Published online Apr 21, 2013. doi: 10.3748/wjg.v19.i15.2388
Revised: April 8, 2013
Accepted: April 9, 2013
Published online: April 21, 2013
Processing time: 56 Days and 6.4 Hours
AIM: To investigate changes in serum ghrelin and obestatin levels before and after Helicobacter pylori (H. pylori) eradication.
METHODS: A total of 92 patients presenting with symptoms of dyspepsia were enrolled in the study. Upper endoscopy was performed on all patients and used to diagnose H. pylori infection according to the presence of characteristic histopathological findings; seventy patients were diagnosed with H. pylori infection and the remaining 22 non-infected patients were classified as healthy controls. H. pylori eradication was accomplished by administering the classical triple therapy drug regimen, consisting of lansoprazole 30 mg bid, amoxicillin 1 g bid, and clarithromycin 500 mg tid for 14 d. The eradication of H. pylori was assessed with C14-urea breath test, which was performed at eight weeks after treatment. Levels of serum active ghrelin and obestatin were assessed at beginning of the study (prior to treatment) and after eight weeks. The levels were comparatively analyzed between the H. pylori negative control group, the H. pylori eradicated group, and the H. pylori non-eradicated group.
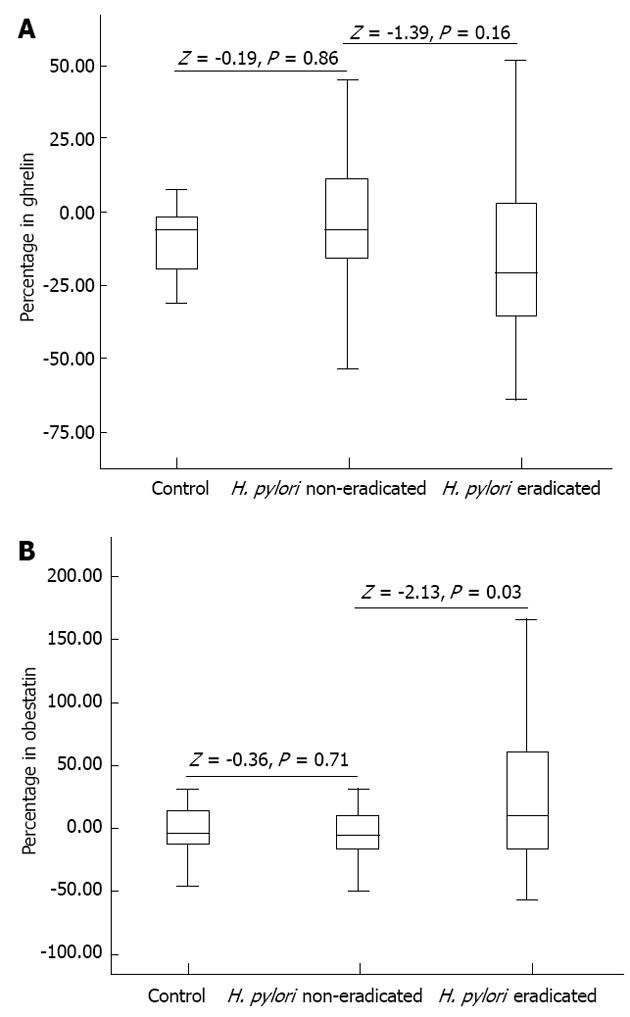
RESULTS: A total of 92 patients, 50 females and 42 males with a mean age of 38.2 ± 11.9 years (range: 19-64), were analyzed. H. pylori eradication success was achieved in 74.3% (52/70) of H. pylori positive patients. The initial levels of ghrelin in the H. pylori positive and control cases were 63.6 ± 19.8 pg/mL and 65.1 ± 19.2 pg/mL (P = 0.78), respectively, and initial obestatin levels were 771 ± 427 pg/mL and 830 ± 296 pg/mL (P = 0.19), respectively. The difference between the initial levels and the week 8 levels of ghrelin and obestatin in the control group was insignificant [4.5% (P = 0.30) and -0.9% (P = 0.65), respectively]. The difference between the initial and week 8 levels of ghrelin and obestatin in the H. pylori non-eradicated group were also insignificant [0.9% (P = 0.64) and 5.3% (P = 0.32), respectively]. The H. pylori eradicated group had a greater change in obestatin levels when compared to the control and the non-eradicated groups (148 ± 381 pg/mL vs -12 ± 138 pg/mL and -72.8 ± 203 pg/mL, respectively, P = 0.015), while decreases in ghrelin levels were insignificant (-7.2 pg/mL vs -1.4 pg/mL and -1.9 pg/mL, respectively, P = 0.52). The ghrelin/obestatin ratio for the initial and week 8 levels changed significantly in only the H. pylori eradicated group (0.11 vs 0.08, respectively, P = 0.015). For overweight patients (as designated by body mass index), we observed significant increases in obestatin levels in the eradicated group as compared to non-eradicated group (201 ± 458 pg/mL vs -5 ± 81 pg/mL, respectively, P = 0.02). In the H. pylori-eradicated group, the levels did not differ between the sexes for ghrelin (-6.3 ± 26.9 pg/mL vs -8.0 ± 24.0 pg/mL, respectively, P = 0.97) or obestatin (210 ± 390 pg/mL vs 96 ± 372 pg/mL, respectively, P = 0.23).
CONCLUSION: Serum levels of ghrelin decreased while obestatin levels increased in H. pylori eradicated subjects, especially in overweight and male patients.
Core tip: Ghrelin and obestatin are peptides that have opposing roles in the regulation of appetite and satiety. Helicobacter pylori (H. pylori), a common cause of gastric inflammation, may have important effects on these peptides and in turn be a potential target of anti-obesity strategies. While the interplay between H. pylori and these peptides are well studied, this study included two novel approaches. First, we collected serum samples at two separate time points for both the experimental and control groups, eliminating potential seasonal problems. Second, we focused on not only to H. pylori positive patients that responded to therapy, but also those who did not. This helped to distinguish the effects of antibiotherapy on ghrelin and obestatin regardless of the effectiveness of H. pylori treatment.
-
Citation: Ulasoglu C, Isbilen B, Doganay L, Ozen F, Kiziltas S, Tuncer I. Effect of
Helicobacter pylori eradication on serum ghrelin and obestatin levels. World J Gastroenterol 2013; 19(15): 2388-2394 - URL: https://www.wjgnet.com/1007-9327/full/v19/i15/2388.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i15.2388
Ghrelin and obestatin are both important peptides that regulate appetite and play roles as orexigenic signals and in satiety pathways. Both are secreted mainly from gastric oxyntic mucosa and are thought to be influenced by Helicobacter pylori (H. pylori)[1-4]. However, the influences of H. pylori on serum concentrations of ghrelin are contradictory, since multiple factors interfere with its serum level. Further, and only a limited number of studies have focused on interaction between obestatin and H. pylori[5,6].
Ghrelin is a 28 amino acid (aa) peptide with a name derived from the root ghre-, which means “grow”. This peptide was discovered in 1999 and is secreted endocrine cells of the stomach and by the brain, bowel, testes, pancreatic islet cells, and kidney[1,7] . Ghrelin has adipogenic properties and is an orexigenic peptide, acting as an appetite stimulant. Moreover, it is a somatotropic peptide involved in regulating body weight, is controlled by the GHRL gene, is derived from preproghrelin (contains 117 aa), and acts as a growth hormone stimulator[1,7]. It has a molecular weight of 3370.9 Da. Before release into the serum, an n-octanyl moiety is attached to a serine residue at position three, thus making the molecule hydrophobic and facilitating penetration into the hypothalamus and hypophysis of the brain[1,4,7].
Obestatin is a 23 aa peptide that is thought to be an appetite suppressant and named from the Latin “obedere”, meaning to devour, and “statin”, which denotes suppression[2]. Both ghrelin and obestatin are controlled by ghrelin/obestatin prepropeptide gene (GHRL), is produced by the post-translational modification by addition of -NH2 and splitting from the same protein precursor that also produces ghrelin (117 aa, preproghrelin), and is secreted mainly by the stomach[2,8]. It has a molecular weight of 2516.84 Da and it activates a rhodopsin type G coupled receptor (GPR-39), which is a member of the ghrelin receptor superfamily[2,3,5].
The underlying purpose for this mechanism that produces two hormones with opposite effects remains unclear, however, this may explain earlier findings that initially seemed ambiguous. For example, removing the ghrelin gene from mice does not significantly reduce appetite, and ghrelin may play a physiological role in the vagal control of gastric function in rats[5,6]. Moreover, obestatin counteracts growth hormone secretion and food intake induced by ghrelin[2]. Additionally, intracerebroventricular and systemic injections of obestatin suppress body weight gain in rats. Some gastrointestinal diseases, such as irritable bowel syndrome[7,8], obesity, Prader-Willi syndrome (chromosome 15-related congenital obesity and hyperphagia)[9], and type II diabetes mellitus[10], may be related to the serum ghrelin/obestatin ratio.
H. pylori is a bacteria that is the main cause of gastric inflammation and peptic ulcer disease worldwide. The exact role of H. pylori on appetite hormones, such as ghrelin and obestatin, remains unclear[11-13]. In this study, we compared the changes in these hormones after a successful H. pylori eradication.
The sample population enrolled in this study consisted of ninety-two consecutive patients (50 female and 42 male patients, with ages between 19 and 65 years) who were treated for H. pylori infections based on histopathological diagnoses after upper endoscopies to investigate dyspepsia. H. pylori positive patients received classical anti-Helicobacter triple therapy as treatment (lansoprazole 30 mg bid, amoxicillin 1 g bid, and clarithromycin 500 mg tid for 14 d). Serum ghrelin and obestatin levels were assessed before treatment and eight weeks after the completion of the eradication therapy. Patients had no comorbidities, no chronic illnesses such as diabetes mellitus, no endocrinological disturbances, were not currently taking any medication, and had no history of gastrointestinal surgery. They were restricted from smoking and exercise on sampling day. The success of the H. pylori eradication therapy was assessed with C14-urea breath test (C-UBT) eight weeks after the cessation of the therapy. Before the initial endoscopy and C-UBT, patients did not use any antibiotic or proton pump inhibitors for one month prior. The body mass index (BMI) for each of the patients was defined as their weight in kilograms divided by the square of height in meters (kg/m2). A cutoff of 25 kg/m2 was used to define normal versus overweight participants. The study was done in accordance with the Declaration of Helsinki and using principles of the Good Clinical Practice. The Goztepe Education and Research Hospital approved these studies (18/H-2012). Each patient gave conscious, written, and informed consent before participating in the study.
Fastingblood samples (12 h fast) were collected on the day of upper endoscopy and 8 wk later. The blood was allowedto clot at room temperature without any chemical treatment with protease inhibitors. Within one hour of the blood draw, serum was obtained by centrifugation at 2000 ×g for 10 min and stored at -80 °C until needed. ELISA kits were used for the measurement of active (acylated) serum ghrelin (EMD Millipore, Billerica, MA, United States) and serum obestatin (Peninsula Laboratories LLC, San Carlos, CA, United States). Serum levels of both active ghrelin and obestatin were measured and calculated according to the manufacturers’ instructions. The analytic sensitivity of the active ghrelin test was 25 pg/mL. The intra- and inter-assay coefficients of variation (%CV) for the active ghrelin test when a mean concentration of 65.2 pg/mL was tested were 3.63% and 3.55%, respectively. The obestatin test kit measured human obestatin within the range of 0.412-100 ng/mL, the intra-assay CV was less than 5%, and the inter-assay CV was less than 15%.
The units of measure for these peptides were all converted to pg/mL in order to make comparisons. In some studies ghrelin levels were expressed in fmol/mL and were converted to pg/mL by multiplying by a conversion factor of 3.372. Obestatin levels expressed as pmol/L were converted to pg/mL by multiplying by a conversion factor of 2.5 according to the following formula: pmol/L/0.397 = pg/mL.
Serum ghrelin levels, serum obestatin levels, the ghrelin/obestatin ratios, and the changes in levels as percentage were analyzed according to the patients’ age, sex, BMI, and eradication of H. pylori infection. The Kolmogorov and Shapiro-Wilks tests were used to analyze the normality of the distribution depending on the number of cases (over or under 50, respectively). Independent continuous variables were analyzed using the Mann-Whitney U test. Repeated (paired) measures of serum ghrelin and obestatin levels were analyzed using the Wilcoxon signed-rank test. Alterations in serum ghrelin and obestatin levels after the anti-Helicobacter triple therapy were also calculated as numerical and percentage and then statistically analyzed. The results were given as mean ± SD. All statistics were done using SPSS 20 (Chicago, IL, United States). In all analyses, double sided P values were considered significant if the P value was lower than 0.05.
Successful H. pylori eradication occurred in 52 (74.3%) of 70 infected patients. The remaining 22 patients were not infected with H. pylori at the initial examination and served as the control group. Serum ghrelin and obestatin levels ranged from 28.5-101.4 pg/mL and 180-2230 pg/mL, respectively.
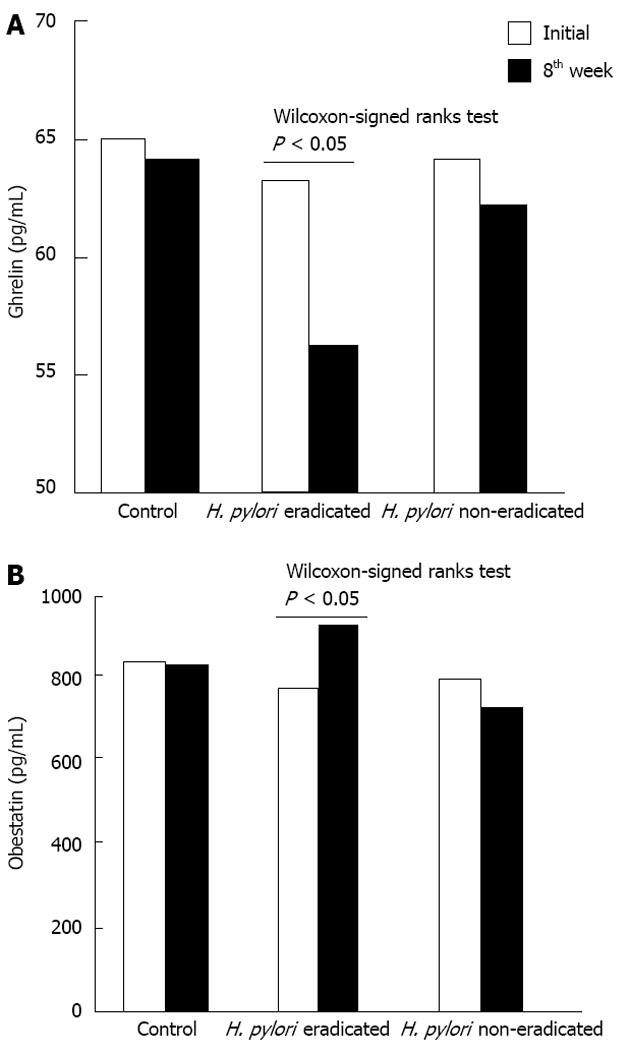
There was no significant difference between the levels of ghrelin or obestatin between the H. pylori infected and control groups. The initial levels of ghrelin in the H. pylori positive cases and the control group were 63.6 ± 19.8 pg/mL and 65.1 ± 19.2 pg/mL, respectively. Initial obestatin levels for these two groups were 771 ± 427 pg/mL and 830 ± 296 pg/mL, respectively (Table 1). Eighth week ghrelin levels were 56.0 ± 19.9 pg/mL, 62.4 ± 19.0 pg/mL, and 63.7 ± 17.4 pg/mL for the eradicated, non-eradicated and the control groups, respectively. Following eight weeks of therapy, obestatin levels were 914 ± 505 pg/mL, 714 ± 269 pg/mL, and 818 ± 291 pg/mL for the eradicated, non-eradicated and the control groups, respectively (Table 1 and Figure 1).
| Controls (n = 22) | H. pylori infected (n = 70) | P value1 | H. pylori eradicated (n = 52) | H. pylori non-eradicated (n = 18) | P value1 | |
| Sex, M/F | 10/12 | 32/38 | 0.98 | 24/28 | 8/10 | 0.99 |
| NW/OW | 9/13 | 31/39 | 0.89 | 23/29 | 8/10 | 0.99 |
| Age (yr) | 40 ± 13 | 38 ± 12 | 0.30 | 38 ± 12 | 37 ± 11 | 0.74 |
| Ghr initial, pg/mL | 65 ± 19 | 64 ± 20 | 0.78 | 63 ± 20 | 64 ± 20 | 0.82 |
| Ghr week 8, pg/mL | 64 ± 17 | 58 ± 20 | 0.16 | 56 ± 20 | 62 ± 19 | 0.35 |
| P value2 | 0.30 | 0.013a | 0.012a | 0.64 | ||
| Ob initial, pg/mL | 830 ± 296 | 771 ± 427 | 0.19 | 765 ± 461 | 787 ± 322 | 0.35 |
| Ob week 8, pg/mL | 818 ± 291 | 863 ± 462 | 0.70 | 914 ± 505 | 714 ± 269 | 0.22 |
| P value2 | 0.65 | 0.07 | 0.01a | 0.32 | ||
| Ghr/Ob ratio initial | 0.089 | 0.107 | 0.420 | 0.113 | 0.092 | 0.460 |
| Ghr/Ob ratio week 8 | 0.092 | 0.086 | 0.480 | 0.081 | 0.099 | 0.140 |
| P value2 | 1.0 | 0.06 | 0.01a | 0.64 | ||
| ∆Ghr | -1.4 | -5.9 | 0.49 | -7.2 | -2.0 | 0.19 |
| ∆Ob | -12.3 | 91.7 | 0.16 | 148.7 | -72.8 | 0.02a |
| ∆Ghr_% | 4.5 | -2.6 | 0.32 | -3.9 | 0.9 | 0.16 |
| ∆Ob_% | -0.9 | 29.4 | 0.13 | 41.5 | -5.3 | 0.03a |
No significant difference between the initial and 8th week measurements of ghrelin and obestatin was observed in the control group (Table 1). The H. pylori eradicated group demonstrated a significant increase in obestatin after eight weeks of treatment, while the control and non-eradicated groups showed only slight decreases. Similarly, ghrelin levels also slightly decreased in the control and non-eradicated groups. Interestingly however, ghrelin levels only showed an insignificant decrease in the eradicated group (Figure 2). The Mann-Whitney U test revealed significant differences in the ghrelin and obestatin levels in the H. pylori eradicated group as compared to the non-eradicated group (Table 1). The ghrelin/obestatin ratio at the initial assessment and the 8th week changed significantly only in the H. pylori eradicated group (Table 1). Overweight patients in the eradicated group demonstrated a significant rise in obestatin levels as compared to that of the overweight patients in the non-eradicated group (Table 2). For all participants with a BMI over 25 kg/m2, the Mann-Whitney U test revealed higher ghrelin and obestatin levels as compared to participants with normal BMIs (Table 2).
| Ghrelin initial, pg/mL | Ghrelin week 8, pg/mL | P value1 | Obestatin initial, pg/mL | Obestatin week 8, pg/mL | P value1 | |
| H. pylori eradicated BMI-NW | 58.8 ± 18.6 | 57.5 ± 22.0 | 0.46 | 759 ± 452 | 865 ± 456 | 0.08 |
| H. pylori eradicated BMI-OW | 68.8 ± 20.3 | 54.1 ± 17.1 | 0.002a | 774 ± 413 | 975 ± 565 | 0.05a |
| P value2 | 0.07 | 0.67 | 0.17 | 0.62 | ||
| H. pylori non-eradicated BMI-NW | 68.7 ± 19.7 | 60.8 ± 22.0 | 0.57 | 780 ± 397 | 653 ± 258 | 0.20 |
| H. pylori non-eradicated BMI-OW | 69.0 ± 21.0 | 64.4 ± 15.6 | 0.88 | 796 ± 221 | 791 ± 280 | 0.77 |
| P value2 | 0.40 | 0.69 | 0.76 | 0.46 |
The differences in ghrelin and obestatin levels in males and females were insignificant in the H. pylori-eradicated group (-6.3 ± 26.9 pg/mL vs -8.0 ± 24.0 pg/mL, respectively, P = 0.97 for ghrelin and 210 ± 390 pg/mL vs 96 ± 372 pg/mL, respectively, P = 0.23 for obestatin). We observed no significant differences in ghrelin or obestatin levels between males and females in non-eradicated and control groups. In the H. pylori-eradicated group, males demonstrated an insignificantly higher percentage change in obestatin than females (51% vs 33%, respectively, P = 0.90), but the males in the H. pylori-eradicated group demonstrated a significantly higher percentage change in obestatin than males in the non-eradicated group (51% vs -8.5%, respectively, P = 0.03).
This study compared the differences in serum acyl-ghrelinand obestatin levels according to H. pylori eradication status, sex, and BMI. The results revealed a significant decrease in ghrelin levels and increase in obestatin levels in the H. pylori eradicated group after treatment. Despite to reported in some studies, no significant sex differences in ghrelin and obestatin levels were observed between any of the subgroups, including the control group.
The normal levels of these peptides can vary widely; the reported ranges for ghrelin and obestatin are between 5.78 to 1732 pg/mL and 200 to 1156 pg/mL , respectively[10,14-22]. In fact, the expected normal serum levels for the different forms of ghrelin are very different: 32.61-65.2 pg/mL for octanoylated ghrelin, 300-430 pg/mL for non-octanoylated ghrelin, and 326-489 pg/mL for total ghrelin, plasma inactive ghrelin (without the n-octanoyl modification) accounts for > 90% of total circulating ghrelin and the ratio of inactive to active ghrelin can be modified under some physiological or pathological conditions. These wide ranges may be due to several reasons including the methodological differences in measurement, the ethnic differences in the study populations, the commercial kit used, the differences in the pre-treatment procedures, the presence of cytotoxin-associated gene A protein positive H. pylori, the nutritional and eating habits of the sample population, and the presence of diabetes or other metabolic syndromes[22]. Therefore, these wide ranges in values should be the subject of further evaluations. To acquire accurate data on ghrelin concentrations, this study recommends a standard procedure for the collection of blood samples: (1) the collection of blood samples with ethylenediaminetetraacetic acid-aprotinin is preferred; (2) blood samples should be chilled and centrifuged as soon as possible, at least within 30 min after collection; and (3) because acidification is the best method for the preservation of plasma ghrelin, 1 mol/L HCl (10% of sample volume) can be added to the plasma sample for adjustment to pH 4[23]. Ghrelin binds to almost 50% to the high density lipoprotein (HDL) in circulation, and the HDL level varies considerably in different ethnic groups[24,25]. Thus, changes in the serum levels of HDL may also alter the ghrelin levels. All patients in our study were of Turkish descent and HDL was not considered, though it would be an interesting area of further study to evaluate how these variables potentially affected the peptide levels.
Small intestine bacterial overgrowth (SIBO) is another possible factor that may explain the variation in ghrelin and obestatin levels, a variable that has been largely neglected to date. Antibiotics used in H. pylori eradication may alter the intestinal flora and may trigger any effects by SIBO on appetite hormones. Any comparison of the H. pylori eradicated and non-eradicated groups may need to overcome the possible effect of SIBO on these peptides[26,27]. Moreover, the half-life of ghrelin is very short (about 60 min), and serum esterase easily breaks ghrelin down to des-octanoyl-ghrelin, the inactive form[28,29]. The differences in these factors and the activity of ghrelin O-acyltransferase may also lead to different results. These technological disadvantages could partially be diminished by including compatible healthy control individuals in each assay[13]. In a study, two popular commercial RIA kits were compared on the same sample set, and a 10-fold difference in the measured total ghrelin levels was seen[30].
In our study, no difference was observed in the ghrelin/obestatin ratio of H. pylori negative and H. pylori positive patients. There are conflicting views of the role of ghrelin and obestatin in the literature. In some reports, the presence of H. pylori was associated with decreasing ghrelin, and its eradication was associated with increased ghrelin levels[31,32]. In contrast, ghrelin decreased after H. pylori eradication in some studies; in fact, Osawa et al[32] reported ghrelin decrease in a majority of patients, while only 50 out of 134 patients demonstrated an increase in this hormone. In Chinese adults,a reduction in the ghrelin/obestatin ratio was associated with patients who were H. pylori positive as compared to uninfected controls[5].
Our results showed that males had insignificantly higher ghrelin and obestatin levels than females (P = 0.66 and P = 0.73, respectively). Even though a number of studies have reported higher ghrelin levels in females[33], fluctuating levels of estrogen related to the different phases of the menstrual cycle may influence serum ghrelin levels[34].
Overweight participants in the H. pylori eradication group demonstrated significant changes in ghrelin and obestatin levels in this study. They showed a decrease in ghrelin levels, an increase in obestatin levels, and a decrease in the ghrelin/obestatin ratio. Thus, overweight cases demonstrated opposite changes that what was expected in terms of their serum ghrelin levels (Table 2).
This study revealed no significant sex and age group (cut-off of 40 years) differences in ghrelin levels, obestatin levels, ghrelin/obestatin ratios, and H. pylori eradication. Changes in the ghrelin/obestatin ratio were also insignificant in comparison to the H. pylori eradicated, non-eradicated, and control groups. According to our results, obestatin, not ghrelin, seems to be more influenced by H. pylori eradication; in fact, this was prominent in overweight and male patients.
The results of these study are valid since influencing factors, errors and procedures were identical for all cases. However, these results suggest a variety of useful, future studies. For example a larger study population would allow generalization of the results to be applicable more than just a small subset of ethnically similar subjects. In addition, it will be interesting to explore several additional variables that were not studied here, including studying subjects in a non-fasting state to allow a comparison of the postprandial or diurnal changes in these hormones. Moreover, including an assessment of the satiety threshold of the patients, taking into account the menstrual status of the female patients and testing for SIBO would also be of great interest.
H. pylori eradication was associated with an significant decrease in ghrelin levels and significant increase in obestatin levels in our study. Due to the contradictory results reported in the literature, the effect of H. pylori on these appetite hormones should be the subject of future studies, and the results may provide important insight for anti-obesity treatment strategies.
Helicobacter pylori (H. pylori) is a major cause of stomach inflammation also worldwide. The pathophysiology of some appetite and satiety peptides such as ghrelin and obestatin secreted from gastric mucosa may be altered by this infection.
Overweight and obesity are major risk factors for a number of chronic diseases, including diabetes, cardiovascular diseases and cancer according to the World Health Organization reports. Worldwide, obesity has increased 82% in the last two decades. A number of studies report the relation of H. pylori with some appetite hormones as ghrelin and obestatin, but the results are not consistent. Some reveal increase and some no change of these peptides related with H. pylori. The reported levels of these hormones vary in a wide spectrum thus all studies need to be accompanied with a control group. In this study, the authors report the influence of H. pylori on these appetite peptides.
In this study, the change of appetite peptides were measured in in three subgroups of human volunteers: H. pylori infected/eradicated, H. pylori infected/non-eradicated and non-infected control. The monitoring of the non-eradicated group eliminated of a number of factors allowing us to focus only on the effect of H.pylori. In this study, comparison of ghrelin and obestatin change between eradicated and non-eradicated group gave the advantage of discarding the effect of receiving antibiotherapy. Also, including the control group provided the exclusion of possible external factors as month, season and placebo effect of being under examination. Finally, as a novel proposal, the effect of small intestinal bacterial overgrowth, considering menstrual cycles and high density lipoprotein influence on these peptides, are also discussed.
The study results suggest that appetite hormones may be related with H. pylori and this may be important in anti-obesity strategies.
Appetite hormones ghrelin and obestatin, two opposite acting peptides involved in appetite and satiety and H. pylori infection and eradication were the main terminological parameters.
The study intends to investigate the changes in serum ghrelin and obestatin levels before and after H. pylori eradication. The authors found that serum ghrelin and obestatin levels decreased and increased in H. pylori eradication groups compared to non-eradicated patients and controls, respectively. These changes were more prominent in overweight and male patients. The manuscript is well written. The methods are adequate. The results justify the conclusions drawn.
P- Reviewers Unger M, Bian ZX, D’Elios MM S- Editor Wen LL L- Editor A E- Editor Li JY
| 1. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. [PubMed] |
| 2. | Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005;310:996-999. [PubMed] |
| 3. | Lagaud GJ, Young A, Acena A, Morton MF, Barrett TD, Shankley NP. Obestatin reduces food intake and suppresses body weight gain in rodents. Biochem Biophys Res Commun. 2007;357:264-269. [PubMed] |
| 4. | Gourcerol G, Taché Y. Obestatin--a ghrelin-associated peptide that does not hold its promise to suppress food intake and motility. Neurogastroenterol Motil. 2007;19:161-165. [PubMed] |
| 5. | Gao XY, Kuang HY, Liu XM, Duan P, Yang Y, Ma ZB. Circulating ghrelin/obestatin ratio in subjects with Helicobacter pylori infection. Nutrition. 2009;25:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, Hosoda H, Kojima M, Kangawa K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276:905-908. [PubMed] |
| 7. | Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495-522. [PubMed] |
| 8. | Hassouna R, Zizzari P, Viltart O, Yang SK, Gardette R, Videau C, Badoer E, Epelbaum J, Tolle V. A natural variant of obestatin, Q90L, inhibits ghrelin’s action on food intake and GH secretion and targets NPY and GHRH neurons in mice. PLoS One. 2012;7:e51135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | DelParigi A, Tschöp M, Heiman ML, Salbe AD, Vozarova B, Sell SM, Bunt JC, Tataranni PA. High circulating ghrelin: a potential cause for hyperphagia and obesity in prader-willi syndrome. J Clin Endocrinol Metab. 2002;87:5461-5464. [PubMed] |
| 10. | Cindoruk M, Yetkin I, Deger SM, Karakan T, Kan E, Unal S. Influence of H pylori on plasma ghrelin in patients without atrophic gastritis. World J Gastroenterol. 2007;13:1595-1598. [PubMed] |
| 11. | Konturek PC, Cześnikiewicz-Guzik M, Bielanski W, Konturek SJ. Involvement of Helicobacter pylori infection in neuro-hormonal control of food intake. J Physiol Pharmacol. 2006;57 Suppl 5:67-81. [PubMed] |
| 12. | Isomoto H, Ueno H, Saenko VA, Mondal MS, Nishi Y, Kawano N, Ohnita K, Mizuta Y, Ohtsuru A, Yamashita S. Impact of Helicobacter pylori infection on gastric and plasma ghrelin dynamics in humans. Am J Gastroenterol. 2005;100:1711-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Kosowicz J, Baumann-Antczak A, Zamysłowska H, Sowiński J. Technological difficulties in ghrelin and obestatin assays. Endokrynol Pol. 2011;62:336-339. [PubMed] |
| 14. | Polat Z, Kilciler G, Ozel AM, Kara M, Kantarcioglu M, Uygun A, Bagci S. Plasma ghrelin levels in patients with familial Mediterranean fever. Dig Dis Sci. 2012;57:1660-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Sawicka B, Bossowski A, Urban M, Szalecki M, Wysocka J, Koput A, Zelazowska-Rutkowska B, Tobolczyk J, Skrzydło M, Rogowski F. Analysis of serum levels of ghrelin and obestatin in children and adolescents with autoimmune thyroid diseases. Pediatr Endocrinol Diabetes Metab. 2009;15:20-27. [PubMed] |
| 16. | Deng ZH, Chu B, Xu YZ, Zhang B, Jiang LR. Influence of Helicobacter pylori infection on ghrelin levels in children. World J Gastroenterol. 2012;18:5096-5100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Gutierrez-Grobe Y, Villalobos-Blasquez I, Sánchez-Lara K, Villa AR, Ponciano-Rodríguez G, Ramos MH, Chavez-Tapia NC, Uribe M, Méndez-Sánchez N. High ghrelin and obestatin levels and low risk of developing fatty liver. Ann Hepatol. 2010;9:52-57. [PubMed] |
| 18. | Komarowska H, Jaskula M, Stangierski A, Wasko R, Sowinski J, Ruchala M. Influence of ghrelin on energy balance and endocrine physiology. Neuro Endocrinol Lett. 2012;33:749-756. [PubMed] |
| 19. | Riis AL, Hansen TK, Møller N, Weeke J, Jørgensen JO. Hyperthyroidism is associated with suppressed circulating ghrelin levels. J Clin Endocrinol Metab. 2003;88:853-857. [PubMed] |
| 20. | Granata R, Settanni F, Gallo D, Trovato L, Biancone L, Cantaluppi V, Nano R, Annunziata M, Campiglia P, Arnoletti E. Obestatin promotes survival of pancreatic beta-cells and human islets and induces expression of genes involved in the regulation of beta-cell mass and function. Diabetes. 2008;57:967-979. [PubMed] |
| 21. | Zamrazilová H, Hainer V, Sedlácková D, Papezová H, Kunesová M, Bellisle F, Hill M, Nedvídková J. Plasma obestatin levels in normal weight, obese and anorectic women. Physiol Res. 2008;57 Suppl 1:S49-S55. [PubMed] |
| 22. | Zou CC, Liang L, Wang CL, Fu JF, Zhao ZY. The change in ghrelin and obestatin levels in obese children after weight reduction. Acta Paediatr. 2009;98:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Hosoda H, Doi K, Nagaya N, Okumura H, Nakagawa E, Enomoto M, Ono F, Kangawa K. Optimum collection and storage conditions for ghrelin measurements: octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples. Clin Chem. 2004;50:1077-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 179] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Hassouna R, Zizzari P, Tolle V. The ghrelin/obestatin balance in the physiological and pathological control of growth hormone secretion, body composition and food intake. J Neuroendocrinol. 2010;22:793-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Ujcic-Voortman JK, Bos G, Baan CA, Uitenbroek DG, Verhoeff AP, Seidell JC. Ethnic differences in total and HDL cholesterol among Turkish, Moroccan and Dutch ethnic groups living in Amsterdam, the Netherlands. BMC Public Health. 2010;10:740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Sajjad A, Mottershead M, Syn WK, Jones R, Smith S, Nwokolo CU. Ciprofloxacin suppresses bacterial overgrowth, increases fasting insulin but does not correct low acylated ghrelin concentration in non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;22:291-299. [PubMed] |
| 27. | Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V, Kopacova M. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16:2978-2990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 383] [Cited by in RCA: 364] [Article Influence: 24.3] [Reference Citation Analysis (4)] |
| 28. | Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753-4758. [PubMed] |
| 29. | Tolle V, Bassant MH, Zizzari P, Poindessous-Jazat F, Tomasetto C, Epelbaum J, Bluet-Pajot MT. Ultradian rhythmicity of ghrelin secretion in relation with GH, feeding behavior, and sleep-wake patterns in rats. Endocrinology. 2002;143:1353-1361. [PubMed] |
| 30. | Gröschl M, Uhr M, Kraus T. Evaluation of the comparability of commercial ghrelin assays. Clin Chem. 2004;50:457-458. [PubMed] |
| 31. | Nwokolo CU, Freshwater DA, O’Hare P, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut. 2003;52:637-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Osawa H, Kita H, Ohnishi H, Nakazato M, Date Y, Bowlus CL, Ishino Y, Watanabe E, Shiiya T, Ueno H. Changes in plasma ghrelin levels, gastric ghrelin production, and body weight after Helicobacter pylori cure. J Gastroenterol. 2006;41:954-961. [PubMed] |
| 33. | Stec-Michalska K, Malicki S, Michalski B, Peczek L, Wisniewska-Jarosinska M, Nawrot B. Gastric ghrelin in relation to gender, stomach topography and Helicobacter pylori in dyspeptic patients. World J Gastroenterol. 2009;15:5409-5417. [PubMed] |
| 34. | De Souza MJ, Leidy HJ, O’Donnell E, Lasley B, Williams NI. Fasting ghrelin levels in physically active women: relationship with menstrual disturbances and metabolic hormones. J Clin Endocrinol Metab. 2004;89:3536-3542. [PubMed] |