Published online Sep 28, 2013. doi: 10.3748/wjg.v19.i36.6055
Revised: July 20, 2013
Accepted: August 8, 2013
Published online: September 28, 2013
Processing time: 143 Days and 6.1 Hours
AIM: To investigate differences between common gastric cancer and α-fetoprotein (AFP)-producing gastric cancer according to the presence or absence of liver metastasis.
METHODS: Between 1997 and 2011, 1299 patients underwent gastrectomy for gastric cancer (GC) at our institute and their hospital records were reviewed retrospectively. Patients were immunohistochemically divided into two groups: 23 patients (1.8%) with AFP-producing GC and 1276 patients (98.2%) without it.
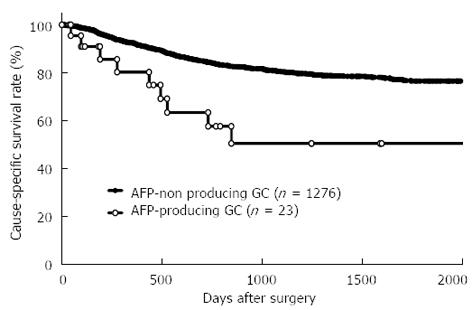
RESULTS: AFP-producing GC patients had a significantly higher incidence of deeper tumors, venous invasion, lymphatic invasion, lymph node metastasis, and liver metastasis and a poorer prognosis (P < 0.005) than those without AFP-producing GC. However, multivariate analysis revealed that AFP-positivity was not an independent prognostic factor. The prognosis of AFP-producing GC was similar to that of AFP-non producing GC according to the presence or absence of liver metastasis. Concerning recurrence, 47.8% of patients (11/23) with AFP-producing GC and 20.0% of patients (256/1276) without AFP-producing GC exhibited recurrence. Liver metastasis [90.9% (10/11)] was the most prevalent in AFP-producing GC patients. Multivariate analysis revealed that liver metastasis was the only independent prognostic factor in AFP-producing GC (HR = 17.6, 95%CI: 2.1-147.1; P = 0.0081).
CONCLUSION: AFP-producing GC is similar to common GC without liver metastasis, which should be specifically targeted in an effort to improve the prognosis of AFP-producing GC patients.
Core tip: In the present study, we re-evaluated the clinicopathological characteristics and clinical outcomes of consecutive patients with α-fetoprotein (AFP)-producing gastric cancer (GC). The results obtained clearly demonstrated that clinical behaviors were different between patients with and without AFP-producing GC. However, the prognosis according to the presence or absence of liver metastasis was similar between patients with and without AFP-producing GC. Our results show that liver metastasis should be specifically targeted in an effort to improve the prognosis of AFP-producing GC.
- Citation: Hirajima S, Komatsu S, Ichikawa D, Kubota T, Okamoto K, Shiozaki A, Fujiwara H, Konishi H, Ikoma H, Otsuji E. Liver metastasis is the only independent prognostic factor in AFP-producing gastric cancer. World J Gastroenterol 2013; 19(36): 6055-6061
- URL: https://www.wjgnet.com/1007-9327/full/v19/i36/6055.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i36.6055
α-fetoprotein (AFP) was initially found in the human fetus in 1956 and is normally produced in the fetal liver and yolk sac[1]. AFP is considered to be a useful tumor marker in screening or monitoring patients with hepatocellular carcinomas or yolk sac tumors. Previous studies have shown that AFP may be produced in other cancers including primary gastric cancer (GC)[2-6].
The incidence of AFP-producing GC is low and has been reported to be 1.3%-15% in GC[7-11]. Since AFP-producing GC was first described in 1970 by Bourrille et al[12], several cases of early and advanced AFP-producing GC have since been reported. Most of them exhibited a poor prognosis with a high incidence of lymphatic invasion, venous invasion, and synchronous and metachronous liver metastasis. Therefore, AFP-producing GC has been associated with a poorer prognosis than AFP-non producing GC[12-16]. However, most of these studies were restricted to the overall prognosis, and few studies conducted subgroup analyses with special reference to the presence or absence of liver metastasis.
In the present study, we re-evaluated the clinicopathological characteristics and clinical outcomes of consecutive patients with AFP-producing GC. The results obtained clearly demonstrated that clinical behaviors were different between patients with and without AFP-producing GC. However, the prognosis according to the presence or absence of liver metastasis was similar between patients with and without AFP-producing GC. It is important to note that the overall poorer prognosis of AFP-producing GC is not related to difficulties in treating it, but from the lack of effective and recommended treatments for liver metastasis of common GC. Our results show that liver metastasis should be specifically targeted in an effort to improve the prognosis of AFP-producing GC.
Between 1997 and 2011, 1375 patients with histologically confirmed primary gastric adenocarcinoma underwent gastrectomy at the Department of Digestive Surgery, Kyoto Prefectural University of Medicine. A total of 76 GC patients with active or chronic hepatitis, fatty liver, and hepatocellular carcinoma were excluded from this analysis, and 1299 patients were enrolled in this study and their hospital records were reviewed retrospectively.
Resected specimens were examined by pathologists based on classifications of the 14th Japanese Classification of Gastric Carcinomas (JCGC)[17] and 7th tumor-node-metastasis (TNM)[18]. The clinicopathological findings of these patients were determined retrospectively on the basis of their hospital records. Histological types were classified as differentiated (papillary, moderately, and well-differentiated adenocarcinoma) and undifferentiated (poorly, undifferentiated, signet-ring cell carcinoma, and mucinous adenocarcinoma) based on the 14th JCGC.
In this study, specimens were fixed in a 10% neutral buffered formaldehyde solution and embedded in paraffin. They were then stained with hematoxylin and eosin and examined histologically. They were also examined immunohistochemically to determine the presence of the tumor-associated antigen AFP. Immunohistochemical analyses were performed using the streptavidin-biotin method. Sections were dewaxed in xylene, passed through ethanol, and incubated with 3% hydrogen peroxide in methanol for 4 min to block endogenous peroxidase activity. Sections were washed in phosphate-buffered saline (PBS) and 10% normal rabbit serum was applied for 20 min to reduce nonspecific antibody binding. AFP, a rabbit polyclonal antibody purchased from the DAKO Corporation, was diluted 1:100 and reacted with tissue specimens at room temperature for 2 h. Specimens were washed three times with PBS. Sections were incubated with biotinylated rabbit antimouse immunoglobulin G at a dilution of 1:100 for 30 min followed by three washes. Slides were reacted with a streptavidin-biotin peroxidase reagent for 30 min at a dilution of 1:100 and washed with PBS three times. Hepatocellular carcinoma cell tissues containing AFP were used as positive controls. The first antibody was replaced with a sodium phosphate buffer for negative controls. Consequently, the immunoreactivity of AFP was demonstrated in 23 primary lesions of GC patients.
The follow-up program schedule for all patients comprised a regular physical examination and laboratory blood tests including tumor markers such as CEA and CA19-9, chest X-rays every 3 mo in the first postoperative year, every 6 mo in the second post-operative year, and annually thereafter for at least 5 years. Computer tomography (CT) was performed annually in patients with pathological stage I-II tumors and every 6 mo in patients with more than pathological stage III tumors for the first 5 years. Otherwise, CT was performed within one month when elevations in tumor markers and/or symptoms were detected in follow-up laboratory blood tests and regular physical examinations. Endoscopy was performed in all patients annually to screen for cancer in the gastric remnant.
The χ2 test and Fisher’s exact probability test were performed to compare clinicopathological characteristics between each group. Cause-specific death was recorded when the cause of death was specified as recurrent gastric cancer. Post-recurrence survival from recurrence to death, and overall survival from curative gastrectomy to death were estimated using the Kaplan-Meier method, and the log-rank test was used to assess differences between clinical factors. A P-value less than 0.05 was considered significant.
The mean patient age was 64.5 (range 27-94) years, and the male: female ratio was 2.1:1. Clinicopathological characteristics were compared between AFP-producing GC and AFP-non producing GC groups (Table 1). Patients with AFP-producing GC had a significantly higher incidence of venous invasion (P < 0.0001), lymphatic invasion (P = 0.0071), larger tumor size (P = 0.0073), lymph node metastasis (P = 0.0007), liver metastasis (P < 0.0001), and advanced stage (P = 0.0002) than those with AFP-non producing GC. The postoperative cause-specific 5-year survival rate of patients with AFP-producing GC was significantly poorer than that of patients without AFP-producing GC (5-year survival rate: 50.3% and 76.5%, P = 0.002) (Figure 1).
| Characteristic | n | Gastric cancer | P value1 | |
| AFP-producing | AFP-non producing | |||
| Total | 1299 | 23 | 1276 | |
| Sex | 0.9974 | |||
| Male | 875 | 16 (70) | 859 (67) | |
| Female | 424 | 7 (30) | 417 (33) | |
| Age (yr) | 0.5835 | |||
| Mean | 64.5 (range: 27-94) | |||
| < 65 | 610 | 9 (39) | 601 (47) | |
| ≥ 65 | 689 | 14 (61) | 675 (53) | |
| Location | 0.8753 | |||
| Upper | 292 | 4 (17) | 288 (23) | |
| Middle | 591 | 10 (44) | 581 (45) | |
| Lower | 416 | 9 (39) | 407 (32) | |
| Macroscopic type | < 0.0001 | |||
| Type 0-I/II/III | 690 | 2 (9) | 688 (54) | |
| Type 1/2/3/4 | 609 | 21 (91) | 588 (46) | |
| Histopathological grading | 0.8155 | |||
| Differentiated | 596 | 10 (43) | 586 (46) | |
| Undifferentiated | 703 | 13 (57) | 690 (54) | |
| Tumor size (mm) | 0.0073 | |||
| < 40 | 614 | 4 (17) | 610 (48) | |
| ≥ 40 | 685 | 19 (83) | 666 (52) | |
| Venous invasion | < 0.0001 | |||
| Negative | 937 | 5 (22) | 932 (73) | |
| Positive | 362 | 18 (78) | 344 (27) | |
| Lymphatic invasion | 0.0071 | |||
| Negative | 726 | 6 (26) | 720 (56) | |
| Positive | 573 | 17 (74) | 556 (44) | |
| TNM classification | ||||
| pT categories | 0.036 | |||
| T1 | 664 | 4 (17) | 660 (52) | |
| T2 | 122 | 3 (13) | 119 (9) | |
| T3 | 217 | 7 (31) | 210 (16) | |
| T4 | 296 | 9 (39) | 287 (23) | |
| pN categories | 0.0007 | |||
| N0 | 821 | 9 (39) | 812 (63) | |
| N1 | 140 | 9 (39) | 131 (10) | |
| N2 | 137 | 3 (13) | 134 (11) | |
| N3 | 201 | 2 (9) | 199 (16) | |
| pStage | ||||
| I | 769 | 3 (13) | 766 (60) | 0.0002 |
| II | 137 | 3 (13) | 134 (11) | |
| III | 195 | 9 (39) | 186 (14) | |
| IV | 198 | 8 (35) | 190 (15) | |
| Absent | 955 | 12 (52) | 943 (74) | 0.0192 |
| Present | 344 | 11 (48) | 333 (26) | |
| Liver metastasis | < 0.0001 | |||
| Absent | 1245 | 13 (57) | 1232 (97) | |
| Present | 54 | 10 (43) | 44 (3) | |
Of all 1299 GC patients analyzed, univariate analysis showed that age, location, macroscopic type, tumor size, histological type, venous invasion, lymphatic invasion, pT categories, pN categories, the presence of AFP-producing GC, and liver metastasis were prognostic factors (Table 2). Furthermore, multivariate analysis using stepwise Cox regression procedures demonstrated that macroscopic type, tumor size, venous invasion, lymphatic invasion, pN categories, and liver metastasis were independent prognostic factors. However, the presence of AFP-producing GC was not an independent prognostic factor (Table 2).
| Factor | Univariate1 | Multivariate2 | ||
| P value | HR | 95%CI | P value | |
| Sex | ||||
| Male vs female | 0.3121 | |||
| Age (yr) | ||||
| 65 ≤vs < 65 | 0.0033 | |||
| Location | ||||
| U vs ML | 0.0027 | |||
| Macroscopic type | ||||
| Type 1/2/3/4 vs Type 0-I/II/III | < 0.0001 | 3.760 | 2.012-7.013 | < 0.0001 |
| Tumor size (mm) | ||||
| 40 ≤vs < 40 | < 0.0001 | 2.128 | 1.259-3.597 | 0.0048 |
| Histological type | ||||
| Undifferentiated vs Differentiated | 0.014 | |||
| pT categories | ||||
| T2/3/4 vs T1 | < 0.0001 | |||
| Venous invasion | ||||
| Positive vs Negative | < 0.0001 | 1.721 | 1.247-2.375 | 0.0010 |
| Lymphatic invasion | ||||
| Positive vs Negative | < 0.0001 | 2.793 | 1.656-4.717 | 0.0001 |
| pN categories | ||||
| Positive vs Negative | < 0.0001 | 2.513 | 1.614-3.951 | < 0.0001 |
| AFP | ||||
| producing vs non producing | 0.0018 | |||
| Liver metastasis | ||||
| Positive vs Negative | < 0.0001 | 3.152 | 2.116-4.558 | < 0.0001 |
To develop surveillance programs and treatment strategies, we compared recurrence patterns between patients with and without AFP-producing GC. Distant metastasis and recurrence developed in 47.8% of patients (11/23) with AFP-producing GC and 20.0% of patients (256/1276) without AFP-producing GC. Of these, liver metastasis [90.9% (10/11)] was the most prevalent in AFP-producing GC patients, while peritoneal recurrence [44.1% (113/256)] was the most common in patients without AFP-producing GC (Table 3).
| Site | GC patients with recurrence | |
| AFP-producing GC (n = 11) | AFP-non producing GC (n = 256) | |
| Peritoneum | 1 (9.1) | 113 (44.1) |
| Liver | 10 (90.9) | 44 (17.2) |
| Lung | 1 (9.1) | 3 (1.2) |
| Bone | 0 (0.0) | 13 (5.1) |
| Lymph nodes | 0 (0.0) | 48 (18.8) |
| Others | 0 (0.0) | 46 (18.0) |
We investigated prognostic factors affecting prognosis in AFP-producing GC. Univariate and multivariate analyses using stepwise Cox regression procedures revealed that the presence of liver metastasis was the only independent prognostic factor (HR = 17.6, 95%CI: 2.1-147.1; P = 0.0081) (Table 4). However, no significantly different prognostic factor was observed between patients with or without liver metastasis in AFP-producing GC (Table 5).
| Factor | Univariate1 | Multivariate2 | ||
| P value | HR | 95%CI | P value | |
| Sex | ||||
| Male vs female | 0.3574 | |||
| Age (yr) | ||||
| 65 ≤vs < 65 | 0.0917 | |||
| Location | ||||
| U vs ML | 0.4311 | |||
| Macroscopic type | ||||
| Type 1/2/3/4 vs Type 0-I/II/III | 0.3422 | |||
| Tumor size (mm) | ||||
| 40 ≤vs < 40 | 0.3539 | |||
| Histological type | ||||
| Undifferentiated vs Differentiated | 0.0503 | |||
| pT categories | ||||
| T2/3/4 vs T1 | 0.1428 | |||
| Venous invasion | ||||
| Positive vs negative | 0.6696 | |||
| Lymphatic invasion | ||||
| Positive vs negative | 0.6188 | |||
| pN categories | ||||
| Positive vs negative | 0.9855 | |||
| Liver metastasis | ||||
| Positive vs negative | 0.0006 | 17.587 | 2.112-147.12 | 0.0081 |
| Factor | n | Liver metastasis | P value1 | |
| Absence | Presence | |||
| Total | 23 | 13 | 10 | |
| Sex | 0.1203 | |||
| Male | 14 | 10 (77) | 4 (40) | |
| Female | 19 | 13 (23) | 6 (60) | |
| Age (yr) | 0.4173 | |||
| < 65 | 9 | 4 (31) | 5 (50) | |
| ≥ 65 | 14 | 9 (69) | 5 (50) | |
| Location | 0.1717 | |||
| Upper | 4 | 0 (0) | 4 (40) | |
| Middle | 10 | 7 (54) | 3 (30) | |
| Lower | 9 | 6 (46) | 3 (30) | |
| Macroscopic type | 0.4862 | |||
| Type 0-I/II/III | 2 | 2 (15) | 0 (0) | |
| Type 1/2/3/4 | 21 | 11 (85) | 10 (100) | |
| Histopathological grading | 0.2215 | |||
| Differentiated | 10 | 4 (31) | 6 (60) | |
| Undifferentiated | 13 | 9 (69) | 4 (40) | |
| Tumor size (mm) | 0.6036 | |||
| < 40 | 4 | 3 (23) | 1 (10) | |
| ≥ 40 | 19 | 10 (77) | 9 (90) | |
| Venous invasion | 1.0000 | |||
| Negative | 5 | 3 (23) | 2 (20) | |
| Positive | 18 | 10 (77) | 8 (80) | |
| Lymphatic invasion | 0.0886 | |||
| Negative | 7 | 6 (46) | 1 (10) | |
| Positive | 16 | 7 (54) | 9 (90) | |
| TNM classification | ||||
| pT categories | 0.7007 | |||
| T1 | 4 | 3 (23) | 1 (10) | |
| T2 | 3 | 2 (15) | 1 (10) | |
| T3 | 7 | 5 (39) | 2 (20) | |
| T4 | 9 | 3 (23) | 6 (60) | |
| pN categories | 0.9049 | |||
| N0 | 9 | 5 (39) | 4 (40) | |
| N1 | 9 | 4 (31) | 5 (50) | |
| N2 | 3 | 2 (15) | 1 (10) | |
| N3 | 2 | 2 (15) | 0 (0) | |
| pStage | 0.6736 | |||
| I | 3 | 3 (23) | 0 (0) | |
| II | 3 | 2 (15) | 1 (10) | |
| III | 9 | 5 (39) | 4 (40) | |
| IV | 8 | 3 (23) | 5 (50) | |
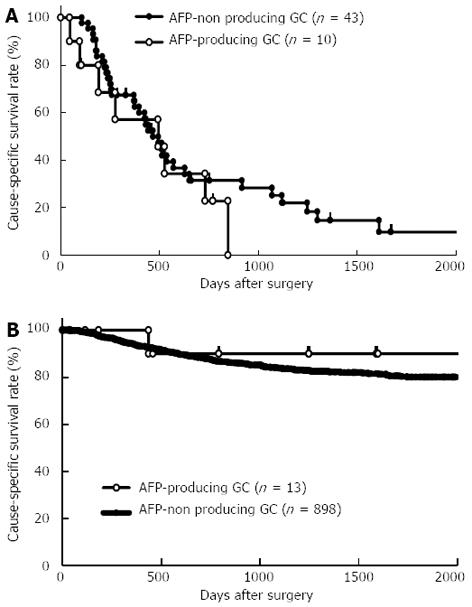
To confirm that the presence of AFP-producing GC was not an independent prognostic factor in GC, we performed subgroup analysis according to the presence or absence of liver metastasis between patients with and without AFP-producing GC. The results obtained showed that the prognosis of AFP-producing GC was similar to that of AFP-non producing GC according to the presence (Figure 2A, P = 0.3778) or absence (Figure 2B, P = 0.5024) of liver metastasis.
AFP-producing GC has been shown to exhibit more aggressive behavior and a poorer prognosis than common GC because of its high incidence of liver metastasis. Liu and his colleagues reported that the 1-, 3- and 5-year survival rates of AFP-producing gastric cancer were 53%, 35%, and 28%, respectively[10]. Chang and his colleagues examined 24 patients with AFP-producing gastric cancer and showed that the prognosis was poor due to the high incidence of synchronous and metachronous liver metastasis[8]. In Japan, one study reported that the 5-year survival rate of AFP-producing GC was 28.4%[7]. However, most of these studies have been restricted to overall prognosis in AFP-producing GC. To date, few subgroup analyses have been performed with special reference to liver metastasis and its related prognosis in AFP-producing GC.
In this study, we clearly demonstrated that the clinical behaviors of patients with and without AFP-producing GC were different (Table 1), and also that prognoses between patients with and without AFP-producing GC regarding the presence or absence of liver metastasis were similar in subgroup analyses (Figure 2). At first, we hypothesized that the presence of AFP-producing GC was an independent prognostic factor in GC because the prognosis of AFP-producing GC is known to be poorer than that of common GC. Indeed, the 5-year survival rate of patients with AFP-producing GC was significantly poorer than that of patients without AFP-producing GC. Patients with AFP-producing GC also had a significantly higher incidence of lymph-venous invasion, larger tumor size, lymph node metastasis, liver metastasis, and advanced stage. However, multivariate analysis using stepwise Cox regression procedures demonstrated that the presence of AFP-producing GC was not an independent prognostic factor. These findings suggested that AFP-producing GC is not different from common gastric cancer. AFP-producing GC without liver metastasis does not necessary have a poor prognosis.
The reason why the prognosis of AFP-producing GC is still poor is that there are currently no standard or recommended treatments for liver metastasis of GC. Even in recent prospective randomized control trials, such as JCOG9912, SPIRITS, TOGA, and START studies using S-1 based regimens, a survival benefit was found particularly in non-measurable tumor recurrences such as peritoneal recurrence[19-22]. Therefore, it is important to note that the poorer prognosis of AFP-producing GC is not related to difficulties in treating it, but from the lack of effective and recommended treatments for liver metastasis of common GC. If a treatment strategy for liver metastasis of GC was established similar to that for colorectal cancer, the prognosis of AFP-producing GC may be markedly improved because of its distinct metastatic pattern.
Concerning patterns of recurrences, distant metastasis and recurrence developed in 47.8% of patients (11/23) with AFP-producing GC and 20.0% of patients (256/1276) without AFP-producing GC. In AFP-producing GC patients, 90.9% of patients (10/11) developed liver metastasis, while peritoneal recurrence occurred in only 9.1% (1/11). In contrast, peritoneal recurrence [44.1% (113/256)]was the most prevalent in patients without AFP-producing GC, with liver metastasis accounting for 17.2% (44/256) (Table 3). Regarding the prognoses of 23 patients with AFP-producing GC, 5-year survival rates between patients with and without liver metastasis were 0% and 90.0%, respectively (data not shown). Moreover, univariate and multivariate analyses in AFP-producing GC revealed that liver metastasis was the only independent prognostic factor (Table 4).
Our results show that liver metastasis should be specifically targeted in an effort to improve the prognosis of AFP-producing GC. However, our study included a small number of patients with AFP-producing GC. Therefore, a larger sample size is needed to confirm these clinical features of AFP-producing gastric cancer.
In the present study, we demonstrated that liver metastasis was the only independent prognostic factor in AFP-producing GC. The absence of standard or recommended treatments for liver metastasis of GC also means that there is no effective treatment strategy for AFP-producing GC. Considering other rarely developing recurrences excluding liver metastasis in AFP-producing GC, the use of hepatic infusion chemotherapy or systemic chemotherapy following resection of hepatic metastasis may be a more efficient treatment than that for liver metastasis of common GC[23-25]. In conclusion, clinical behaviors between patients with and without AFP-producing GC were different. However, prognoses were similar according to the presence or absence of liver metastasis. Liver metastasis should be specifically targeted in an effort to improve the prognosis of AFP-producing GC.
α-fetoprotein (AFP)-producing gastric cancer (GC) is generally known to exhibit more aggressive behavior and a poorer prognosis than common GC because of its high incidence of liver metastasis. However, little is known about the clinical behavior of AFP-producing GC with regard to the presence or absence of liver metastasis.
The authors re-evaluated the clinicopathological characteristics and clinical outcomes of patients with AFP-producing GC. The results obtained clearly demonstrated that clinical behaviors were different between patients with and without AFP-producing GC. Concerning recurrence, while the incidence of peritoneal recurrence was the highest in AFP-producing GC, liver metastasis was the most prevalent in AFP-non producing GC. Moreover, the prognosis according to the presence or absence of liver metastasis was similar between patients with and without AFP-producing GC.
Multivariate analysis revealed that AFP-positivity was not an independent prognostic factor and also that liver metastasis was the only independent prognostic factor in AFP-producing GC. It is important to note that the overall poorer prognosis of AFP-producing GC is not related to difficulties in treating it, but from the lack of effective and recommended treatments for liver metastasis of common GC.
The prognosis according to the presence or absence of liver metastasis was similar between patients with and without AFP-producing GC. The results show that liver metastasis should be specifically targeted in an effort to improve the prognosis of AFP-producing GC.
AFP was initially found in the human fetus and is normally produced in the fetal liver and yolk sac. AFP is considered to be a useful tumor marker in screening or monitoring patients with hepatocellular carcinomas or yolk sac tumors. Some studies showed that AFP could be produced in other cancers including primary GC. Liver metastasis refers to cancerous tumors that have spread to the liver from somewhere else in the body. The risk of cancer spreading to the liver depends on the site of the original cancer. Liver metastasis may be present when the original (primary) cancer is diagnosed, or it may occur months or years after the primary tumor is removed.
This is an interesting manuscript from a group that has done excellent work on gastric cancer. The authors evaluated the effect of AFP-producing gastric carcinoma on survival. Their conclusion that AFP production was not an independent predictor of poor survival is in contradistinction to other reports.
P- Reviewers Balzan SMP, Leitman M, Pucci S S- Editor Zhai HH L- Editor A E- Editor Zhang DN
| 1. | Bergstrand CG, Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest. 1956;8:174. [PubMed] |
| 2. | Nishimura H, Okamoto Y, Takahashi M, Fujita T. Occurrence of alpha-fetoprotein, Regan isoenzyme, and variant alkaline phosphatase in the serum of a patient with gastric cancer. Gastroenterology. 1976;71:497-499. [PubMed] |
| 3. | Masuzawa M, Lee PK, Kamada T, Akeyama T, Abe H, Shimano T, Mori T, Morino H, Ishiguro S. Carcinoembryonic antigen, alpha-fetoprotein and carcinoplacental alkaline phosphatase in gastric carcinoma metastatic to the liver. Cancer. 1977;39:1175-1180. [PubMed] |
| 4. | Kodama T, Kameya T, Hirota T, Shimosato Y, Ohkura H, Mukojima T, Kitaoka H. Production of alpha-fetoprotein, normal serum proteins, and human chorionic gonadotropin in stomach cancer: histologic and immunohistochemical analyses of 35 cases. Cancer. 1981;48:1647-1655. [PubMed] |
| 5. | Ooi A, Okada Y, Minamoto T, Imabori T, Shima K. [A case of alpha-fetoprotein-producing gastric carcinoma with histologic features of embryonal carcinoma]. Gan No Rinsho. 1985;31:334-340. [PubMed] |
| 6. | Koyama S, Ebihara T, Osuga T. Histologic and immunohistochemical studies of alpha-fetoprotein (AFP)-producing gastric carcinoma. Gastroenterol Jpn. 1987;22:419-427. [PubMed] |
| 7. | Kono K, Amemiya H, Sekikawa T, Iizuka H, Takahashi A, Fujii H, Matsumoto Y. Clinicopathologic features of gastric cancers producing alpha-fetoprotein. Dig Surg. 2002;19:359-365; discussion 365. [PubMed] |
| 8. | Chang YC, Nagasue N, Kohno H, Taniura H, Uchida M, Yamanoi A, Kimoto T, Nakamura T. Clinicopathologic features and long-term results of alpha-fetoprotein-producing gastric cancer. Am J Gastroenterol. 1990;85:1480-1485. [PubMed] |
| 9. | Chun H, Kwon SJ. Clinicopathological characteristics of alpha-fetoprotein-producing gastric cancer. J Gastric Cancer. 2011;11:23-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long Z, Zhu H, Wang Y. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol. 2010;102:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | McIntire KR, Waldmann TA, Moertel CG, Go VL. Serum alpha-fetoprotein in patients with neoplasms of the gastrointestinal tract. Cancer Res. 1975;35:991-996. [PubMed] |
| 12. | Bourreille J, Metayer P, Sauger F, Matray F, Fondimare A. [Existence of alpha feto protein during gastric-origin secondary cancer of the liver]. Presse Med. 1970;78:1277-1278. [PubMed] |
| 13. | Chang YC, Nagasue N, Abe S, Kohno H, Yamanoi A, Uchida M, Nakamura T. [The characters of AFP-producing early gastric cancer]. Nihon Geka Gakkai Zasshi. 1990;91:1574-1580. [PubMed] |
| 14. | Motoyama T, Aizawa K, Watanabe H, Fukase M, Saito K. alpha-Fetoprotein producing gastric carcinomas: a comparative study of three different subtypes. Acta Pathol Jpn. 1993;43:654-661. [PubMed] |
| 15. | Chang YC, Nagasue N, Abe S, Taniura H, Kumar DD, Nakamura T. Comparison between the clinicopathologic features of AFP-positive and AFP-negative gastric cancers. Am J Gastroenterol. 1992;87:321-325. [PubMed] |
| 16. | Koide N, Nishio A, Igarashi J, Kajikawa S, Adachi W, Amano J. Alpha-fetoprotein-producing gastric cancer: histochemical analysis of cell proliferation, apoptosis, and angiogenesis. Am J Gastroenterol. 1999;94:1658-1663. [PubMed] |
| 17. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [PubMed] |
| 18. | Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of Malignant Tumors. 7th ed. New York: Wiley-Liss 2009; . |
| 19. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5285] [Article Influence: 352.3] [Reference Citation Analysis (3)] |
| 20. | Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 474] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 21. | Kim JH, Jang YJ, Park SS, Park SH, Mok YJ. Benefit of post-operative surveillance for recurrence after curative resection for gastric cancer. J Gastrointest Surg. 2010;14:969-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1416] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 23. | Yabusaki H, Nashimoto A, Tanaka O. [A case of AFP-producing gastric cancer after curative operation effectively treated with chemotherapies including hepatic arterial infusion therapy]. Gan To Kagaku Ryoho. 2000;27:735-738. [PubMed] |
| 24. | Sobajima J, Murata N, Ishida H, Hashimoto D, Adachi A, Itoyama S. [A case of postoperative liver metastasis of alpha-fetoprotein producing gastric cancer successfully treated with intrahepatic chemotherapy (adriamycin, cisplatin, irinotecan hydrochloride)]. Gan To Kagaku Ryoho. 2002;29:2132-2134. [PubMed] |
| 25. | Takada J, Kenno S, Aoki T, Hamada H, Katsuki Y. [A case in which intra-arterial chemotherapy for simultaneous hepatic metastases markedly improved AFP-producing gastric cancer]. Gan To Kagaku Ryoho. 2009;36:2326-2329. [PubMed] |