Published online Oct 28, 2013. doi: 10.3748/wjg.v19.i40.6863
Revised: August 18, 2013
Accepted: September 4, 2013
Published online: October 28, 2013
Processing time: 136 Days and 19 Hours
AIM: To evaluate the effects of estrogen (E2) on systemic and splanchnic hyperdynamic circulation in portal hypertensive rats.
METHODS: Fifty castrated female Sprague-Dawley rats were divided into five groups: sham operation (SO), partial portal vein ligation (PPVL) + placebo (PLAC), PPVL + E2, PPVL + ICI and PPVL + E2 + ICI. Hemodynamic measurements were performed using ultrasonography. Mesenteric arteriole contractility in response to norepinephrine was determined using a vessel perfusion system. Oxidative stress in the mesenteric artery was investigated by in situ detection of the superoxide anion (O2•−) and hydrogen peroxide (H2O2) concentrations.
RESULTS: Treatment with E2 resulted in a significant decrease of portal pressure (P < 0.01) and portal venous inflow (P < 0.05), and higher systemic vascular resistance (P < 0.05) and splanchnic arteriolar resistance (P < 0.01) in PPVL + E2 rats compared to PPVL + PLAC rats. In the mesenteric arterioles of PPVL + E2 rats, the dose-response curve was shifted left, and the EC50 was decreased (P < 0.01). E2 reduced O2•− production and H2O2 concentration in the mesenteric artery. However, ICI182, 780 reversed the beneficial effects of E2, therefore, the systemic and splanchnic hyperdynamic circulation were more deteriorated in ICI182, 780-treated rats.
CONCLUSION: Treatment with estrogen improved the systemic and splanchnic hyperdynamic circulation in PPVL rats, in part due to the alleviation of oxidative stress.
Core tip: Vascular hyporeactivity is affected by gender and estrogen. The aim of the present study was to investigate whether estrogen could attenuate the severity of hyperdynamic circulation and the underlying mechanism in pre-hepatic portal hypertensive rats without cirrhosis, with a focus on oxidative stress. The authors proposed that treatment with estrogen could improve the systemic and splanchnic hyperdynamic circulation in partial portal vein ligation rats, in part due to the alleviation of oxidative stress.
- Citation: Zhang B, Zhang CG, Zhou QB, Chen W, Wu ZY. Estrogen improves the hyperdynamic circulation and hyporeactivity of mesenteric arteries by alleviating oxidative stress in partial portal vein ligated rats. World J Gastroenterol 2013; 19(40): 6863-6868
- URL: https://www.wjgnet.com/1007-9327/full/v19/i40/6863.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i40.6863
Portal hypertension (PHT) is a major complication of liver cirrhosis and is associated with the development of hyperdynamic circulation, characterized by elevated portal pressure (PP), increased splanchnic (small intestine) blood flow, increased cardiac output, and development of an extensive network of portosystemic collaterals[1]. PP is determined by extrahepatic factors such as portal blood flow and collateral resistance in addition to intrahepatic resistance. Therefore, it would be triggered by persistent splanchnic vasodilation. Functional changes can be found in the portal hypertensive splanchnic vasculature, including splanchnic vasodilation and decreased responsiveness to vasoconstrictors as a result of endothelial dysfunction and impaired activation of vasoconstrictive mechanisms[2]. Although the levels of vasoconstrictors such as norepinephrine (NE) and angiotensin-II (Ang-II) are increased in the blood circulation and are accompanied by enhanced sympathetic excitability in PHT, the splanchnic vasculature remains dilated[3]. Hyporeactivity of the artery in response to vasoconstrictors plays a key role in blood vessel dilation and hyperdynamic circulation[4,5]. Vascular hyporeactivity has been observed in PHT animals in both the systemic circulation and in the mesenteric artery, and it is also affected by gender and estrogen[6]. In addition, experimental studies have shown that estradiol (E2) reduces PP and increases hepatic blood flow in castrated and cirrhotic model rats, but the effect is significantly inhibited by the estrogen antagonist ICI182, 780[7,8].
Both acute and chronic liver diseases are characterized by an increased formation of reactive oxygen species (ROS), as indicated by increased superoxide anion in the whole blood of cirrhotic patients with decompensated liver and increased urinary F2-isoprostanes and lipoperoxidation and decreased superoxide dismutase in experimental PHT models[9]. Increased oxidative stress may lead to impaired endothelium dysfunction and be involved in the pathogenesis of PHT. It has therefore been a subject of interest[10,11].
The aim of the present study was to investigate whether estrogen could attenuate the severity of hyperdynamic circulation and the underlying mechanism in PHT rats without cirrhosis, with a focus on oxidative stress. Thus, to exclude the hepatoprotective and antifibrogenic effects of estrogen, the PHT was induced by partial portal vein ligation (PPVL) but not by the injection of hepatotoxic drugs, such as CCl4 and dimethylnitrosamine, or by bile duct ligation.
This animal study was approved by the local animal ethics committee of Renji Hospital and performed according to the guidelines of the Laboratory Animal Care and Use Committee at School of Medicine, Shanghai Jiao Tong University (Shanghai, China). Fifty female Sprague-Dawley rats weighing 200-220 g underwent ovariectomy before the study. Three weeks after the initial surgery, the rats underwent PPVL or sham operation (SO) as previously described by Reiberger et al[1] and were divided into five groups: SO, PPVL + placebo (PLAC), PPVL + E2, PPVL + ICI and PPVL + E2 + ICI. Briefly, under ketamine anesthesia (intramuscular injection of 10 mg/100 g body weight), the portal vein was isolated, and stenosis was induced by a single ligature of 3-0 silk placed around both the portal vein and a 20-gauge blunt-tipped needle. The needle was then removed, leaving a calibrated stenosis of the portal vein. Portal hypertension was considered present at 14 d after surgery. In SO animals, the portal vein was isolated and similarly manipulated but not ligated. Starting on the day of surgery, the PPVL + E2, PPVL + ICI and PPVL + E2 + ICI groups were subcutaneously administered β-estradiol (2 μg/100 g body weight/d, R and D Systems, United States) or/and ICI182, 780 (2 μg/100 g body weight/d, R and D Systems, United States) for 14 d. The SO and PPVL groups were subcutaneously administered the same volume of the placebo (ethanol).
Under anesthesia induced by an intramuscular injection of ketamine (10 mg/100 g body weight), the velocity and inside diameter of the portal vein (PV) proximal to the ligation and stroke volume (SV) were obtained using a Vevo770 High-Resolution Imaging System (Visual Sonics, Canada). A 22 G catheter filled with heparin saline was inserted into the femoral artery to obtain the mean arterial pressure (MAP) and heart rate (HR). Another 22 G catheter was introduced into the portal vein to measure PP, and one was inserted into the inferior vena cava to measure central venous pressure (CVP) after making an incision at the midline of the abdomen. All parameters were recorded using an SP840 pressure transducer and a multichannel recorder (Philips, United States).
Cardiac output (CO), cardiac index (CI), PV blood flow, portal venous inflow (PVI), systemic vascular resistance (SVR) and splanchnic arteriolar resistance (SAR) were calculated as follows: CO = SV × HR; CI = CO × 100/body weight (g); PVI = PV blood flow × 100/body weight (g); SVR = (MAP - CVP)/CI; SAR = (MAP - PP)/PVI. PV blood flow and CO were normalized by body weight and presented as CI and PVI. Resistances in the vascular systems were calculated from the ratio between the perfusion pressure (P) and blood flow (Q) of each vascular territory.
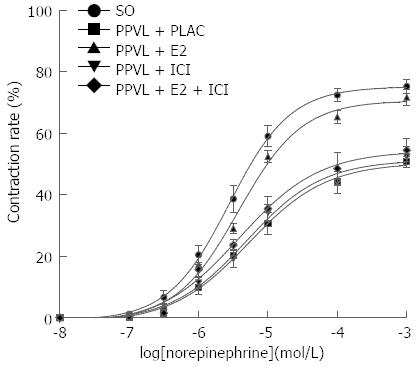
Following the determination of hemodynamic measurements, the mesenteric arteries and the mesentery were removed. Using an SMZ-168 dissecting microscope (Motic, China), the third-order arterioles in the mesentery were carefully dissected and transferred to a vascular perfusion system containing a 3-(N-morpholino) propanesulfonic acid-buffered physiological salt solution (MOPS-PSS, 0-4 °C, pH 7.4; NaCl 145 mmol/L, KCl 5.0 mmol/L, CaCl2 2.0 mmol/L, MgSO4 1.0 mmol/L, NaH2PO4 1.0 mmol/L, glucose 5.0 mmol/L, pyruvate 2.0 mmol/L, EDTA 0.02 mmol/L and MOPS 3.0 mmol/L). A glass micropipette containing MOPS-PSS (top diameter, 50 μm) was inserted into one end of the arteriole and fixed with 11-0 single strands. Blood was flushed out at a perfusion pressure of 8 mmHg. Another glass micropipette was then inserted into the other end of the arteriole and fixed. The two glass micropipettes were suspended in organ baths containing 60 mL of MOPS-PSS (37 °C, pH 7.4). The arteriole was equilibrated under a pressure of 80 mmHg for 30 min prior to the experiments. After the equilibration period, cumulative NE concentration response curves (10-8 mol/L-10-4 mol/L) were obtained by increasing the concentration in quarter-log increments. The inner diameter was measured using a BA310 microscope camera system (Motic, China). The vasoconstriction rate and the logarithm of the NE concentration were used as the vertical axis and the abscissa, respectively.
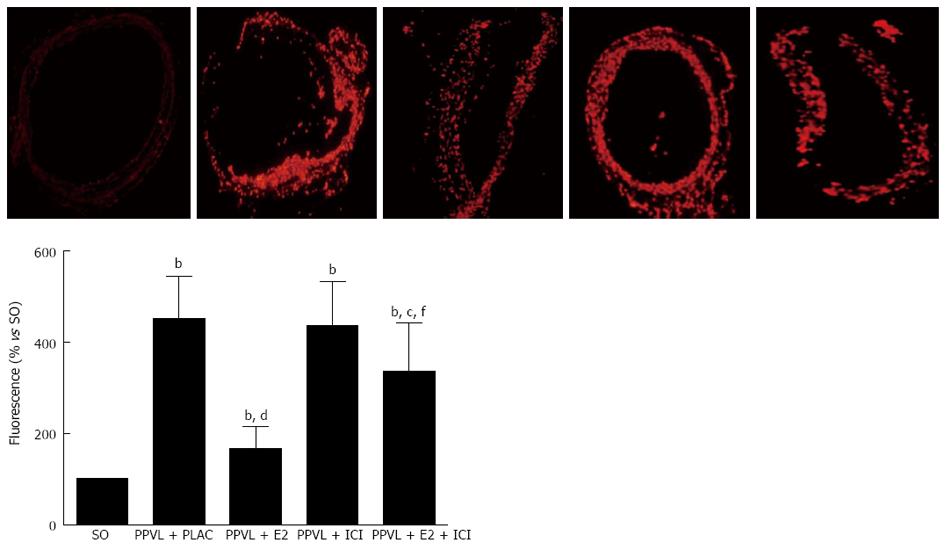
The oxidative fluorescent dihydroethidium (DHE, Sigma, United States) was used to evaluate in situ production of superoxide anion (O2•−)[12]. Frozen, enzymatically intact, 10-μm-thick sections of the superior mesenteric arteries were incubated with DHE (10 μmol/L) in PBS for 30 min at 37 °C in a humidified chamber protected from light. DHE is freely permeable to cells and is oxidized in a reaction with O2•− to ethidium bromide, which binds to DNA in the nucleus and fluoresces red. Images were obtained with an IX71 fluorescence microscope (Olympus, Japan).
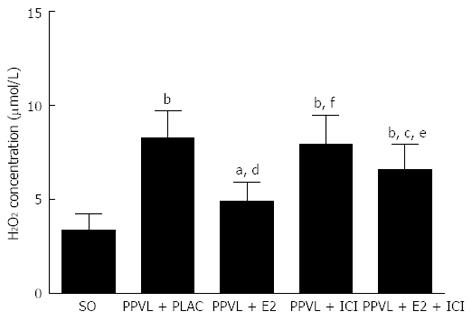
The level of hydrogen peroxide (H2O2) was measured using a hydrogen peroxide assay kit (Abcam, United States). In the presence of horseradish peroxidase (HRP), the OxiRed Probe reacts with H2O2 to produce product with color. The superior mesenteric artery was cleaned of connective tissue, precipitated with RIPA (Beyotime, China; 200 μL RIPA/20 mg tissue) for 15 min and then centrifuged for 15 min at 1000 g. A total of 5 μL of the supernatant was diluted with 46 μL of assay buffer, mixed with 50 μL of the Reaction Mix (assay buffer 46 μL, OxiRed Probe 2 μL, HRP 2 μL) and then incubated at room temperature for 10 min. The OD570 nm was read with a Synergy 4 Multi-Mode Microplate Reader (BioTek, United States), and the H2O2 concentration was calculated according to a standard concentration curve.
The change in the reactivity of the mesenteric arteriole in response to NE was presented as a dose-response curve, which was fitted by a nonlinear regression analysis (GraphPad Software Inc., San Diego, CA, United States). Maximal responses (Emax) and effective concentrations causing half maximum responses (EC50, calculated by regression analysis) were obtained from concentration response curves. Values are expressed as the means ± SD. Statistical comparisons were performed using one-way ANOVA. P values < 0.05 were considered significant. All statistical analyses were performed using the Statistical Package for the Social Sciences 13.0 (SPSS Inc., United States).
Compared to SO, PPVL resulted in a lowered HR (P < 0.05) and MBP (P < 0.05) and significantly increased PP (P < 0.01) in the four groups of prehepatic PHT. However, measurement of MAP and HR revealed no significant differences between the four PPVL groups (P > 0.05). PP significantly decreased by 18.5% in PPVL + E2 rats with a mean PP of 12.2 ± 1.6 mmHg compared to a mean PP of 14.9 ± 1.4 mmHg in PPVL + PLAC rats (P < 0.01) (Table 1).
| SO | PPVL + PLAC | PPVL + E2 | PPVL + ICI | PPVL + E2 + ICI | |
| Body weight (g) | 248 ± 15 | 261 ± 22 | 245 ± 18 | 231 ± 23ac | 244 ± 13 |
| HR (bpm) | 440 ± 32 | 412 ± 25a | 415 ± 35a | 419 ± 21a | 406 ± 28a |
| MAP (mmHg) | 95 ± 6 | 85 ± 11a | 88 ± 9a | 89 ± 5a | 84 ± 8a |
| PP (mmHg) | 4.6 ± 1.2 | 14.9 ± 0.9b | 12.2 ± 1.6b | 14.2 ± 1.5b | 13.9 ± 1.3b |
| PV flow (mL/min) | 8.52 ± 2.3 | 12.71 ± 3.1 | 9.76 ± 2.8 | 11.87 ± 3.5 | 13.04 ± 3.8 |
| PVI (mL/min/100 g) | 3.43 ± 1.2 | 4.87 ± 1.6a | 3.98 ± 1.4c | 5.14 ± 1.8a | 5.34 ± 1.9a |
| CO (mL ∙ min-1) | 54.68 ± 4.9 | 61.79 ± 5.9 | 52.07 ± 5.1 | 56.82 ± 5.6 | 57.91 ± 6.0 |
| CI (Ml/min/100 g) | 22.05 ± 1.9 | 23.67 ± 2.1 | 21.25 ± 2.0 | 24.60 ± 2.2 | 23.79 ± 2.5 |
| SVR (mmHg∙min∙100 g/mL) | 4.08 ± 0.48 | 3.38 ± 0.35b | 3.95 ± 0.41c | 3.50 ± 0.38a | 3.32 ± 0.33a |
| SAR (mmHg∙min∙100 g/mL) | 26.33 ± 2.7 | 14.39 ± 2.2b | 19.29 ± 1.8bd | 14.94 ± 1.7b | 13.12 ± 1.5b |
| Emax | 75.18% ± 4.52% | 50.47% ± 3.48%a | 70.65% ± 2.42%ad | 51.37% ± 4.12%b | 54.33% ± 4.71%b |
| EC50 (10-6 mol/L) | 2.77 ± 0.74 | 5.27 ± 0.88b | 3.77 ± 0.69bd | 4.89 ± 0.76b | 3.85 ± 0.52bd |
PVI was significantly increased in the four groups of rats that underwent PPVL compared to the corresponding SO group (P < 0.01). Treatment with E2 resulted in a reduction in PVI in PPVL + E2 rats compared to PPVL + PLAC rats (3.98 ± 1.4 mL/min per 100 g vs 4.87 ± 1.6 mL/min per 100 g, P < 0.05) (Table 1).
Although CI was slightly increased in the four groups of PPVL rats compared to the SO group, there were no significant differences among the five different groups (P > 0.05). SVR was lower in the four groups of PPVL rats compared to SO rats due to the decreased MBP (P < 0.05), which was increased 16.9% by E2 treatment in PPVL + E2 rats compared to PPVL + PLAC rats (3.95 ± 0.41 mmHg∙min∙100 g/mL vs 3.38 ± 0.35 mmHg∙min∙100 g/mL, P < 0.05) (Table 1).
SAR was significantly lower in the four groups of PPVL rats compared to SO rats (P < 0.01). Rats treated with E2 showed significantly higher SAR, with a 34.1% increase compared to PPVL + PLAC rats (19.29 ± 1.8 mmHg∙min∙100 g/mL vs 14.39 ± 2.2 mmHg∙min∙100 g/mL, P < 0.01) (Table 1).
ICI182, 780 treatment alone did not influence PP, PVI, CI, SVR or SAR compared to PPVL + PLAC rats, except that the body weight was slightly lower (P < 0.05). However, ICI182, 780 reversed the beneficial effects of E2 on PVI, CI, SVR and SAR. Thereby, the systemic circulation and splanchnic hyperdynamic circulation were more deteriorated (Table 1).
Compared with the SO group, the dose-response curve of the mesenteric arteriole in response to NE was lower and shifted right, with decreased Emax (P < 0.01) and increased EC50 (P < 0.01, Figure 1; Table 1) in the four PPVL groups. In the mesenteric arterioles of PPVL rats treated with E2, the dose-response curve was shifted left, and the EC50 was decreased (P < 0.01), compared with the PPVL + PLAC rats. ICI182, 780 alone did not influence the dose-response curve or EC50 compared to PPVL rats treated with placebo (P > 0.05). However, ICI182, 780 decreased the response to NE of arterioles treated with E2, thereby the dose-response curve was shifted right.
Low-intensity fluorescence was observed throughout SO vessels, confirming that all layers of the normal vessel produced low amounts of O2•−. Fourteen d after the surgery, O2•− production was increased in the four groups of PPVL rats (Figure 2). However, the staining was particularly weak in PPVL + E2 rats, and the administration of ICI182, 780 blocked the beneficial effect that E2 provided.
In the superior mesenteric arteries, H2O2 generation was nearly 2.5-fold higher in PPVL + PLAC rats than in SO rats (8.2 ± 1.5 μmol/L vs 3.3 ± 0.9 μmol/L, P < 0.01; Figure 3). Exogenous E2 reduced H2O2 levels in PPVL + E2 rats when compared with those in PPVL + PLAC rats (4.9 ± 1.0 μmol/L vs 8.2 ± 1.5 μmol/L, P < 0.01). The H2O2 levels in mesenteric arteries treated with ICI182, 780 alone were similar to those found in PPVL + PLAC rats (7.9 ± 1.8 μmol/L vs 3.3 ± 0.9 μmol/L, P < 0.01). However, the administration of ICI182, 780 to PPVL + E2 rats suppressed the antioxidant effect of E2 (6.6 ± 1.3 μmol/L vs 4.9 ± 1.0 μmol/L, P < 0.05).
Recent studies have already provided evidence of the importance of vascular hyporeactivity in the development and maintenance of PHT. Aortic ring hypocontractility has been investigated both in bile duct ligation and CCl4-induced cirrhotic models[13-15]. Ferlitsch et al[16] have shown that forearm artery responses to NE and Ang-II are decreased in patients with cirrhosis. Clear sex differences have also been observed in the vasoconstrictor responsiveness of aortic rings from rats with and without PHT[17]. In contrast to male rats, PHT does not induce vascular hyporesponsiveness in female rats. Estrogen has been shown to be effective in animal models of established PHT with cirrhosis by suppressing hepatic fibrosis and relaxation of the hepatic sinusoid[7,18]. However, the efficacy of estrogen in the setting of de novo PHT and the underlying mechanism had not been characterized in detail. To exclude the effect of cirrhosis on oxidative status and inflammatory cytokines, we established this animal model of prehepatic PHT to further describe both the hemodynamic and anti-oxidant effects of estrogen treatment in the PPVL rat model. The hypothesis of our study was that estrogen could reverse the severity of hyperdynamic circulation and the vascular hyporeactivity of the mesenteric arteries by alleviating oxidative stress in portal hypertensive rats without cirrhosis in which liver function was normal.
The results of our study suggested that mesenteric arteriole sensitivity and contractility in response to NE were decreased in PPVL rats, indicating the hyporesponsiveness of the splanchnic vessels to vasoconstrictors, in accordance with deteriorative splanchnic hemodynamics. Treatment with E2 significantly ameliorated the hyperdynamic splanchnic circulation in PPVL rats. The reduction in splanchnic blood inflow represented by decreased PVI could be explained by an improved contractile reaction of the splanchnic vessels to the vasoconstrictor and a subsequently increased SAR. Additionally, the production of ROS was decreased in PPVL-E2 rats compared to PPVL-SA rats, indicating a profound amelioration of oxidative stress and corresponding to the improvement of systemic and splanchnic hyperdynamic circulation.
The fact that PPVL resulted in oxidant injury was first demonstrated by Fernando et al[18], who concluded that the formation of ROS may be important in the pathogenesis of hemodynamic changes and that anti-oxidants can ameliorate oxidant injury and prevent the development of hyperdynamic circulation[19]. Estrogen played a protective role by acting as an antioxidant, which granted it action as a scavenger of free radicals, decreasing the formation of ROS. In castrated rats, significant increases in the activity of antioxidant enzymes were observed, which may have occurred to compensate for the absence of circulating E2 promoted by castration; even then, it was not effective in reducing lipid peroxidase levels[8,20,21]. Estrogen replacement can reverse this effect, reducing lipid peroxidase to the values of control animals[21]. In addition, estrogen stimulates eNOS expression in SECs and increases NO production, contributing to a reduction in portal pressure in a model of intrahepatic PHT[7].
In summary, we conclude that improvements in oxidative stress after estrogen administration manifest as a functional improvement in the contractile response to vasoconstrictors. Indeed, we observed improvements in both the systemic and splanchnic hyperdynamic circulation. Further studies on the clinical administration of estrogen should be performed.
Portal hypertension, a major complication of liver cirrhosis, is associated with the development of hyperdynamic circulation and would be triggered by persistent splanchnic vasodilation. Functional changes can be found in the portal hypertensive splanchnic vasculature, including splanchnic vasodilation and decreased responsiveness to vasoconstrictors as a result of endothelial dysfunction and impaired activation of vasoconstrictive mechanisms.
Increased oxidative stress may lead to impaired endothelium dysfunction and be involved in the pathogenesis of portal hypertension. It has therefore been a subject of interest
The authors found that improvements in oxidative stress after estrogen administration manifest as a functional improvement in the contractile response of mesenteric arteries to vasoconstrictors in partial portal vein ligation (PPVL) rats.
The authors proposed that treatment with estrogen could improve the systemic and splanchnic hyperdynamic circulation in PPVL rats.
The manuscript is an interesting manuscript with novel observations. The authors delineated the effect of estrogen treatment on the systemic and splanchnic hyperdynamic circulation in portal hypertensive rats. The findings are straight forward and manuscript is well written and nicely discussed.
P- Reviewers Ozdemir S, Rojas A, Sahu RP S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Reiberger T, Angermayr B, Schwabl P, Rohr-Udilova N, Mitterhauser M, Gangl A, Peck-Radosavljevic M. Sorafenib attenuates the portal hypertensive syndrome in partial portal vein ligated rats. J Hepatol. 2009;51:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Hennenberg M, Trebicka J, Sauerbruch T, Heller J. Mechanisms of extrahepatic vasodilation in portal hypertension. Gut. 2008;57:1300-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Trebicka J, Leifeld L, Hennenberg M, Biecker E, Eckhardt A, Fischer N, Pröbsting AS, Clemens C, Lammert F, Sauerbruch T. Hemodynamic effects of urotensin II and its specific receptor antagonist palosuran in cirrhotic rats. Hepatology. 2008;47:1264-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Hadoke PW. Cirrhosis of the liver and receptor-mediated function in vascular smooth muscle. Pharmacol Ther. 2001;89:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Schepke M, Heller J, Paschke S, Thomas J, Wolff M, Neef M, Malago M, Molderings GJ, Spengler U, Sauerbruch T. Contractile hyporesponsiveness of hepatic arteries in humans with cirrhosis: evidence for a receptor-specific mechanism. Hepatology. 2001;34:884-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Tsai MH, Iwakiri Y, Cadelina G, Sessa WC, Groszmann RJ. Mesenteric vasoconstriction triggers nitric oxide overproduction in the superior mesenteric artery of portal hypertensive rats. Gastroenterology. 2003;125:1452-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Sakamoto M, Ueno T, Nakamura T, Sakata R, Hasimoto O, Torimura T, Sata M. Improvement of portal hypertension and hepatic blood flow in cirrhotic rats by oestrogen. Eur J Clin Invest. 2005;35:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Morgan-Martins MI, Jacques SI, Hartmann RM, Marques CM, Marroni CA, Marroni NP. Protection of estrogen in portal hypertension gastropathy: an experimental model. Arq Gastroenterol. 2011;48:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Dal-Ros S, Oswald-Mammosser M, Pestrikova T, Schott C, Boehm N, Bronner C, Chataigneau T, Gény B, Schini-Kerth VB. Losartan prevents portal hypertension-induced, redox-mediated endothelial dysfunction in the mesenteric artery in rats. Gastroenterology. 2010;138:1574-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Vuppalanchi R, Juluri R, Bell LN, Ghabril M, Kamendulis L, Klaunig JE, Saxena R, Agarwal D, Johnson MS, Chalasani N. Oxidative stress in chronic liver disease: relationship between peripheral and hepatic measurements. Am J Med Sci. 2011;342:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Vujanac A, Jakovljevic V, Djordjevic D, Zivkovic V, Stojkovic M, Celikovic D, Andjelkovic N, Skevin AJ, Djuric D. Nitroglycerine effects on portal vein mechanics and oxidative stress in portal hypertension. World J Gastroenterol. 2012;18:331-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Szöcs K, Lassègue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, Griendling KK. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol. 2002;22:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 301] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 13. | Hennenberg M, Trebicka J, Biecker E, Schepke M, Sauerbruch T, Heller J. Vascular dysfunction in human and rat cirrhosis: role of receptor-desensitizing and calcium-sensitizing proteins. Hepatology. 2007;45:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Hennenberg M, Biecker E, Trebicka J, Jochem K, Zhou Q, Schmidt M, Jakobs KH, Sauerbruch T, Heller J. Defective RhoA/Rho-kinase signaling contributes to vascular hypocontractility and vasodilation in cirrhotic rats. Gastroenterology. 2006;130:838-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Hennenberg M, Trebicka J, Kohistani AZ, Heller J, Sauerbruch T. Vascular hyporesponsiveness to angiotensin II in rats with CCl(4)-induced liver cirrhosis. Eur J Clin Invest. 2009;39:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Ferlitsch A, Pleiner J, Mittermayer F, Schaller G, Homoncik M, Peck-Radosavljevic M, Wolzt M. Vasoconstrictor hyporeactivity can be reversed by antioxidants in patients with advanced alcoholic cirrhosis of the liver and ascites. Crit Care Med. 2005;33:2028-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Robert R, Chagneau-Derrode C, Carretier M, Mauco G, Silvain C. Gender differences in vascular reactivity of aortas from rats with and without portal hypertension. J Gastroenterol Hepatol. 2005;20:890-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Fernando B, Marley R, Holt S, Anand R, Harry D, Sanderson P, Smith R, Hamilton G, Moore K. N-acetylcysteine prevents development of the hyperdynamic circulation in the portal hypertensive rat. Hepatology. 1998;28:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Moreira AJ, Fraga C, Alonso M, Collado PS, Zetller C, Marroni C, Marroni N, González-Gallego J. Quercetin prevents oxidative stress and NF-kappaB activation in gastric mucosa of portal hypertensive rats. Biochem Pharmacol. 2004;68:1939-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Ozgönül M, Oge A, Sezer ED, Bayraktar F, Sözmen EY. The effects of estrogen and raloxifene treatment on antioxidant enzymes in brain and liver of ovarectomized female rats. Endocr Res. 2003;29:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Strehlow K, Rotter S, Wassmann S, Adam O, Grohé C, Laufs K, Böhm M, Nickenig G. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003;93:170-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |