diabetes mellitus: A risk factor for Helicobacter pylori infection: A hospital based case-control study. Int J Diabetes Dev Ctries. 2010;30:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Bener A, Micallef R, Afifi M, Derbala M, Al-Mulla HM, Usmani MA. Association between type 2 diabetes mellitus and Helicobacter pylori infection. Turk J Gastroenterol. 2007;18:225-229. [PubMed] |
| 26. | Gulcelik NE, Kaya E, Demirbas B, Culha C, Koc G, Ozkaya M, Cakal E, Serter R, Aral Y. Helicobacter pylori prevalence in diabetic patients and its relationship with dyspepsia and autonomic neuropathy. J Endocrinol Invest. 2005;28:214-217. [PubMed] |
| 27. | Anastasios R, Goritsas C, Papamihail C, Trigidou R, Garzonis P, Ferti A. Helicobacter pylori infection in diabetic patients: prevalence and endoscopic findings. Eur J Intern Med. 2002;13:376. [PubMed] |
| 28. | Ko GT, Chan FK, Chan WB, Sung JJ, Tsoi CL, To KF, Lai CW, Cockram CS. Helicobacter pylori infection in Chinese subjects with type 2 diabetes. Endocr Res. 2001;27:171-177. [PubMed] |
| 29. | Stanciu OG, Trifan A, Sfarti C, Cojocariu C, Stanciu C. Helicobacter pylori infection in patients with diabetes mellitus. Rev Med Chir Soc Med Nat Iasi. 2003;107:59-65. [PubMed] |
| 30. | Simon L, Tornóczky J, Tóth M, Jámbor M, Sudár Z. [The significance of Campylobacter pylori infection in gastroenterologic and diabetic practice]. Orv Hetil. 1989;130:1325-1329. [PubMed] |
| 31. | Oldenburg B, Diepersloot RJ, Hoekstra JB. High seroprevalence of Helicobacter pylori in diabetes mellitus patients. Dig Dis Sci. 1996;41:458-461. [PubMed] |
| 32. | Quadri R, Rossi C, Catalfamo E, Masoero G, Lombardo L, Della Monica P, Rovera L, Pera A, Cavello Perin P. Helicobacter pylori infection in type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2000;10:263-266. [PubMed] |
| 33. | Longo-Mbenza B, Nkondi Nsenga J, Vangu Ngoma D. Prevention of the metabolic syndrome insulin resistance and the atherosclerotic diseases in Africans infected by Helicobacter pylori infection and treated by antibiotics. Int J Cardiol. 2007;121:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Zhou X, Zhang C, Wu J, Zhang G. Association between Helicobacter pylori infection and diabetes mellitus: a meta-analysis of observational studies. Diabetes Res Clin Pract. 2013;99:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 35. | Jeon CY, Haan MN, Cheng C, Clayton ER, Mayeda ER, Miller JW, Aiello AE. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care. 2012;35:520-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 36. | Buell C, Kermah D, Davidson MB. Utility of A1C for diabetes screening in the 1999 2004 NHANES population. Diabetes Care. 2007;30:2233-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 37. | Herman WH, Engelgau MM, Zhang Y, Brown MB. Use of GHb (HbA(1c)) to screen for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000;23:1207-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Rohlfing CL, Little RR, Wiedmeyer HM, England JD, Madsen R, Harris MI, Flegal KM, Eberhardt MS, Goldstein DE. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000;23:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 259] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 39. | Chen Y, Blaser MJ. Association between gastric Helicobacter pylori colonization and glycated hemoglobin levels. J Infect Dis. 2012;205:1195-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Hsieh MC, Wang SS, Hsieh YT, Kuo FC, Soon MS, Wu DC. Helicobacter pylori infection associated with high HbA1c and type 2 diabetes. Eur J Clin Invest. 2013;43:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Lutsey PL, Pankow JS, Bertoni AG, Szklo M, Folsom AR. Serological evidence of infections and Type 2 diabetes: the MultiEthnic Study of Atherosclerosis. Diabet Med. 2009;26:149-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Xia HH, Talley NJ, Kam EP, Young LJ, Hammer J, Horowitz M. Helicobacter pylori infection is not associated with diabetes mellitus, nor with upper gastrointestinal symptoms in diabetes mellitus. Am J Gastroenterol. 2001;96:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Oluyemi A, Anomneze E, Smith S, Fasanmade O. Prevalence of a marker of active helicobacter pylori infection among patients with type 2 diabetes mellitus in Lagos, Nigeria. BMC Res Notes. 2012;5:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Dore MP, Bilotta M, Malaty HM, Pacifico A, Maioli M, Graham DY, Realdi G. Diabetes mellitus and Helicobacter pylori infection. Nutrition. 2000;16:407-410. [PubMed] |
| 45. | Demir M, Gokturk HS, Ozturk NA, Kulaksizoglu M, Serin E, Yilmaz U. Helicobacter pylori prevalence in diabetes mellitus patients with dyspeptic symptoms and its relationship to glycemic control and late complications. Dig Dis Sci. 2008;53:2646-2649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Bowlin SJ, Morrill BD, Nafziger AN, Lewis C, Pearson TA. Reliability and changes in validity of self-reported cardiovascular disease risk factors using dual response: the behavioral risk factor survey. J Clin Epidemiol. 1996;49:511-517. [PubMed] |
| 47. | Borody T, Ren Z, Pang G, Clancy R. Impaired host immunity contributes to Helicobacter pylori eradication failure. Am J Gastroenterol. 2002;97:3032-3037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | de Luis DA, de la Calle H, Roy G, de Argila CM, Valdezate S, Canton R, Boixeda D. Helicobacter pylori infection and insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1998;39:143-146. [PubMed] |
| 49. | Gentile S, Turco S, Oliviero B, Torella R. The role of autonomic neuropathy as a risk factor of Helicobacter pylori infection in dyspeptic patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 1998;42:41-48. [PubMed] |
| 50. | Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1582] [Cited by in RCA: 1609] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 51. | Polyzos SA, Kountouras J, Zavos C, Deretzi G. The association between Helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter. 2011;16:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 52. | Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, Urabe A. Helicobacter pylori infection significantly increases insulin resistance in the asymptomatic Japanese population. Helicobacter. 2009;14:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 53. | Aydemir S, Bayraktaroglu T, Sert M, Sokmen C, Atmaca H, Mungan G, Gun BD, Borazan A, Ustundag Y. The effect of Helicobacter pylori on insulin resistance. Dig Dis Sci. 2005;50:2090-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Eshraghian A, Hashemi SA, Hamidian Jahromi A, Eshraghian H, Masoompour SM, Davarpanah MA, Eshraghian K, Taghavi SA. Helicobacter pylori infection as a risk factor for insulin resistance. Dig Dis Sci. 2009;54:1966-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Dinneen S, Gerich J, Rizza R. Carbohydrate metabolism in non-insulin-dependent diabetes mellitus. N Engl J Med. 1992;327:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 168] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Moller DE, Flier JS. Insulin resistance--mechanisms, syndromes, and implications. N Engl J Med. 1991;325:938-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 487] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 57. | Gillum RF. Infection with Helicobacter pylori, coronary heart disease, cardiovascular risk factors, and systemic inflammation: the Third National Health and Nutrition Examination Survey. J Natl Med Assoc. 2004;96:1470-1476. [PubMed] |
| 58. | Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, Sung IK, Sohn CI, Kim BI, Keum DK. Helicobacter pylori eradication has no effect on metabolic and inflammatory parameters. J Natl Med Assoc. 2005;97:508-513. [PubMed] |
| 59. | Ricci C, Holton J, Vaira D. Diagnosis of Helicobacter pylori: invasive and non-invasive tests. Best Pract Res Clin Gastroenterol. 2007;21:299-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 60. | Eshraghian A. The continuous story of Helicobacter pylori infection and insulin resistance: this time in Japan. Helicobacter. 2010;15:160; author reply 161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Shinohara K, Shoji T, Emoto M, Tahara H, Koyama H, Ishimura E, Miki T, Tabata T, Nishizawa Y. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:1894-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | Moss SF, Legon S, Bishop AE, Polak JM, Calam J. Effect of Helicobacter pylori on gastric somatostatin in duodenal ulcer disease. Lancet. 1992;340:930-932. [PubMed] |
| 63. | Fernández-Real JM, Pickup JC. Innate immunity, insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2008;19:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 64. | Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 261] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 65. | Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2974] [Cited by in RCA: 3028] [Article Influence: 126.2] [Reference Citation Analysis (1)] |
| 66. | Vaarala O, Yki-Järvinen H. Diabetes: Should we treat infection or inflammation to prevent T2DM? Nat Rev Endocrinol. 2012;8:323-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 2106] [Article Influence: 140.4] [Reference Citation Analysis (1)] |
| 68. | Liu S, Tinker L, Song Y, Rifai N, Bonds DE, Cook NR, Heiss G, Howard BV, Hotamisligil GS, Hu FB. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med. 2007;167:1676-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 69. | Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1102] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 70. | Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2726] [Cited by in RCA: 3083] [Article Influence: 162.3] [Reference Citation Analysis (0)] |
| 71. | Fernández-Real JM, López-Bermejo A, Vendrell J, Ferri MJ, Recasens M, Ricart W. Burden of infection and insulin resistance in healthy middle-aged men. Diabetes Care. 2006;29:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Howard BV, Best L, Comuzzie A, Ebbesson SO, Epstein SE, Fabsitz RR, Howard WJ, Silverman A, Wang H, Zhu J. C-Reactive protein, insulin resistance, and metabolic syndrome in a population with a high burden of subclinical infection: insights from the Genetics of Coronary Artery Disease in Alaska Natives (GOCADAN) study. Diabetes Care. 2008;31:2312-2314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 73. | Misiewicz JJ. Current insights in the pathogenesis of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1995;7:701-703. [PubMed] |
| 74. | Diomedi M, Stanzione P, Sallustio F, Leone G, Renna A, Misaggi G, Fontana C, Pasqualetti P, Pietroiusti A. Cytotoxin-associated Gene-A-positive Helicobacter pylori strains infection increases the risk of recurrent atherosclerotic stroke. Helicobacter. 2008;13:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Oshima T, Ozono R, Yano Y, Oishi Y, Teragawa H, Higashi Y, Yoshizumi M, Kambe M. Association of Helicobacter pylori infection with systemic inflammation and endothelial dysfunction in healthy male subjects. J Am Coll Cardiol. 2005;45:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 76. | Jackson L, Britton J, Lewis SA, McKeever TM, Atherton J, Fullerton D, Fogarty AW. A population-based epidemiologic study of Helicobacter pylori infection and its association with systemic inflammation. Helicobacter. 2009;14:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Hamed SA, Amine NF, Galal GM, Helal SR, Tag El-Din LM, Shawky OA, Ahmed EA, Abdel Rahman MS. Vascular risks and complications in diabetes mellitus: the role of helicobacter pylori infection. J Stroke Cerebrovasc Dis. 2008;17:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 2346] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 79. | Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, Tracy RP. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50:2384-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 377] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 80. | Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 774] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 81. | Freeman DJ, Norrie J, Caslake MJ, Gaw A, Ford I, Lowe GD, O’Reilly DS, Packard CJ, Sattar N. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51:1596-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 521] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 82. | Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 561] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 83. | Thorand B, Löwel H, Schneider A, Kolb H, Meisinger C, Fröhlich M, Koenig W. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study, 1984-1998. Arch Intern Med. 2003;163:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 264] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 84. | Laaksonen DE, Niskanen L, Nyyssönen K, Punnonen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia. 2004;47:1403-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 85. | Festa A, D’Agostino R, Tracy RP, Haffner SM. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 757] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 86. | Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean ME, Haffner SM. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care. 2002;25:2016-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 356] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 87. | Krakoff J, Funahashi T, Stehouwer CD, Schalkwijk CG, Tanaka S, Matsuzawa Y, Kobes S, Tataranni PA, Hanson RL, Knowler WC. Inflammatory markers, adiponectin, and risk of type 2 diabetes in the Pima Indian. Diabetes Care. 2003;26:1745-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 228] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 88. | Snijder MB, Dekker JM, Visser M, Stehouwer CD, Yudkin JS, Bouter LM, Heine RJ, Nijpels G, Seidell JC. Prospective relation of C-reactive protein with type 2 diabetes: response to Han et al. Diabetes Care. 2003;26:1656-167; author reply 1656-167;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196-4200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 466] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 90. | Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209-214. [PubMed] |
| 91. | Greenberg AS, Nordan RP, McIntosh J, Calvo JC, Scow RO, Jablons D. Interleukin 6 reduces lipoprotein lipase activity in adipose tissue of mice in vivo and in 3T3-L1 adipocytes: a possible role for interleukin 6 in cancer cachexia. Cancer Res. 1992;52:4113-4116. [PubMed] |
| 92. | Berg M, Fraker DL, Alexander HR. Characterization of differentiation factor/leukaemia inhibitory factor effect on lipoprotein lipase activity and mRNA in 3T3-L1 adipocytes. Cytokine. 1994;6:425-432. [PubMed] |
| 93. | Orban Z, Remaley AT, Sampson M, Trajanoski Z, Chrousos GP. The differential effect of food intake and beta-adrenergic stimulation on adipose-derived hormones and cytokines in man. J Clin Endocrinol Metab. 1999;84:2126-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 94. | Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5334] [Cited by in RCA: 5421] [Article Influence: 169.4] [Reference Citation Analysis (0)] |
| 95. | Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31:817-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 357] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 96. | Lassenius MI, Pietiläinen KH, Kaartinen K, Pussinen PJ, Syrjänen J, Forsblom C, Pörsti I, Rissanen A, Kaprio J, Mustonen J. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care. 2011;34:1809-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 293] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 97. | Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, Gallimore JR, Pepys MB. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 1138] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 98. | Ridker PM, Danesh J, Youngman L, Collins R, Stampfer MJ, Peto R, Hennekens CH. A prospective study of Helicobacter pylori seropositivity and the risk for future myocardial infarction among socioeconomically similar U.S. men. Ann Intern Med. 2001;135:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 99. | Jeffery PL, McGuckin MA, Linden SK. Endocrine impact of Helicobacter pylori: focus on ghrelin and ghrelin o-acyltransferase. World J Gastroenterol. 2011;17:1249-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 100. | Roper J, Francois F, Shue PL, Mourad MS, Pei Z, Olivares de Perez AZ, Perez-Perez GI, Tseng CH, Blaser MJ. Leptin and ghrelin in relation to Helicobacter pylori status in adult males. J Clin Endocrinol Metab. 2008;93:2350-2357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 101. | Kaneko H, Konagaya T, Kusugami K. Helicobacter pylori and gut hormones. J Gastroenterol. 2002;37:77-86. [PubMed] |
| 102. | Peach HG, Barnett NE. Helicobacter pylori infection and fasting plasma glucose concentration. J Clin Pathol. 2001;54:466-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 103. | Rehfeld JF, Stadil F. The effect of gastrin on basal- and glucose-stimulated insulin secretion in man. J Clin Invest. 1973;52:1415-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 110] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 104. | Açbay O, Celik AF, Gündoğdu S. Does Helicobacter pylori-induced gastritis enhance food-stimulated insulin release? Dig Dis Sci. 1996;41:1327-1331. [PubMed] |
| 105. | Colturi TJ, Unger RH, Feldman M. Role of circulating somatostatin in regulation of gastric acid secretion, gastrin release, and islet cell function. Studies in healthy subjects and duodenal ulcer patients. J Clin Invest. 1984;74:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 106. | Calam J. Helicobacter pylori modulation of gastric acid. Yale J Biol Med. 1999;72:195-202. [PubMed] |
| 107. | Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1056] [Cited by in RCA: 1052] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 108. | Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, Hayashi T, Inoue G, Hosoda K, Kojima M. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 569] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 109. | Williams J, Mobarhan S. A critical interaction: leptin and ghrelin. Nutr Rev. 2003;61:391-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 110. | Verhulst PJ, Depoortere I. Ghrelin’s second life: from appetite stimulator to glucose regulator. World J Gastroenterol. 2012;18:3183-3195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (1)] |
| 111. | Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, Urabe A. Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population. Am J Gastroenterol. 2008;103:3005-3010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 112. | Isomoto H, Ueno H, Nishi Y, Wen CY, Nakazato M, Kohno S. Impact of Helicobacter pylori infection on ghrelin and various neuroendocrine hormones in plasma. World J Gastroenterol. 2005;11:1644-1648. [PubMed] |
| 113. | Nwokolo CU, Freshwater DA, O’Hare P, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut. 2003;52:637-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 114. | Francois F, Roper J, Joseph N, Pei Z, Chhada A, Shak JR, de Perez AZ, Perez-Perez GI, Blaser MJ. The effect of H. pylori eradication on meal-associated changes in plasma ghrelin and leptin. BMC Gastroenterol. 2011;11:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 115. | Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2834] [Cited by in RCA: 2779] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 116. | Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3184] [Cited by in RCA: 3072] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 117. | Osawa H, Nakazato M, Date Y, Kita H, Ohnishi H, Ueno H, Shiiya T, Satoh K, Ishino Y, Sugano K. Impaired production of gastric ghrelin in chronic gastritis associated with Helicobacter pylori. J Clin Endocrinol Metab. 2005;90:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 118. | Osawa H. Ghrelin and Helicobacter pylori infection. World J Gastroenterol. 2008;14:6327-6333. [PubMed] |
| 119. | Nishi Y, Isomoto H, Uotani S, Wen CY, Shikuwa S, Ohnita K, Mizuta Y, Kawaguchi A, Inoue K, Kohno S. Enhanced production of leptin in gastric fundic mucosa with Helicobacter pylori infection. World J Gastroenterol. 2005;11:695-699. [PubMed] |
| 120. | Pöykkö SM, Kellokoski E, Hörkkö S, Kauma H, Kesäniemi YA, Ukkola O. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes. 2003;52:2546-2553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 368] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 121. | Cohen B, Novick D, Rubinstein M. Modulation of insulin activities by leptin. Science. 1996;274:1185-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 463] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 122. | Segal KR, Landt M, Klein S. Relationship between insulin sensitivity and plasma leptin concentration in lean and obese men. Diabetes. 1996;45:988-991. [PubMed] |
| 123. | Fischer S, Hanefeld M, Haffner SM, Fusch C, Schwanebeck U, Köhler C, Fücker K, Julius U. Insulin-resistant patients with type 2 diabetes mellitus have higher serum leptin levels independently of body fat mass. Acta Diabetol. 2002;39:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 124. | Guldstrand M, Ahrén B, Adamson U. Improved beta-cell function after standardized weight reduction in severely obese subjects. Am J Physiol Endocrinol Metab. 2003;284:E557-E565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 125. | Hennige AM, Stefan N, Kapp K, Lehmann R, Weigert C, Beck A, Moeschel K, Mushack J, Schleicher E, Häring HU. Leptin down-regulates insulin action through phosphorylation of serine-318 in insulin receptor substrate 1. FASEB J. 2006;20:1206-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 126. | Maedler K, Schulthess FT, Bielman C, Berney T, Bonny C, Prentki M, Donath MY, Roduit R. Glucose and leptin induce apoptosis in human beta-cells and impair glucose-stimulated insulin secretion through activation of c-Jun N-terminal kinases. FASEB J. 2008;22:1905-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 127. | Brown JE, Dunmore SJ. Leptin decreases apoptosis and alters BCL-2: Bax ratio in clonal rodent pancreatic beta-cells. Diabetes Metab Res Rev. 2007;23:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 128. | Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1372] [Cited by in RCA: 1276] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 129. | So WY, Tong PC, Ko GT, Ma RC, Ozaki R, Kong AP, Yang X, Ho CS, Lam CC, Chan JC. Low plasma adiponectin level, white blood cell count and Helicobacter pylori titre independently predict abnormal pancreatic beta-cell function. Diabetes Res Clin Pract. 2009;86:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 130. | Rahman MA, Cope MB, Sarker SA, Garvey WT, Chaudhury HS, Khaled MA. Helicobacter pylori Infection and Inflammation: Implication for the Pathophysiology of Diabetes and Coronary Heart Disease in Asian Indians. J. Life Sci. 2009;1:45-50. [PubMed] |
| 131. | Fosslien E. Mitochondrial medicine--molecular pathology of defective oxidative phosphorylation. Ann Clin Lab Sci. 2001;31:25-67. [PubMed] |
| 132. | Lee YH, Magkos F, Mantzoros CS, Kang ES. Effects of leptin and adiponectin on pancreatic β-cell function. Metabolism. 2011;60:1664-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 133. | Wang C, Guan Y, Yang J. Cytokines in the Progression of Pancreatic β-Cell Dysfunction. Int J Endocrinol. 2010;2010:515136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 134. | Omori K, Mitsuhashi M, Ishiyama K, Nair I, Rawson J, Todorov I, Kandeel F, Mullen Y. mRNA of the pro-apoptotic gene BBC3 serves as a molecular marker for TNF-α-induced islet damage in humans. Diabetologia. 2011;54:2056-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 135. | Wada Y, Hamamoto Y, Kawasaki Y, Honjo S, Fujimoto K, Tatsuoka H, Matsuoka A, Ikeda H, Fujikawa J, Koshiyama H. The Eradication of Helicobacter pylori does not Affect Glycemic Control in Japanese Subjects with Type 2 Diabetes. Jpn Clin Med. 2013;4:41-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 136. | Zojaji H, Ataei E, Sherafat SJ, Ghobakhlou M, SR F. The effect of the treatment of Helicobacter pylori infection on the glycemic control in type 2 diabetes mellitus. Gastroenterol Hepatol Bed Bench. 2013;6:36-40. |
| 137. | Gen R, Demir M, Ataseven H. Effect of Helicobacter pylori eradication on insulin resistance, serum lipids and low-grade inflammation. South Med J. 2010;103:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 138. | Vafaeimanesh J, Rajabzadeh R, Ahmadi A, Moshtaghi M, Banikarim S, Hajiebrahimi S, Seyyedmajidi M. Effect of Helicobacter pylori eradication on glycaemia control in patients with type 2 diabetes mellitus and comparison of two therapeutic regimens. Arab J Gastroenterol. 2013;14:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 139. | Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med. 2009;169:798-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 242] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 140. | Ojetti V, Pellicano R, Fagoonee S, Migneco A, Berrutti M, Gasbarrini A. Helicobacter pylori infection and diabetes. Minerva Med. 2010;101:115-119. [PubMed] |
| 141. | Burghen GA, Murrell LR, Whitington GL, Klyce MK, Burstein S. Acid peptic disease in children with type I diabetes mellitus. A complicating relationship. Am J Dis Child. 1992;146:718-722. [PubMed] |
| 142. | Chang J, Rayner CK, Jones KL, Horowitz M. Diabetic gastroparesis and its impact on glycemia. Endocrinol Metab Clin North Am. 2010;39:745-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 143. | Jones KL, Wishart JM, Berry M, Russo A, Xia HH, Talley NJ, Horowitz M. Helicobacter pylori infection is not associated with delayed gastric emptying or upper gastrointestinal symptoms in diabetes mellitus. Dig Dis Sci. 2002;47:704-709. [PubMed] |
| 144. | Polyzos SA, Kountouras J, Zavos C, Deretzi G. Comment on: Jeon et al. Helicobacter pylori Infection Is Associated With an Increased Rate of Diabetes. Diabetes Care 2012; 35: 520-525. Diabetes Care. 2012;35:e53; author reply e54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 145. | Astrup A, Finer N. Redefining type 2 diabetes: ‘diabesity’ or ‘obesity dependent diabetes mellitus’? Obes Rev. 2000;1:57-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 253] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 146. | Perdichizzi G, Bottari M, Pallio S, Fera MT, Carbone M, Barresi G. Gastric infection by Helicobacter pylori and antral gastritis in hyperglycemic obese and in diabetic subjects. New Microbiol. 1996;19:149-154. [PubMed] |
| 147. | Cohen D, Muhsen K. Association between Helicobacter pylori colonization and glycated hemoglobin levels: is this another reason to eradicate H. pylori in adulthood? J Infect Dis. 2012;205:1183-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 148. | Ioannou GN, Weiss NS, Kearney DJ. Is Helicobacter pylori seropositivity related to body mass index in the United States? Aliment Pharmacol Ther. 2005;21:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 149. | Cho I, Blaser MJ, François F, Mathew JP, Ye XY, Goldberg JD, Bini EJ. Helicobacter pylori and overweight status in the United States: data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2005;162:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 150. | Wu MS, Lee WJ, Wang HH, Huang SP, Lin JT. A case-control study of association of Helicobacter pylori infection with morbid obesity in Taiwan. Arch Intern Med. 2005;165:1552-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 151. | Fujiwara Y, Higuchi K, Arafa UA, Uchida T, Tominaga K, Watanabe T, Arakawa T. Long-term effect of Helicobacter pylori eradication on quality of life, body mass index, and newly developed diseases in Japanese patients with peptic ulcer disease. Hepatogastroenterology. 2002;49:1298-1302. [PubMed] |
| 152. | Kamada T, Hata J, Kusunoki H, Ito M, Tanaka S, Kawamura Y, Chayama K, Haruma K. Eradication of Helicobacter pylori increases the incidence of hyperlipidaemia and obesity in peptic ulcer patients. Dig Liver Dis. 2005;37:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 153. | Konturek PC, Cześnikiewicz-Guzik M, Bielanski W, Konturek SJ. Involvement of Helicobacter pylori infection in neuro-hormonal control of food intake. J Physiol Pharmacol. 2006;57 Suppl 5:67-81. [PubMed] |
| 154. | Goldberg IJ. Clinical review 124: Diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab. 2001;86:965-971. [PubMed] |
| 155. | Chahil TJ, Ginsberg HN. Diabetic dyslipidemia. Endocrinol Metab Clin North Am. 2006;35:491-510, vii-viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 156. | Taskinen MR. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003;46:733-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 576] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 157. | Majka J, Róg T, Konturek PC, Konturek SJ, Bielański W, Kowalsky M, Szczudlik A. Influence of chronic Helicobacter pylori infection on ischemic cerebral stroke risk factors. Med Sci Monit. 2002;8:CR675-CR684. [PubMed] |
| 158. | Chimienti G, Russo F, Lamanuzzi BL, Nardulli M, Messa C, Di Leo A, Correale M, Giannuzzi V, Pepe G. Helicobacter pylori is associated with modified lipid profile: impact on Lipoprotein(a). Clin Biochem. 2003;36:359-365. [PubMed] |
| 159. | Laurila A, Bloigu A, Näyhä S, Hassi J, Leinonen M, Saikku P. Association of Helicobacter pylori infection with elevated serum lipids. Atherosclerosis. 1999;142:207-210. [PubMed] |
| 160. | Hoffmeister A, Rothenbacher D, Bode G, Persson K, März W, Nauck MA, Brenner H, Hombach V, Koenig W. Current infection with Helicobacter pylori, but not seropositivity to Chlamydia pneumoniae or cytomegalovirus, is associated with an atherogenic, modified lipid profile. Arterioscler Thromb Vasc Biol. 2001;21:427-432. [PubMed] |
| 161. | Takashima T, Adachi K, Kawamura A, Yuki M, Fujishiro H, Rumi MA, Ishihara S, Watanabe M, Kinoshita Y. Cardiovascular risk factors in subjects with Helicobacter pylori infection. Helicobacter. 2002;7:86-90. [PubMed] |
| 162. | Feingold KR, Grunfeld C. Role of cytokines in inducing hyperlipidemia. Diabetes. 1992;41 Suppl 2:97-101. [PubMed] |
| 163. | Ando TMM, Ishiguro K, Maeda O, Watanabe O, Mizuno T, Fujita T, Takahashi H, Noshiro M, Goto H. Changes in biochemical parameters related to atherosclerosis after Helicobacter pylori eradication. Aliment Pharmaco Ther. 2006;24 Suppl 4:58-64. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 164. | Elizalde JI, Piqué JM, Moreno V, Morillas JD, Elizalde I, Bujanda L, De Argila CM, Cosme A, Castiella A, Ros E. Influence of Helicobacter pylori infection and eradication on blood lipids and fibrinogen. Aliment Pharmacol Ther. 2002;16:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 165. | Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J Clin Invest. 2009;119:2475-2487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 395] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 166. | Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 282] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 167. | Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 291] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 168. | Perry S, de Jong BC, Solnick JV, de la Luz Sanchez M, Yang S, Lin PL, Hansen LM, Talat N, Hill PC, Hussain R. Infection with Helicobacter pylori is associated with protection against tuberculosis. PLoS One. 2010;5:e8804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 169. | Rothenbacher D, Blaser MJ, Bode G, Brenner H. Inverse relationship between gastric colonization of Helicobacter pylori and diarrheal illnesses in children: results of a population-based cross-sectional study. J Infect Dis. 2000;182:1446-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 170. | de Martel C, Llosa AE, Farr SM, Friedman GD, Vogelman JH, Orentreich N, Corley DA, Parsonnet J. Helicobacter pylori infection and the risk of development of esophageal adenocarcinoma. J Infect Dis. 2005;191:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 171. | Vaezi MF, Falk GW, Peek RM, Vicari JJ, Goldblum JR, Perez-Perez GI, Rice TW, Blaser MJ, Richter JE. CagA-positive strains of Helicobacter pylori may protect against Barrett’s esophagus. Am J Gastroenterol. 2000;95:2206-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 172. | Ye W, Held M, Lagergren J, Engstrand L, Blot WJ, McLaughlin JK, Nyrén O. Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia. J Natl Cancer Inst. 2004;96:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |