Published online Oct 21, 2013. doi: 10.3748/wjg.v19.i39.6559
Revised: August 4, 2013
Accepted: August 20, 2013
Published online: October 21, 2013
Processing time: 121 Days and 15.7 Hours
AIM: To investigate the effects of photodynamic therapy with quantum dots-arginine-glycine-aspartic acid (RGD) probe as photosensitizer on the proliferation and apoptosis of pancreatic carcinoma cells.
METHODS: Construction of quantum dots-RGD probe as photosensitizer for integrin-targeted photodynamic therapy was accomplished. After cells were treated with photodynamic therapy (PDT), the proliferation of SW1990 cells were measured by methyl thiazolyl tetrazolium assay. Morphologic changes, cell cycle retardance and apoptosis were observed under fluoroscope and flow cytometry. The expression of myeloid cell leukemia-1 (Mcl-1), protein kinase B (Akt) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mRNA were detected by reverse transcription-polymerase chain reaction. The amount of reactive oxygen species were also evaluated by fluorescence probe.
RESULTS: The photodynamic therapy with quantum dots-RGD probe as photosensitizer significantly inhibited cell proliferation (P < 0.01). Apoptotic cells and morphologic changes could be found under optical microscope. The FCM revealed PDT group had more significant cell apoptosis rate compared to control cells (F = 130.617, P < 0.01) and cell cycle G0/G1 and S retardance (P < 0.05) compared to control cells. The expression of Mcl-1 and Akt mRNA were down-regulated, while expression of TRAIL mRNA was up-regulated after cells treated with PDT. PDT group had more significant number of cells producing reactive oxygen species compared to control cells (F = 3262.559, P < 0.01).
CONCLUSION: The photodynamic therapy with quantum dots-RGD probe as photosensitizer significantly inhibits cell proliferation and increases apoptosis in SW1990 cells.
Core tip: Arginine-glycine-aspartic acid (RGD), sequence of small peptide, is an integrin antagonist. Quantum dots are characterized by conjugation with antibodies, peptides, or small molecules. Therefore, the construction of RGD-coupled quantum dots fluorescence probe allows for successful combination to integrin. Photodynamic therapy with a quantum dots-RGD probe, as a photosensitizer, significantly inhibits cell proliferation and increases apoptosis in SW1990 cells.
- Citation: Zhou M, Ni QW, Yang SY, Qu CY, Zhao PC, Zhang JC, Xu LM. Effects of integrin-targeted photodynamic therapy on pancreatic carcinoma cell. World J Gastroenterol 2013; 19(39): 6559-6567
- URL: https://www.wjgnet.com/1007-9327/full/v19/i39/6559.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i39.6559
Pancreatic adenocarcinoma ranks as the fourth most common cause of cancer death in the United States. Patients usually present late with advanced disease, limiting attempted curative surgery to 10% of cases. Overall prognosis is poor with one-year survival rates of less than 10% with palliative chemotherapy and/or radiotherapy[1-3]. Given these dismal results, a minimally invasive treatment capable of local destruction of tumor tissue with low morbidity may have a place in the treatment of this disease. The photodynamic therapy is a treatment that has high specificity, minimal invasiveness and good cosmetic outcome, which produces local necrosis of tissue with light after prior administration of a photosensitising agent[4]. In the past few years, little studies have been reported in the literature about the photodynamic therapy (PDT) on pancreatic cancer treatment, especially for the targeted-PDT[5-7]. Integrins αvβ3 plays a critical role in regulating tumor growth and metastasis as well as tumor angiogenesis[8,9]. Arginine-glycine-aspartic acid (RGD) peptides is the integrin antagonist which can link to integrin[10-12]. Therefore, it makes the RGD coupled quantum dots (QDs) probe successful to image the pancreatic cancer. In order to further discuss the possibility of PDT applied to pancreatic cancer treatment and its mechanism, we investigated the effects of photodynamic therapy with quantum dots-RGD probe as photosensitizer on the proliferation and apoptosis of pancreatic carcinoma cells to provide a new prospect for the clinical treatment of pancreatic cancer.
Quantum dots-RGD probe synthesized by School of Materials Science and Engineering, Shanghai University. Pancreatic cell line SW1990 purchased from Chinese Academy of Science. DEPC, Trizol, anhydrous ethanol, DAPI, MTT, DMSO, reactive oxygen species assay kit purchased from Beyotime institute of Biotechnology. Annexin V-FITC/PI purchased from invitrogen. RPMI 1640, 0.25% trypsin, fetal bovine serum,phosphate buffered saline purchased from Gibco. Reverse transcription-polymerase chain reaction (RT-PCR) assay kit purchased from Takara.
Quantum dots-RGD probe targeting research on pancreatic cancer cells: For laser confocal microscopy, cells grown on 35-mm two imaging dishes (Cat no. P35G-0-14-C, Ashland, MA, United States) were washed with PBS and then were incubated at 37 °C in the presence of 5 nmol/L quantum dots-RGD probe and probe with excessive RGD 1 μmol for 1 h. Afterward, cells were washed in ice-cold PBS, fixed by 4% paraformaldehyde and examined with simultaneous 543-nm excitation for laser confocal microscopy imaging. DAPI 2 μg/mL was utilized for the nuclear labeling localization which was incubated dark at room temperature for 15 min.
Methyl thiazolyl tetrazolium assay test: Cells grown on 96-well plates were divided into four groups, without QDs and light treated cells as blank control, pure light treated group, photosensitizer group, PDT group. Each group were set 10 double holes. Photosensitizer, PDT groups were incubated at 37 °C in the presence of 10 nmol/L Qds-RGD integrin-targeted probe. Pure light treated and PDT groups were treated with the wavelength of 690 nm laser, 20 J/cm2 for 20 min. Absorbance (A) value were measured at the wavelength of 490 nm. Cells growth were observed after treated 24, 48 and 72 h. Cells relative inhibition rate (%) = (1 - the average A value of experimental group/the average A value of control group) × 100%.
Morphologic changes in fluorescence microscopy: After treated 48 h, cells were fixed by 4% paraformaldehyde and incubated with DAPI 2 μg/mL. The morphological changes of cells were examined under fluorescence microscope.
Cell cycle retardance and apoptosis observed by flow cytometry: Each group were set 5 double holes. After treated 48 h, cells apoptosis and cell cycle retardance were observed by flow cytometry.
Reactive oxygen species test: Each group were set 3 double holes. After treated 48 h, DCFH-DA probe was applied to detect the expression of reactive oxygen species of four groups respectively.
Relative gene expression by RT-PCR test: According to the RT-PCR test kit procedure from Takara company, we observed the relative gene expression after PDT therapy. Gene primer sequences were synthesized by Shanghai Shenggong. Myeloid cell leukemia-1 (Mcl-1) primer forward 5’-AAAGCCTGTCTGCCAAAT-3’, reverse 5’-TATAAACCCACCACTCCC-3’, product fragment 196 bp. Protein kinase B (Akt) primer: forward 5’-AAGCACCGCGTGACCATGAA-3’, reverse 5’-TCTTAATGTGCCCGTCCTTG-3’, product fragment 445 bp. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) primer: forward 5’-TGGCTAACTGACCTGGAA-3’, reverse5’-GGAAACCTGGAGGCTACT-3’, product fragment 415 bp. Internal reference primer glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward 5’-CATGGTCTACACGTTCCAGT-3’. Reverse 5’-GGCTAAGCAGTTGGTGGTGC-3’, product fragment 349 bp. PCR reaction condition: 94 °C 3 min predenaturation, 94 °C 30 s, 55 °C 30 s, 72 °C 1 min (× 35 cycles) 72 °C extend for 5 min. Afterwards, grayscale scanning analysis was made by means of gel imaging analysis system. mRNA expression of the target gene = average gray value of specimens target gene/average gray value of the same specimen GAPDH.
Quantitative data in accordance with the normal distribution were analyzed using the One-way ANOVA and Student-Neuman-Keuls, otherwise using the non-parametric tests. SPSS 17.0 statistical software was used for analysis, and the significance level α was set at 0.05.
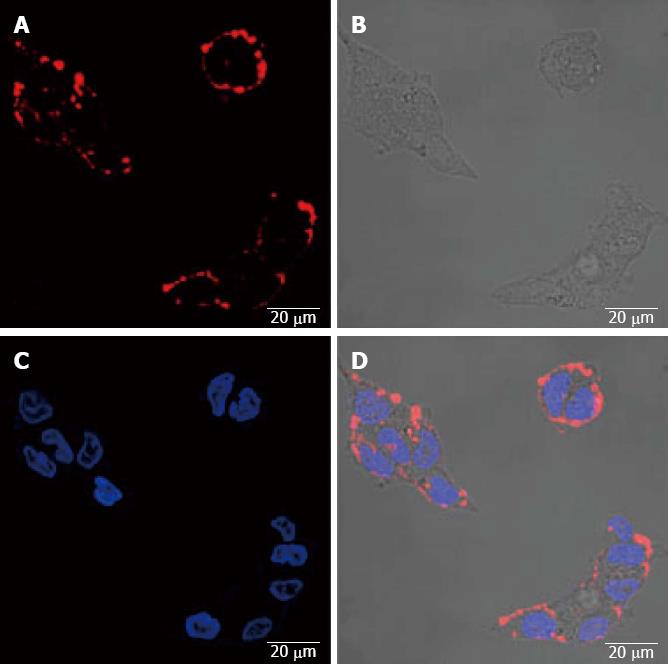
The fluorescence expression of SW1990 cells with the imaging of QDS-RGD probe: QDS-RGD probe imaging, original image, DAPI dyeing, and integrated image (Figure 1).
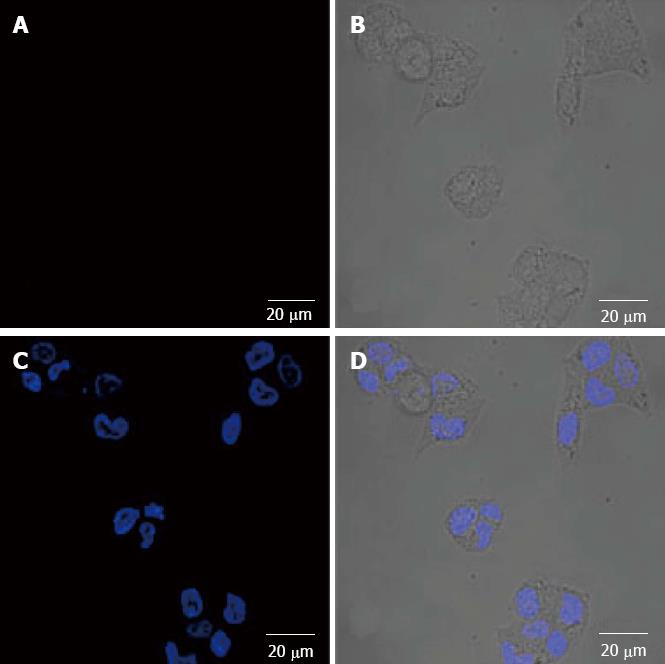
The fluorescence imaging of SW1990 cells with QDS-RGD probe at the block of excess RGD peptide: QDS-RGD probe imaging, original image, DAPI dyeing, and integrated image (Figure 2).
After 24, 48 or 72 h incubation, the PDT group cells growth inhibition rate was all higher than that of the control group, which makes statistically significant difference (F = 73.00, 85.10, 126.58, P < 0.01). While compared with the control group, the cell growth inhibition rate of light group and photosensitizer group had no significant difference (P > 0.05) (Table 1).
| Treat grouping | 24 h | 48 h | 72 h | G0/G1 | S |
| Control group | 0.213% ± 0.079% | 0.216% ± 0.071% | 0.274% ± 0.065% | 55.74% ± 2.82% | 37.47% ± 0.74% |
| Light group | 0.217% ± 0.053% | 0.242% ± 0.057% | 0.281% ± 0.042% | 53.73% ± 1.37% | 37.88% ± 3.60% |
| Photosensitizer group | 0.280% ± 0.061% | 0.273% ± 0.055% | 0.300% ± 0.050% | 57.72% ± 0.91% | 33.90% ± 3.39% |
| PDT group | 0.566% ± 0.052%b | 0.600% ± 0.062%b | 0.651% ± 0.045%b | 69.14% ± 2.63%b | 24.41% ± 2.67%b |
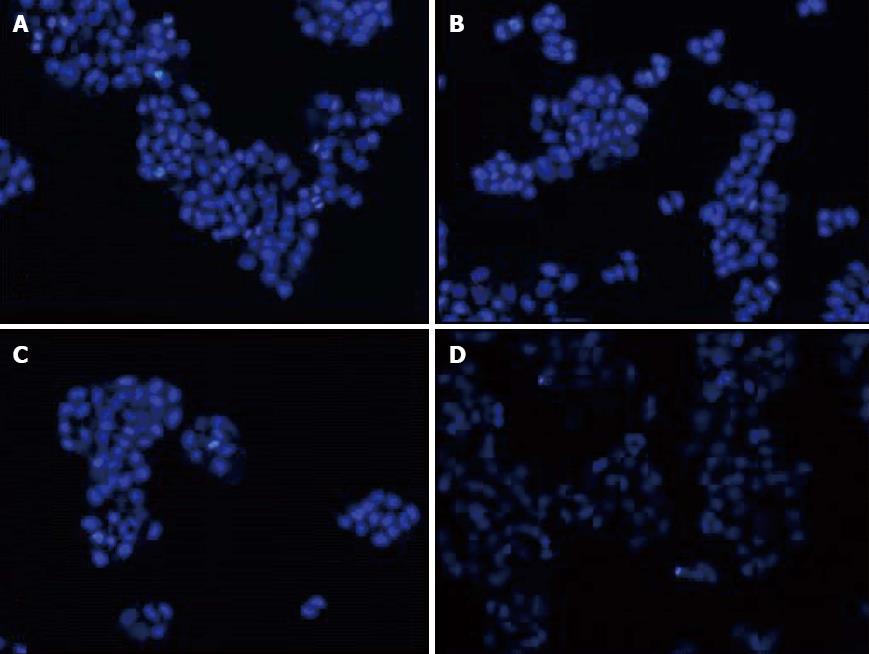
The cells nuclear morphologic changes in PDT group were corrugated under fluorescence microscopy. The nuclear of partial cells became staining deepen, bright blue, fuzzy and obviously pyknotic. With time prolonged, a large number of nuclear began to became dark, pyknotic and fragmentation. The remaining cells showed no significant change (Figure 3).
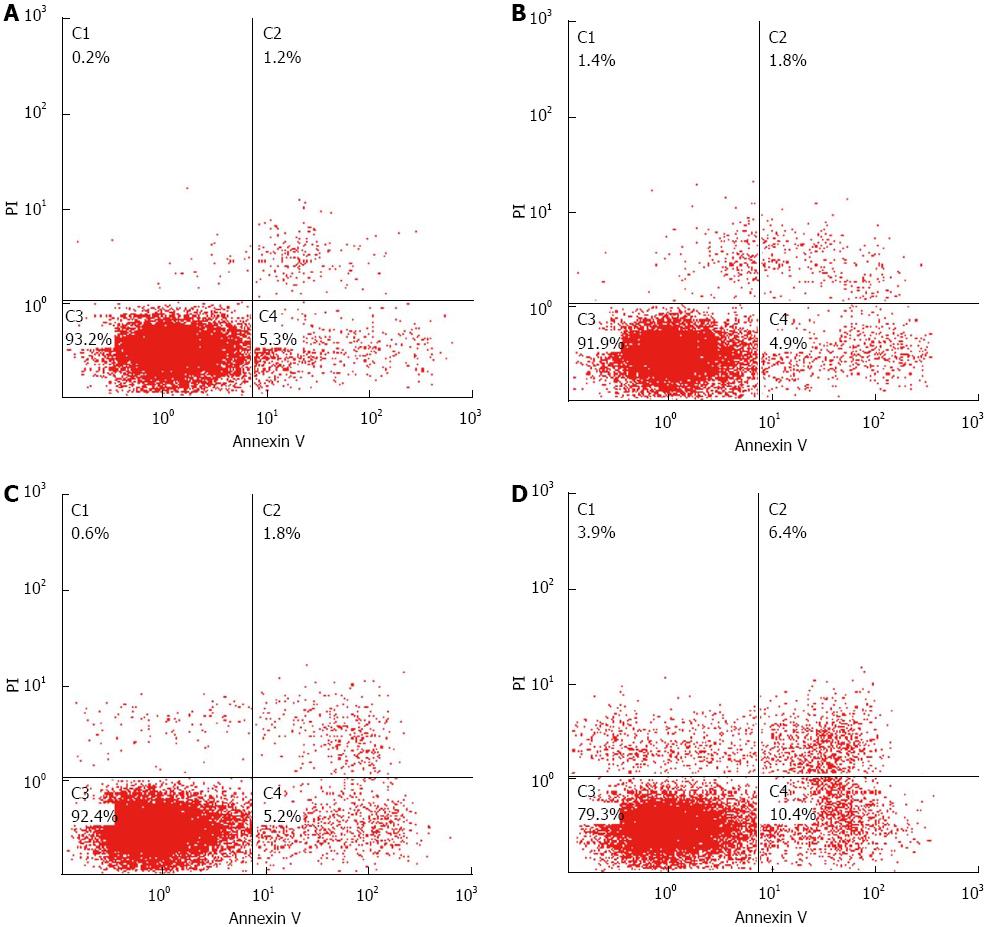
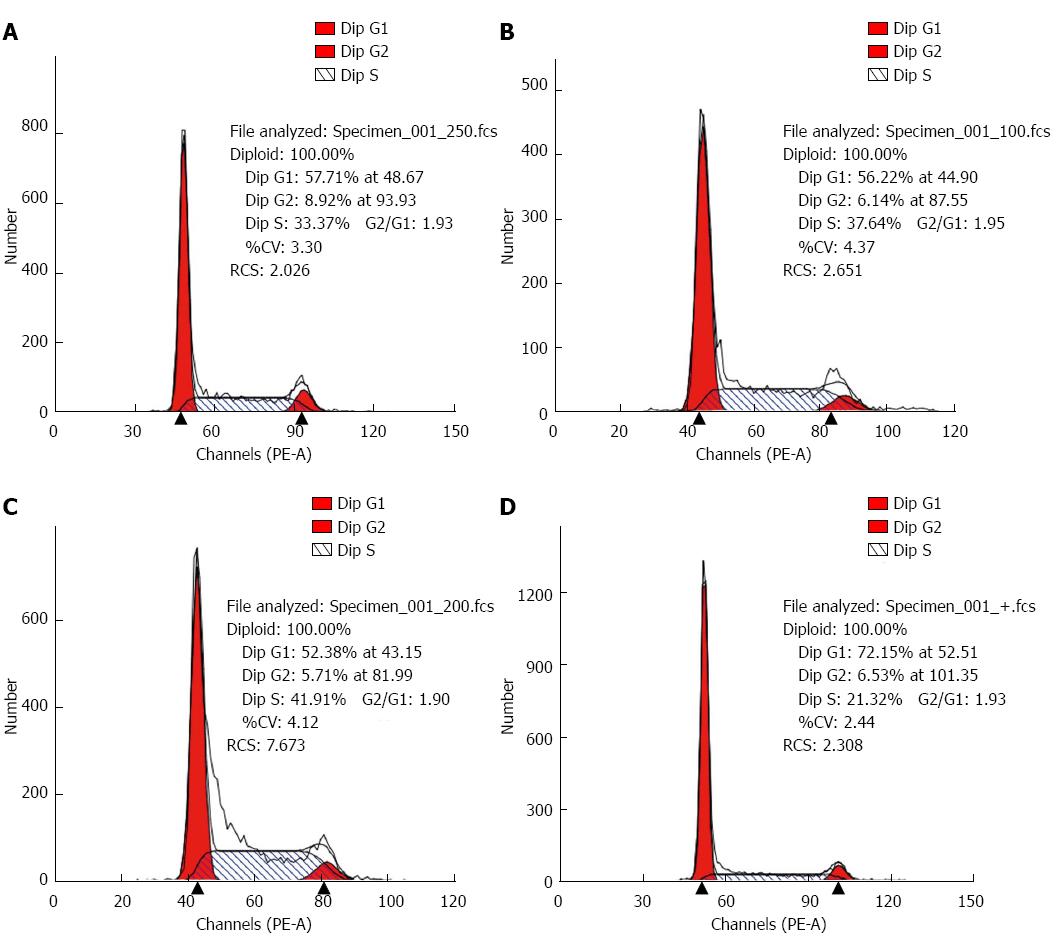
After treated for 48 h, the cell apoptotic rate of PDT group (17.86% ± 1.230%) was higher than the control (7.62% ± 1.219%), the light group (8.38% ± 0.277%) and the photosensitizer group (8.96% ± 0.673%), which made statistical difference (F = 130.617, P < 0.01, Figure 4). Compared to the control, PDT group had cell cycle G0/G1 and S retardance (G0/G1 69.14% ± 2.63% vs 55.74% ± 2.82%, S 24.41% ± 2.67% vs 37.47% ± 0.74%, all P < 0.05, Figure 5 and Table 1).
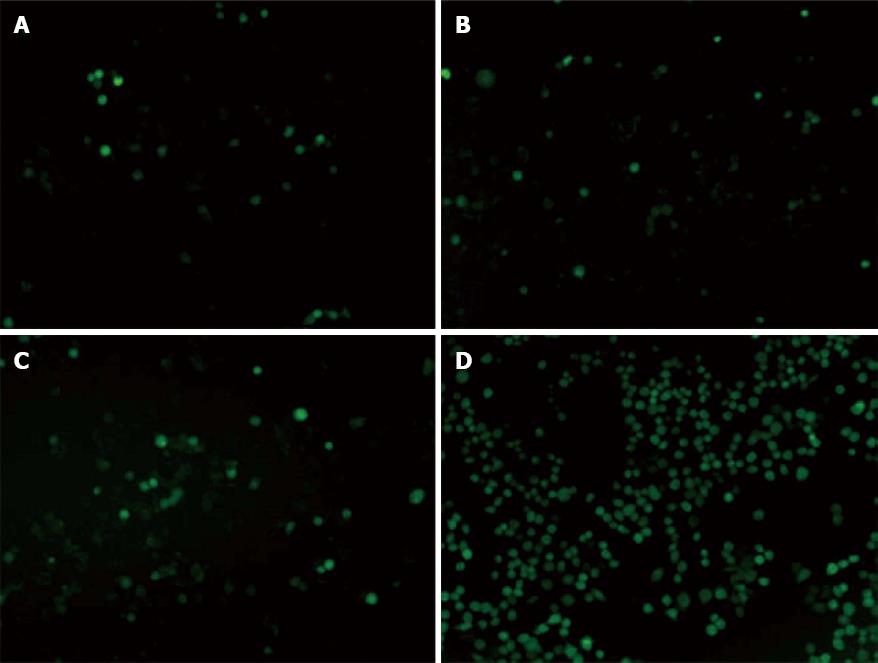
Each group was observed five high fields and figured out the mean value of the cells. The cells of each HPF expressing fluorescence in PDT group (286 ± 5.508) were higher than the control (28 ± 2.646), the light group (34 ± 2.082) and the photosensitizer group (36 ± 4.163), which made statistical difference (F = 3262.559, P < 0.01) (Figure 6).
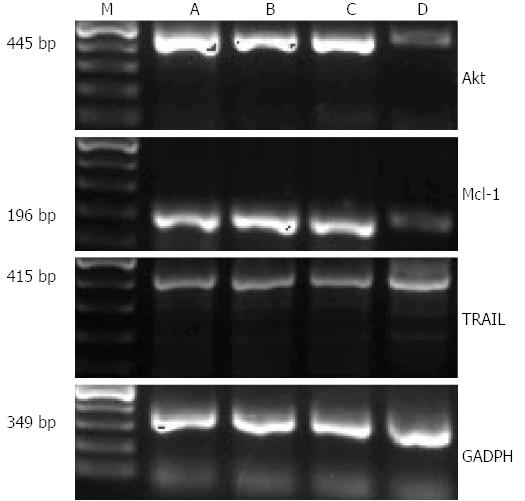
The grayscale ratio of Akt mRNA and GADPH mRNA of the control, the light group, the photosensitizer group and PDT group were 1.3319 ± 0.0482, 1.3137 ± 0.0100, 1.2900 ± 0.0116, 0.6161 ± 0.0032, respectively. The ratio of PDT group was lower than the others, which made statistical difference (F = 567.456, P < 0.05); The relative expression level of Mcl-1 was 0.9864 ± 0.0056, 1.0467 ± 0.0248, 1.0187 ± 0.0205, 0.3394 ± 0.0451, respectively. The ratio of these two mRNA in PDT group were lower than the others, which made statistical difference (F = 446.817, P < 0.05); the relative expression level of TRAIL was 0.6221 ± 0.0076, 0.6075 ± 0.0072, 0.6189 ± 0.0114, 0.7239 ± 0.0017, respectively. The ratio of PDT group was higher than the others, which made statistical difference (F = 145.238, P < 0.05) (Figure 7).
Since its discovery in the early 1900s, photodynamic therapy has developed from an emerging cancer treatment to an Food and Drug Administration-approved therapy for different malignancies. Two major challenges of current PDT are the limited tissue penetration of excitation light and poor tumor-selectivity of the photosensitizer. To address these issues, we developed a multifunctional nanoconstruct consisting of Semiconductor quantum dots and RGD peptide. RGD peptide was coated on the surface of QDs to anchor the probe close to SW1990 cells, thereby facilitating tumor imaging and targeted therapy.
Semiconductor QDs, after surface modification to render them water soluble and biocompatible, have a promising future in biomedical applications. QDs have size- and composition-adjustable fluorescence emission wavelengths, narrow emission bands, and very high levels of brightness and photostability. For in vitro studies, QDs have been used for cell labeling, fluorescence in situ hybridization, cell tracking, fluorescence resonance energy transfer, photodynamic therapy and many other applications[13-16]. QDs can serve as energy donors to conventional photosensitizers through FRET or interact directly with molecular oxygen via energy transfer mechanisms, to generate reactive 1O2 species that can be exploited for PDT[17,18].
Members of the integrin family influence several aspects of tumor progression and metastasis, including cell survival, proliferation, and angiogenesis. The αvβ3 integrin is the most prominent receptor class affecting tumor growth, tumor invasiveness, metastasis, tumor-induced angiogenesis, inflammation, osteoporosis, and rheumatoid arthritis. The αvβ3 integrin is strongly expressed on tumor cells and activated endothelial cells. In contrast, expression of αvβ3 integrin is weak on resting endothelial cells and most normal tissues[8,9,19]. The αvβ3 antagonists are being studied as anti-tumor and anti-angiogenic agents. A tripeptide sequence consisting of Arg-Gly-Asp (RGD) has been identified as a recognition motif used by extracellular matrix proteins (vitronectin, fibrinogen, laminin, and collagen) to bind to a variety of integrins, including αvβ3.
Confocal microscopy demonstrated the enhanced tumor-selectivity of the nanoconstructs to cancer cells that overexpressed integrin receptor. The integrin-positive SW1990 cells were clearly visualized in fluorescence images from QDs-RGD binding. The fluorescence intensity correlated with the integrin expression level of the cell lines. This binding was blocked effectively by excessive 1 μmol/L RGD. Unconjugated QDs showed no significant binding to the cell lines.
In vitro, the light-triggered PDT based on the nanoconstructs possessed remarkable therapeutic efficacy with tumor cell inhibition. The result of MTT showed that the PDT group cells growth inhibition rate was all higher than that of the control group, which made statistically significant difference (P < 0.01). While dealed with quantum dots or light irradiation alone, the cells did not showed significant destruction (P > 0.05). Apoptotic cells and morphologic changes could be found under optical microscope. The FCM revealed PDT group had more significant cell apoptosis rate compared to control cells (F = 130.617, P < 0.01) and cell cycle G0/G1 and S retardance(P < 0.05) compared to control cells. All the results demonstrated that photodynamic therapy based on integrin targeted probe could really inhibit pancreatic cancer cell proliferation and induce apoptosis, which were similar to the results of Yu et al[20] and Liu et al[7]. Xie et al[21] also reported that in vivo PDT combined with gemcitabine chemotherapy drugs could effectively inhibit the proliferation of pancreatic tumors and kill tumor tissue, which confirmed the inhibition and killing effect of PDT treatment in pancreatic cancer.
Akt is an important molecule involved in cell growth and invasion. Bcl-2 gene family have close relationship with cell apoptosis, including Mcl-1 (apoptosis inhibition) and TRAIL (apoptosis promotion) among members of the family. In our study, the expression of Mcl-1 and Akt were down-regulated, while expression of TRAIL mRNA was up-regulated after SW1990 cells treated with PDT. All the changes revealed the direction to promoting apoptosis.
The literature indicated that reactive oxygen species (ROS) was the activator of mitochondrial damage and apoptosis, which is highly reactive and toxic to cell membranes, lysosomes, and mitochondria[22]. Our study showed that PDT group had more significant number of SW1990 cells producing reactive oxygen species compared to the control, which revealed that the PDT promoted pancreatic cancer cells generating large amounts of ROS resulting in apoptosis. It had been reported that cancer cells had lower ability to scavenge oxygen free radicals and were more sensitive to ROS than the normal[22]. Though pancreatic cancer cell growth inhibition could also occur with the treatment of laser or photosensitizer alone, the inhibition in PDT group was significantly enhanced. The results revealed that PDT could significantly change the redox state of the pancreatic cancer cells and enhance their level of oxidative stress, which were similar to the others. The literature indicated that the semiconductor nanoparticles were not internalized in the cell nuclei but in the cytoplasm[22]. Furthermore, the nanoparticles were internalized into the lysosomes. Lysosomes were the primary cellular targets of photodynamic therapy. The ruptured lysosomes release cathepsins B or L, which activate initiator caspases or inhibited the Bcl-2 family, which then promoted apoptosis via the mitochondria. The expression changes of apoptosis related genes, Bcl-2 and TRAIL, were consistent with the literature reported[22]. Therefore, with this study, we proved for the first time that the increased cell death by the integrin-targeted photodynamic therapy.
Furthermore, it had been reported that photodynamic therapy could kill the pancreatic tumor in vitro[20] and was also effective to improve the chemotherapy drug gemcitabine resistance[23]. In vivo, it had been reported that PDT and chemotherapy drugs could have synergistic effect on killing tumor. PDT was respected to be a new promising method because of broad application in precancerous lesions and partial pancreas advanced lesions[24].
In summary, these results indicate that the multifunctional nanoconstruct is a promising PDT agent for deep-seated tumor treatment and demonstrate a new paradigm for enhancing PDT efficacy. Photodynamic therapy can be an effective treatment of patients with pancreatic cancer, but more extensive preclinical and clinical trials are needed for further improvement in the clinical application of PDT, especially in avoidance of complications during PDT.
A minimally invasive treatment capable of local destruction of tumor tissue with low morbidity may have a place in the treatment of pancreatic adenocarcinoma. The photodynamic therapy is a treatment that has high specificity, minimal invasiveness and good cosmetic outcome, which produces local necrosis of tissue with light after prior administration of a photosensitising agent. They investigated the effects of photodynamic therapy with quantum dots-arginine-glycine-aspartic acid (RGD) probe as photosensitizer on the proliferation and apoptosis of pancreatic carcinoma cells to provide a new prospect for the clinical treatment of pancreatic cancer.
The photodynamic therapy is a treatment that has high specificity, minimal invasiveness and good cosmetic outcome, which produces local necrosis of tissue with light after prior administration of a photosensitising agent. In the past few years, little studies have been reported in the literature about the photodynamic therapy (PDT) on pancreatic cancer treatment, especially for the targeted-PDT
Two major challenges of current photodynamic therapy (PDT) are the limited tissue penetration of excitation light and poor tumor-selectivity of the photosensitizer. To address these issues, authors developed a multifunctional nanoconstruct consisting of Semiconductor quantum dots and RGD peptide. RGD peptide was coated on the surface of quantum dots to anchor the probe close to SW1990 cells, thereby facilitating tumor imaging and targeted therapy.
These results indicate that the multifunctional nanoconstruct is a promising PDT agent for deep-seated tumor treatment and demonstrate a new paradigm for enhancing PDT efficacy. Photodynamic therapy can be an effective treatment of patients with pancreatic cancer, but more extensive preclinical and clinical trials are needed for further improvement in the clinical application of PDT, especially in avoidance of complications during PDT.
This is a good descriptive study in which authors evaluate the effect of photodynamic therapy with quantum dots-RGD probe as photosensitizer on the proliferation and apoptosis of pancreatic carcinoma cells. The results are interesting and suggest that significantly inhibits cell proliferation and increases apoptosis in SW1990 cells, which provide a new prospect for the clinical treatment of pancreatic cancer.
P- Reviewers Chowdhury P, Goral V, Sperti C, Yan MX S- Editor Gou SX L- Editor A E- Editor Ma S
| 1. | Baron TH, Kozarek RA. Preoperative biliary stents in pancreatic cancer--proceed with caution. N Engl J Med. 2010;362:170-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7953] [Cited by in RCA: 8101] [Article Influence: 506.3] [Reference Citation Analysis (2)] |
| 3. | Hruban RH, Maitra A, Kern SE, Goggins M. Precursors to pancreatic cancer. Gastroenterol Clin North Am. 2007;36:831-849, vi. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Shirasu N, Nam SO, Kuroki M. Tumor-targeted photodynamic therapy. Anticancer Res. 2013;33:2823-2831. [PubMed] |
| 5. | Bown SG, Rogowska AZ, Whitelaw DE, Lees WR, Lovat LB, Ripley P, Jones L, Wyld P, Gillams A, Hatfield AW. Photodynamic therapy for cancer of the pancreas. Gut. 2002;50:549-557. [PubMed] |
| 6. | Fan BG, Andrén-Sandberg A. Photodynamic therapy for pancreatic cancer. Pancreas. 2007;34:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Liu CD, Kwan D, Saxton RE, McFadden DW. Hypericin and photodynamic therapy decreases human pancreatic cancer in vitro and in vivo. J Surg Res. 2000;93:137-143. [PubMed] |
| 8. | De S, Razorenova O, McCabe NP, O’Toole T, Qin J, Byzova TV. VEGF-integrin interplay controls tumor growth and vascularization. Proc Natl Acad Sci USA. 2005;102:7589-7594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Robinson SD, Reynolds LE, Wyder L, Hicklin DJ, Hodivala-Dilke KM. Beta3-integrin regulates vascular endothelial growth factor-A-dependent permeability. Arterioscler Thromb Vasc Biol. 2004;24:2108-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Zhang X, Xiong Z, Wu Y, Cai W, Tseng JR, Gambhir SS, Chen X. Quantitative PET imaging of tumor integrin alphavbeta3 expression with 18F-FRGD2. J Nucl Med. 2006;47:113-121. [PubMed] |
| 11. | Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1246] [Cited by in RCA: 1247] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 12. | Miyoshi T, Hirohata S, Ogawa H, Doi M, Obika M, Yonezawa T, Sado Y, Kusachi S, Kyo S, Kondo S. Tumor-specific expression of the RGD-alpha3(IV)NC1 domain suppresses endothelial tube formation and tumor growth in mice. FASEB J. 2006;20:1904-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Chen LD, Li Y, Yuan HY, Pang DW. Quantum dots and their applications in cancer research. Ai Zheng. 2006;25:651-656. [PubMed] |
| 14. | Huang HC, Barua S, Sharma G, Dey SK, Rege K. Inorganic nanoparticles for cancer imaging and therapy. J Control Release. 2011;155:344-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 364] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 15. | Shao L, Gao Y, Yan F. Semiconductor quantum dots for biomedicial applications. Sensors (Basel). 2011;11:11736-11751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Luo G, Long J, Zhang B, Liu C, Ji S, Xu J, Yu X, Ni Q. Quantum dots in cancer therapy. Expert Opin Drug Deliv. 2012;9:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Samia AC, Chen X, Burda C. Semiconductor quantum dots for photodynamic therapy. J Am Chem Soc. 2003;125:15736-15737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 492] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 18. | Qi ZD, Li DW, Jiang P, Jiang FL, Li YS, Liu Y, Wong WK, Cheah KW. Biocompatible CdSe quantum dot-based photosensitizer under two-photon excitation for photodynamic therapy. J Mater Chem. 2011;21:2455-2458. [RCA] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Hosotani R, Kawaguchi M, Masui T, Koshiba T, Ida J, Fujimoto K, Wada M, Doi R, Imamura M. Expression of integrin alphaVbeta3 in pancreatic carcinoma: relation to MMP-2 activation and lymph node metastasis. Pancreas. 2002;25:e30-e35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Yu Z, Huang KH, Zhong W, Yang LQ, Chen QK, Zhu ZH. In Vitro Lethal Effect of Photodynamic Therapy on Human Pancreatic Cancer Cells and Its Major Influencing Factors. Clinical Oncology and Cancer Research. 2011;8:155-162. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Xie Q, Jia L, Liu YH, Wei CG. Synergetic anticancer effect of combined gemcitabine and photodynamic therapy on pancreatic cancer in vivo. World J Gastroenterol. 2009;15:737-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Wang L, Yang W, Read P, Larner J, Sheng K. Tumor cell apoptosis induced by nanoparticle conjugate in combination with radiation therapy. Nanotechnology. 2010;21:475103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Celli JP, Solban N, Liang A, Pereira SP, Hasan T. Verteporfin-based photodynamic therapy overcomes gemcitabine insensitivity in a panel of pancreatic cancer cell lines. Lasers Surg Med. 2011;43:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Topazian M, Zhong N, Baron TH, Vege SS, Wang KK. Photodynamic therapy of intraductal papillary mucinous neoplasm. Endoscopy. 2012;44:213-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |